Abstract
OBJECTIVE
Common genetic variants in GCK and TCF7L2 are associated with higher fasting glucose and type 2 diabetes in nonpregnant populations. However, their associations with glucose levels from oral glucose tolerance tests (OGTTs) in pregnancy have not been assessed in a large sample. We hypothesized that these variants are associated with quantitative measures of glycemia in pregnancy.
RESEARCH DESIGN AND METHODS
We analyzed the associations between variants rs1799884 (GCK) and rs7903146 (TCF7L2) and OGTT outcomes at 24–32 weeks' gestation in 3,811 mothers of European (U.K. and Australia) and 1,706 mothers of Asian (Thailand) ancestry from the HAPO cohort. We also tested associations with offspring birth anthropometrics.
RESULTS
The maternal GCK variant was associated with higher fasting glucose in Europeans (P = 0.001) and Thais (P < 0.0001), 1-h glucose in Europeans (P = 0.001), and 2-h glucose in Thais (P = 0.005). It was also associated with higher European offspring birth weight, fat mass, and skinfold thicknesses (P < 0.05). The TCF7L2 variant was associated with all three maternal glucose outcomes (P = 0.03, P < 0.0001, and P < 0.0001 for fasting and 1-h and 2-h glucose, respectively) in the Europeans but not in the Thais (P > 0.05). In both populations, both variants were associated with higher odds of gestational diabetes mellitus according to the new International Association of Diabetes and Pregnancy Study Groups recommendations (P = 0.001–0.08).
CONCLUSIONS
Maternal GCK and TCF7L2 variants are associated with glucose levels known to carry an increased risk of adverse pregnancy outcome in women without overt diabetes. Further studies will be important to determine the variance in maternal glucose explained by all known genetic variants.
Maternal glycemia in pregnancy is associated with adverse pregnancy outcomes including birth weight >90th percentile, delivery by cesarean section, neonatal hypoglycemia, and fetal hyperinsulinemia (1). These associations occur across the full range of maternal glucose levels below those classified as overt diabetes.
In healthy, nondiabetic, nonpregnant populations, approximately one-third of the variation in fasting glucose is genetic (2), and common genetic variants at multiple loci are now robustly associated with fasting glucose (3–10) and with type 2 diabetes and related glycemic traits (11–18). Thus, genetic factors are likely to contribute to variation in glucose levels in pregnancy. However, these variants have not been examined extensively in large studies of pregnant women.
Studies of birth weight in Europeans have provided indirect evidence that two common genetic variants influence maternal glycemia in pregnancy. The T-allele of the rs1799884 variant in the GCK gene is associated with higher fasting glucose in the general population (4) and with type 2 diabetes (10). Pregnant women who carry this allele give birth to babies that are, on average, 32 g (95% CI 11–53) heavier at birth (4). Similarly, each additional T-allele of rs7903146 in the TCF7L2 gene—which is associated with reduced β-cell function, raised fasting glucose, and type 2 diabetes (10,13,19)—is also associated with a 30-g (95% CI 15–45) higher offspring birth weight when carried by the mother (20). We hypothesize that these associations with birth weight reflect higher levels of maternal glucose, which result in greater fetal insulin secretion and a consequent increase in fetal size at birth (21).
There is some evidence from small studies that the GCK and TCF7L2 variants are associated with fasting glucose in pregnancy or gestational diabetes mellitus. The GCK variant was associated with higher fasting glucose in 755 European pregnant women from the U.K. (22) and with gestational diabetes mellitus in a Scandinavian sample (23). Variation at the TCF7L2 locus was not associated with fasting glucose in 921 European pregnant women (20) but was associated with gestational diabetes mellitus in Scandinavian (24,25), Korean (26), and Mexican-American (27) samples.
The large sample size and detailed pregnancy and birth phenotype data available in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study provide a unique opportunity to investigate more thoroughly the associations of the GCK and TCF7L2 variants with maternal glycemia, as measured in an oral glucose tolerance test (OGTT), during pregnancy as well as fetal size at birth and body composition. We used OGTT results from 5,517 pregnant women of European and South East Asian ancestry to assess associations both with quantitative measures of maternal glucose and with the new International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommendations for the diagnosis of gestational diabetes mellitus (28).
RESEARCH DESIGN AND METHODS
We studied 3,811 pregnant women of European ancestry (Manchester and Belfast, U.K., and Newcastle and Brisbane, Australia) and 1,706 pregnant women of South East Asian ancestry (Bangkok, Thailand). The protocol was approved by the institutional review board at each field center, and all participants gave written, informed consent.
Maternal phenotypes and exclusion criteria.
The HAPO study methods have previously been described in detail (1,29,30). Briefly, eligible women (30) underwent a 75-g OGTT at 24–32 weeks' gestation (as close to 28 weeks as possible). Fasting, 1-h, and 2-h glucose levels were measured. Height, weight, and blood pressure were also measured using standardized procedures and calibrated equipment. A sample for random plasma glucose was collected at 34–37 weeks' gestation as a safety measure to identify cases with hyperglycemia above a predefined threshold. Gestational age was determined as previously described (30). Demographic and lifestyle characteristics, age, and parity were collected via questionnaire. Race/ethnicity was self-identified.
Participants, caregivers, and HAPO study staff (except laboratory personnel) remained blinded to glucose values unless fasting plasma glucose was >5.8 mmol/l, 2-h OGTT plasma glucose was >11.1 mmol/l, random plasma glucose was ≥8.9 mmol/l, or any plasma glucose value was <2.5 mmol/l. Although unblinded participants were excluded from the original HAPO study of pregnancy outcome (1), we did not exclude them from maternal glucose analyses in the current study because there was no intervention to alter maternal glucose levels before the OGTT.
Neonatal phenotypes.
Cord blood samples for DNA, plasma glucose, and serum C-peptide were collected from the offspring at delivery. Neonatal anthropometric data were collected within 72 h of delivery. These have been described in detail previously (31). In the current study, we analyzed weight, length, head circumference, triceps, and flank and subscapular skinfold thicknesses. Birth weight, length, and head circumference were available from medical records in addition to the measurements taken by HAPO study personnel. To maximize sample size, we used the medical record birth weight because it was more widely available and highly correlated with the HAPO-measured birth weight (r = 0.98). We used the HAPO study measures for length and head circumference and added medical record values when these were unavailable. Fat mass at birth (grams) was derived using the following formula (32):
where BW is birth weight in grams, FLS is flank skinfold thickness in millimeters, and BL is birth length in centimeters (all measured by HAPO study personnel). From this, percent body fat was derived as follows: 100 × fat mass/birth weight.
Genotyping.
We selected the rs1799884 (GCK) and rs7903146 (TCF7L2) single nucleotide polymorphisms (SNPs) for association analysis. As well as being robustly associated with higher fasting glucose and type 2 diabetes in Europeans (4,10,19), these SNPs, or perfectly correlated (r2 = 1) proxies, show similar associations in East Asian and South Asian populations (33–37). We therefore carried out our primary analyses using only these SNPs. However, we also selected TCF7L2 rs290487 and rs11196218 for analysis in our Thai samples because they have shown independent evidence of association with type 2 diabetes in East Asians (r2 < 0.1 with rs7903146 and r2 = 0.01 with each other in the HapMap Han Chinese from Beijing and Japanese from Tokyo reference samples) (35,38–40) and are more common than rs7903146 in these samples, giving greater power to detect associations.
Genotyping was carried out as part of a larger candidate panel of 1,536 SNPs using the Illumina Golden Gate platform in the Northwestern University Genomics Core. We included DNA samples in our study that were successfully genotyped for over 94% of SNPs. Call rates for the GCK and TCF7L2 SNPs exceeded 99% in these samples, and there was not deviation from Hardy-Weinberg equilibrium (P > 0.01 for the four SNPs in each of the five field centers). The frequency of the T-allele of GCK rs1799884 (associated with higher glucose levels) ranged from 17.1 to 18.7% in the European samples and was 10.3% in the Thai sample. The type 2 diabetes risk allele frequency of TCF7L2 rs7903146 ranged from 29.2 to 30.6% in the Europeans and was 4.7% in the Thai. The rs290487 and rs11196218 risk allele frequencies (risk alleles defined by previous studies) (35,38–40) were 47.4 and 72.4%, respectively, in the Thai sample.
Statistical analyses: associations between maternal genotype and maternal glycemia.
All analyses were carried out using Stata (version 10; Stata, College Station, TX). We analyzed the association between each of the three primary outcome measures (fasting plasma glucose, 1-h OGTT glucose, and 2-h OGTT glucose) and genotype using linear regression under an additive genetic model. We included maternal age, BMI, and mean arterial pressure as covariates in the model. We repeated the analysis including additional covariates (BMI squared, gestational age at OGTT, parity, sex of the baby, and maternal height) to check that they did not change the results. We analyzed the European and Thai samples separately and included field center as a covariate in all analyses of Europeans.
We also sought to investigate the association of each SNP with maternal glucose levels that carry a substantially higher risk of adverse pregnancy outcome. We created a high glucose variable with a value of 1 if the participant had one or more high values in the OGTT (fasting plasma glucose ≥5.1 mmol/l, 1-h glucose ≥10.0 mmol/l, or 2-h glucose ≥8.5 mmol/l) and a value of 0 if the glucose was below these thresholds. The high glucose threshold corresponds to an odds ratio (OR) of 1.75, relative to the mean glucose level, averaged across three outcomes in the HAPO study: offspring birth weight >90th percentile, cord C-peptide >90th percentile, offspring percent body fat >90th percentile, and is the IADPSG-recommended threshold for gestational diabetes mellitus (28). We analyzed the association between each SNP and the odds of high glucose using logistic regression (log-additive genetic model), adjusting for field center (European ancestry samples only) maternal age, BMI, and mean arterial pressure. Using all 5,515 study subjects with both GCK and TCF7L2 genotype available (and adjusting for field center), we then analyzed the association between the odds of high glucose and the combined number of T-alleles at both loci (0, 1, 2, and 3 or 4), with the same covariates as before. We combined individuals with three or four T-alleles into one group because of low numbers in the final category. To test for deviation from a multiplicative trend across the four groups, we compared the results with a full model (including the allele score as indicator variables) using a likelihood ratio test.
To guard against possible population stratification, we generated principal components of ancestry using smartpca from the Eigensoft software package (41) based on 141 ancestry informative markers that were genotyped in the same panel of 1,536 SNPs as the GCK and TCF7L2 SNPs. We repeated our analyses including the first two principal components as covariates.
Associations between maternal genotype and neonatal anthropometric traits.
We analyzed the association between each neonatal outcome and maternal genotype using linear regression (additive genetic model). We performed the analysis twice: 1) a minimally adjusted model with field center (European ancestry only), sex of the baby, and gestational age at delivery as covariates and 2) a fully adjusted model including maternal age at OGTT, maternal BMI at OGTT, maternal BMI at OGTT squared, parity, maternal smoking (yes/no), mean arterial pressure, and maternal height as additional covariates. The first analysis was performed to enable comparison with previously published studies (4,20,22), and the second was performed for comparison with the first to verify that the additional covariates did not change the results. All analyses of neonatal outcomes excluded babies born preterm (before 37 full weeks of gestation) and pregnancies in which caregivers were unblinded to maternal glucose levels.
We performed inverse variance meta-analysis (fixed effects) of the association between each SNP and birth weight to combine the HAPO study data with data from previously published studies (4,20,22). Within each study sample, the association between genotype and birth weight was analyzed using linear regression under an additive genetic model, with sex and gestational age at delivery as covariates. For the meta-analysis, we used the METAN module developed for Stata (42). Heterogeneity between studies was estimated using Cochran's Q test and the I2 statistic (43). We also used Cochran's Q test to assess evidence of heterogeneity between the analyses of maternal glucose outcomes in the European and Thai samples.
Associations between fetal genotype and birth weight.
We analyzed the association between birth weight and fetal genotype using linear regression (additive model), adjusting for field center (European ancestry only), sex, and gestational age and again excluding preterm births and unblinded pregnancies. Since maternal and fetal genotypes are correlated, we then repeated the analysis stratifying by maternal genotype and tested for evidence of a fetal genotype–birth weight association within each stratum. To address whether fetal genotype alters the impact of maternal glucose levels on offspring birth weight, fat mass, or skinfold thickness, we additionally tested for evidence of interaction between fetal genotype and maternal glucose using a likelihood ratio test.
Power calculations.
Our sample of 3,811 Europeans gave us 80% power to detect per-allele differences in an outcome of 0.09 SD and 0.07 SD for the GCK and TCF7L2 SNPs, respectively, at P < 0.05. Due to the lower sample size and allele frequencies in the Thai sample, power to detect these associations was reduced (35 and 14%, respectively). Power calculations were performed using Quanto, version 1.2 (44).
Comparing the discriminatory impact of maternal genotype with measured glucose on birth weight and neonatal adiposity.
To address whether maternal GCK and TCF7L2 genotypes improve prediction of birth weight or neonatal adiposity in the presence of different combinations of other known variables, we used logistic regression to model the odds of birth weight, skinfold sum, and percent body fat >90th percentile against various explanatory variables, including both maternal GCK and TCF7L2 genotypes (included as indicator variables). We constructed receiver operator characteristic (ROC) curves and calculated the area under the curve (AUC) to estimate the discriminatory power of the model. The AUCs were compared for various models using a χ2 test. For this analysis, we combined all of the study subjects and adjusted for study center.
RESULTS
Associations between maternal genotype and maternal glycemia.
Basic characteristics of the study participants are presented in Table 1. The associations between continuous measures of maternal glucose, as determined during an OGTT, and the GCK and TCF7L2 variants are presented in Table 2 and Table 3, respectively. Summary data from each of the field centers with European ancestry women are presented in supplementary Tables 1 and 2, available in an online appendix (http://diabetes.diabetesjournals.org/cgi/content/full/db10-0177/DC1).
TABLE 1.
Basic characteristics of study participants
| Belfast, U.K. | Manchester, U.K. | Brisbane, Australia | Newcastle, Australia | Bangkok, Thailand | |
|---|---|---|---|---|---|
| Pregnant women (n)* | 1,284 | 1,085 | 959 | 483 | 1,706 |
| Gestational age at which OGTT was performed (weeks) | 29.0 ± 1.2 | 28.4 ± 1.0 | 28.1 ± 1.2 | 28.1 ± 1.5 | 28.1 ± 1.7 |
| Age at OGTT (years) | 29.8 ± 5.5 | 30.8 ± 5.6 | 29.2 ± 5.3 | 29.5 ± 5.5 | 27.9 ± 5.5 |
| BMI at OGTT (kg/m2) | 28.4 ± 4.9 | 29.0 ± 5.5 | 29.0 ± 5.7 | 29.7 ± 6.0 | 25.6 ± 3.6 |
| Mean arterial pressure at OGTT (mmHg) | 83.6 ± 7.9 | 83.5 ± 8.0 | 83.8 ± 7.7 | 82.9 ± 8.1 | 80.1 ± 7.8 |
| FPG (mmol/l) | 4.63 ± 0.37 | 4.61 ± 0.40 | 4.43 ± 0.35 | 4.54 ± 0.41 | 4.44 ± 0.37 |
| 1-h plasma glucose (mmol/l) | 7.50 ± 1.70 | 7.46 ± 1.80 | 7.40 ± 1.51 | 7.33 ± 1.66 | 8.27 ± 1.75 |
| 2-h plasma glucose (mmol/l) | 6.08 ± 1.23 | 5.99 ± 1.35 | 6.25 ± 1.20 | 6.21 ± 1.33 | 6.66 ± 1.40 |
| Offspring birth weight in g† | 3,526 ± 489 | 3,511 ± 498 | 3,552 ± 466 | 3,585 ± 466 | 3,142 ± 399 |
| Gestational age at delivery (weeks)† | 40.1 ± 1.1 | 40.0 ± 1.3 | 40.0 ± 1.2 | 40.1 ± 1.2 | 39.4 ± 1.2 |
| Male offspring (%)† | 50.6 | 52.1 | 51.1 | 51.2 | 48.7 |
| Maternal smoking during pregnancy (%)† | 23.7 | 19.2 | 13.2 | 15.5 | 0.6 |
| Primiparous births (%)† | 50.2 | 47.8 | 55.8 | 46.1 | 53.3 |
Data are means ± SD unless otherwise indicated.
*Number of women with fasting glucose, GCK rs1779984 genotype, age, BMI, and mean arterial pressure available. The numbers with TCF7L2 rs7903146 genotype were very similar.
†Excluding births before 37 completed weeks of gestation and pregnancies in which caregivers were not blinded to maternal glucose levels. FPG, fasting plasma glucose.
TABLE 2.
Association between maternal GCK rs1799884 genotype and maternal glucose levels in pregnancy
| Total N | Mean ± SE plasma glucose level by GCK rs1799884 genotype |
Effect size ± SE per T-allele (mmol/l)*† | P† | |||
|---|---|---|---|---|---|---|
| CC | CT | TT | ||||
| FPG (mmol/l) | ||||||
| European | 3,811 | 4.55 ± 0.01 | 4.59 ± 0.01 | 4.63 ± 0.03 | 0.03 ± 0.01 | 0.001 |
| Thai | 1,706 | 4.42 ± 0.01 | 4.51 ± 0.02 | 4.52 ± 0.08 | 0.08 ± 0.02 | <0.0001 |
| 1-h plasma glucose (mmol/l) | ||||||
| European | 3,811 | 7.39 ± 0.03 | 7.54 ± 0.05 | 7.69 ± 0.15 | 0.15 ± 0.05 | 0.001 |
| Thai | 1,706 | 8.25 ± 0.05 | 8.35 ± 0.09 | 8.54 ± 0.37 | 0.11 ± 0.09 | 0.24 |
| 2-h plasma glucose (mmol/l) | ||||||
| European | 3,811 | 6.11 ± 0.02 | 6.12 ± 0.04 | 6.18 ± 0.11 | 0.02 ± 0.04 | 0.56 |
| Thai | 1,706 | 6.61 ± 0.04 | 6.85 ± 0.08 | 6.81 ± 0.30 | 0.21 ± 0.07 | 0.005 |
The European plasma glucose levels by genotype are the mean values across all four field centers. These are presented separately in supplementary Table 1.
*The T-allele of GCK rs1799884 is associated with raised fasting glucose in nondiabetic, nonpregnant populations. The T-allele frequency ranged from 17.1 to 18.7% in the European ancestry samples and was 10.3% in the Thai samples.
†The effect sizes and P values are from linear regression of maternal glucose level against genotype (coded 0, 1, or 2 T-alleles), with field center (European ancestry only), age, BMI, and mean arterial pressure as covariates. Age, BMI, and mean arterial pressure were measured at a median of 28–29 weeks' gestation, depending on the field center. Mean ± SE values are adjusted for these three covariates. FPG, fasting plasma glucose.
TABLE 3.
Association between maternal TCF7L2 rs7903146 genotype and maternal glucose levels in pregnancy
| Total N | Mean ± SE plasma glucose level by TCF7L2 rs7903146 genotype |
Effect size ± SE per T-allele (mmol/l)*† | P† | |||
|---|---|---|---|---|---|---|
| CC | CT | TT | ||||
| FPG (mmol/l) | ||||||
| European | 3,811 | 4.56 ± 0.01 | 4.55 ± 0.01 | 4.63 ± 0.02 | 0.02 ± 0.01 | 0.03 |
| Thai | 1,706 | 4.44 ± 0.01 | 4.45 ± 0.03 | 4.44 ± 0.21 | 0.01 ± 0.03 | 0.66 |
| 1-h plasma glucose (mmol/l) | ||||||
| European | 3,811 | 7.34 ± 0.04 | 7.51 ± 0.04 | 7.65 ± 0.08 | 0.16 ± 0.04 | <0.0001 |
| Thai | 1,706 | 8.25 ± 0.04 | 8.53 ± 0.13 | 7.39 ± 0.96 | 0.23 ± 0.14 | 0.09 |
| 2-h plasma glucose (mmol/l) | ||||||
| European | 3,811 | 6.04 ± 0.03 | 6.18 ± 0.03 | 6.27 ± 0.06 | 0.13 ± 0.03 | <0.0001 |
| Thai | 1,706 | 6.64 ± 0.03 | 6.86 ± 0.11 | 5.81 ± 0.77 | 0.17 ± 0.11 | 0.11 |
The European plasma glucose levels by genotype are the mean values across all four field centers. These are presented separately in supplementary Table 2.
*The T-allele of TCF7L2 rs7903146 is associated with an increased risk of type 2 diabetes. The T-allele frequency ranged from 29.2 to 30.6% in the European ancestry samples and was 4.7% in the Thai samples.
†The effect sizes and P values are from linear regression of maternal glucose level against genotype (coded 0, 1, or 2 T-alleles), with field center (European ancestry only), age, BMI, and mean arterial pressure as covariates. Age, BMI, and mean arterial pressure were measured at a median of 28–29 weeks' gestation, depending on the field center. Mean ± SE values by genotype are adjusted for these three covariates. FPG, fasting plasma glucose.
We observed associations between the GCK rs1799884 variant and fasting glucose in both the European (0.03 mmol/l higher per T-allele [95% CI 0.01–0.05]; P = 0.001) and Thai samples (0.08 mmol/l higher per T-allele [0.04–0.12]; P < 0.0001). We also observed an association with 1-h glucose in the Europeans (P = 0.001) but not in the Thais (P = 0.24). However, the estimated differences were similar (per T-allele increase 0.15 mmol/l [0.06–0.25] in the Europeans and 0.11 mmol/l [−0.07 to 0.29] in the Thais). There was evidence of association with 2-h glucose in the Thai sample (0.21 mmol/l higher per T-allele [95% CI 0.06–0.35]; P = 0.005), but not in the Europeans (0.02 mmol/l per T-allele [−0.05–0.09]; P = 0.56). There was evidence of heterogeneity between the European and Thai samples for the fasting (P = 0.03) and 2-h (P = 0.02) glucose measures but not the 1-h measure (P = 0.70). When we repeated these analyses including additional covariates, we observed similar results (supplementary Table 3).
In the European samples, we observed associations between the TCF7L2 rs7903146 variant and both 1-h (0.16 mmol/l higher per T-allele [95% CI 0.08–0.24]; P < 0.0001) and 2-h (0.13 mmol/l higher per T-allele [0.07–0.19]; P < 0.0001) glucose levels. There was also some evidence for association with fasting glucose (0.02 mmol/l higher per T-allele [0.002–0.03]; P = 0.03). We observed no evidence for association at P < 0.05 between the TCF7L2 variant and the maternal glucose measures in the Thai samples, reflecting either no association in this population or reduced power due to the lower minor allele frequency. The estimated differences were similar to those in the Europeans (all heterogeneity P values >0.6). When we repeated these analyses including additional covariates, we observed similar results (supplementary Table 4). There were also no associations between any of the maternal glucose outcomes and the TCF7L2 rs290487 or rs11196218 SNPs in the Thai sample (P > 0.05; data not shown).
Consistent with the results for continuous glucose measures, we observed associations between both the GCK and TCF7L2 variant and the odds of high glucose, defined by the IADPSG (28) as having one or more of the following: fasting plasma glucose ≥5.1 mmol/l, 1-h glucose ≥10.0 mmol/l, and 2-h glucose ≥8.5 mmol/l (Table 4). In the Europeans, 26% of women with the GCK rs1799884 TT genotype had high glucose, compared with 15% with the CC genotype, and the per-allele OR was 1.29 (95% CI 1.09–1.50). The corresponding OR in the Thai sample was 1.42 (1.06–1.77). For TCF7L2 rs7903146, 20% of women of European ancestry with the TT genotype had high glucose, compared with 16% with the CC genotype. The per-allele ORs were 1.15 (1.00–1.31) in the Europeans and 1.39 (0.88–1.90) in the Thais. Analysis of the complete dataset, combining information from both GCK and TCF7L2, showed strong evidence for a trend to increased odds of high glucose with an increasing number of T-alleles (OR 1.25 [95% CI 1.14–1.37]; P < 0.00001) (supplementary Fig. 1). There was no evidence of deviation from a multiplicative model (P = 0.28).
TABLE 4.
Association between GCK or TCF7L2 genotype and high* maternal glucose levels
| Ancestry and genotype | No. of women with high/normal glucose (% high) | Per T-allele OR (95% CI) for high glucose† | P† |
|---|---|---|---|
| GCK rs1799884 | |||
| European | 1.29 (1.09–1.50) | 0.001 | |
| CC | 388/2,575 (15.1) | ||
| CT | 194/1,114 (17.4) | ||
| TT | 32/122 (26.2) | ||
| Thai | 1.42 (1.06–1.77) | 0.007 | |
| CC | 288/1,375 (20.9) | ||
| CT | 91/311 (29.3) | ||
| TT | 5/20 (25.0) | ||
| TCF7L2 rs7903146 | |||
| European | 1.15 (1.00–1.31) | 0.04 | |
| CC | 293/1,884 (15.6) | ||
| CT | 246/1,557 (15.8) | ||
| TT | 75/370 (20.3) | ||
| Thai | 1.39 (0.88–1.90) | 0.08 | |
| CC | 338/1,549 (21.8) | ||
| CT/TT‡ | 46/157 (29.3) |
*Defined as fasting glucose ≥5.1 mmol/l or 1-h glucose ≥10.0 mmol/l or 2-h glucose ≥8.5 mmol/l.
†ORs and P values are from logistic regression of high glucose status (1 or 0) against genotype (coded 0, 1, or 2 T-alleles), with field center (European ancestry only), age, BMI, and mean arterial pressure as covariates. Age, BMI, and mean arterial pressure were measured at a median of 28–29 weeks' gestation, depending on the field center.
‡Categories are combined because of the small number of TT homozygotes (n = 3).
Inclusion of the first two ancestry principal components as covariates did not change the associations between the GCK and TCF7L2 variant and maternal glucose outcomes (data not shown). A sensitivity analysis including only participants whose caregivers remained blinded to glucose levels gave very similar results for all associations.
Associations between maternal genotype and neonatal anthropometric traits.
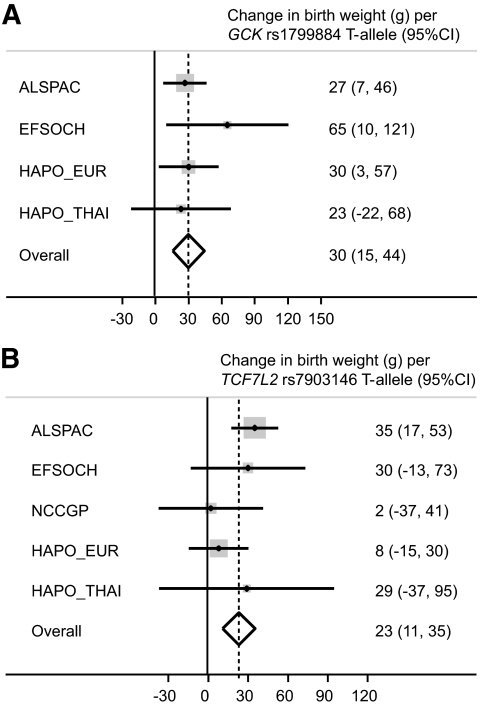
Meta-analysis of the associations between the maternal GCK and TCF7L2 variants and offspring birth weight, using both Thai and European study samples, showed that those in the HAPO study were consistent in size and direction with previously published studies of Europeans (heterogeneity P values >0.05; I2 < 20%) (Fig. 1). The overall P values for association with birth weight were P < 0.0001 for maternal GCK rs1799884 (n = 12,643) and P < 0.001 for maternal TCF7L2 rs7903146 (n = 13,406).
FIG. 1.
Meta-analysis of the association between offspring birth weight and maternal GCK rs1799884 genotype (overall P < 0.0001) (A) or maternal TCF7L2 rs7903146 genotype (overall P < 0.001) (B) in the HAPO study and previously published studies (4,20,22). Analyses were adjusted for sex and gestational age. ALSPAC, Avon Longitudinal Study of Parents and Children; EFSOCH, Exeter Family Study of Childhood Health; NCCGP, North Cumbria Community Genetics Project.
We analyzed the associations between the maternal GCK and TCF7L2 variants and various neonatal traits (supplementary Tables 5 and 6). We did not observe an association with cord C-peptide at either locus (P > 0.05). After Bonferroni adjustment for 10 tests, there was no association at P < 0.05 with neonatal anthropometric traits at either locus. However, in the Europeans, the GCK rs1799884 effect size estimates for all traits were in the direction expected, given the association with maternal glucose, and the unadjusted P values for association with birth weight, fat mass, percent body fat, and skinfold thickness were all <0.05.
Associations between fetal genotype and birth weight.
We observed no overall association between birth weight and the fetal genotype at either locus (P > 0.05; data not shown). To address the correlation between maternal and fetal genotype, we stratified by maternal genotype, but there was no evidence of association (P > 0.05) after multiple testing correction (supplementary Fig. 2). We observed no evidence of interaction between fetal genotype and maternal glucose levels on offspring birth weight, fat mass, or skinfold thickness (P > 0.05; data not shown).
Using ROC curves to evaluate the impact of maternal genotype versus maternal glucose on birth weight and neonatal adiposity.
We next addressed 1) whether maternal GCK and TCF7L2 genotypes improve prediction of birth weight or neonatal adiposity when maternal fasting glucose is not known and 2) whether knowledge of maternal genotypes improves prediction of birth weight or neonatal adiposity when maternal fasting glucose is known but maternal 1- and 2-h glucose values are not known. When maternal fasting glucose was added to a model containing various covariates, the observed area under the ROC curves for birth weight, skinfold sum, and percent body fat >90th percentile increased (P < 0.05). In contrast, the addition of maternal genotype at GCK and TCF7L2 did not result in increased discriminatory ability (P > 0.05). Similarly, genotype did not improve the AUCs when considered alongside fasting glucose, whereas measured 1- and 2-h glucose values did result in improvement (P<0.05; supplementary Table 7).
DISCUSSION
In our study of 5,517 women of European and Thai ancestry, we have shown for the first time associations between variants at the GCK and TCF7L2 loci and continuous OGTT measures of maternal glucose in pregnant women without overt diabetes. An additional new finding in this study is association of these variants with gestational diabetes mellitus, as defined by the new IADPSG consensus recommendation (28), itself associated with higher risk of adverse birth outcomes.
The GCK rs1799884 variant was associated with fasting glucose in both of our study populations. The association in the Thai sample was similar to associations previously observed in nonpregnant East Asian subjects (37), while the estimated fasting glucose difference per allele in the Europeans was smaller than previously observed (P = 0.02) (7). We observed some evidence of heterogeneity, with the Thai sample showing a tendency to larger per-allele differences than the European ancestry sample (P = 0.03). This is consistent with previous observations from East Asian, but not South Asian, versus European analyses (36,37). Changes in glucokinase activity or content in pancreatic β-cells are known to impact primarily on fasting glucose levels (45). However, the rate of β-cell glucose metabolism—and, hence, insulin secretion—is controlled by glucokinase across the full range of glucose levels (46,47), and mutations of the glucokinase gene result in a raising of glucose values throughout the glucose tolerance test compared with non–mutation-carrying family members (48). In keeping with this, GCK rs1799884 was associated with higher 1-h glucose levels in the European population in our study and higher 2-h glucose levels in the Thais. Association of GCK rs1799884 with 2-h glucose was demonstrated previously in a European study of nonpregnant individuals (49).
The TCF7L2 rs7903146 variant showed associations at P < 0.05 with all measures of maternal glucose, but these were largest for 1- and 2-h glucose—in keeping with the known association of this locus with glucose-stimulated insulin secretion and incretin signaling in the islet (13). Despite the lower minor allele frequency in the Thai sample, the glucose differences per allele for all three measures were similar to those observed in the Europeans.
Previous studies of both GCK and TCF7L2 have shown associations with gestational diabetes mellitus (23,24,26,27). In keeping with these, we have shown associations with the new consensus definition of gestational diabetes mellitus (28) in both the European and Thai samples. We observed strong evidence for a trend to higher odds of high glucose with increasing numbers of T-alleles at GCK and TCF7L2 (P < 0.00001) but found no evidence for statistical interaction between these loci (P > 0.2). It is important that we now assess the impact of all confirmed fasting glucose and type 2 diabetes susceptibility loci on maternal glycemia because we are likely to identify extreme groups within the population who are at greatly increased genetic risk of high glucose levels in pregnancy. Due to the adverse impact of glucose levels per se on offspring phenotype, genetic variants that are only associated with steady-state glucose regulation may be associated with potentially harmful outcomes in pregnancy even if they are not associated with disease risk in nonpregnant adults.
It is important to understand the contribution of these variants to nenonatal outcomes. The associations that we observed between the maternal risk allele at both loci and higher offspring birth weight were similar to those previously published (4,20). Our finding that the GCK variant showed adiposity associations consistent with the continuous relationship between glucose and neonatal adiposity (31) is a novel observation that extends the previously demonstrated association of this variant with birth weight. Larger studies or meta-analyses will be necessary to provide enough statistical power with which to investigate thoroughly the associations between maternal genotype and neonatal anthropometrics. Consistent with previous studies (4,20,22), we observed no association between birth weight and fetal genotype, suggesting that, unlike rare fetal mutations in GCK (50), the common variants at GCK and TCF7L2 do not influence fetal growth directly.
Measurement of maternal glucose can help identify women without overt diabetes who have a higher risk of neonatal adiposity >90th percentile (31). We hypothesized that maternal GCK and TCF7L2 genotypes might add useful information for predicting neonatal birth weight, skinfold sum, and percent body fat >90th percentile when maternal glucose is not known. Using ROC curves, we found that maternal genotypes did not improve the discriminatory ability of the models (P > 0.05). This may reflect that genotypes at these variants explain only a small proportion (<1%) of variance in maternal glucose levels. However, a total of 16 genetic variants are now known to explain 3–4% of the variation in fasting glucose in Europeans (10). It will therefore be important to repeat these analyses with all known variants.
To conclude, variants at the GCK and TCF7L2 loci, which predispose to higher fasting glucose and type 2 diabetes in the general population, are associated with 1) higher glucose levels from OGTTs in pregnant women who do not have overt diabetes and 2) gestational diabetes mellitus under the new consensus definition (28). Further well-powered studies will be important to assess fully the contribution of known genetic variants to maternal glycemia in pregnancy, pregnancy outcomes, and neonatal phenotypes.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by National Institute of Child Health and Human Development and National Institute of Diabetes and Digestive and Kidney Diseases grants R01-HD34242, R01-HD34243, and R01-DK067459 and by the American Diabetes Association. R.M.F. is funded by a Sir Henry Wellcome Postdoctoral Fellowship (Wellcome Trust grant 085541/Z/08/Z).
No potential conflicts of interest relevant to this article were reported.
R.M.F. carried out analyses, wrote the manuscript, reviewed and edited the manuscript, and contributed to discussion. M.G.H. researched data, wrote the manuscript, reviewed and edited the manuscript, and contributed to discussion. M.U. researched data, reviewed and edited the manuscript, and contributed to discussion. L.P.L. researched data, reviewed and edited the manuscript, and contributed to discussion. H.L. carried out analyses and contributed to discussion. C.A. researched data and contributed to discussion. T.M.F. reviewed and edited the manuscript and contributed to discussion. N.J.C. researched data and contributed to discussion. D.B.D. researched data and contributed to discussion. A.R.D. researched data, reviewed and edited the manuscript, and contributed to discussion. A.T.H. researched data and contributed to discussion. B.E.M. researched data, reviewed and edited the manuscript, and contributed to discussion. W.L.L. researched data, wrote the manuscript, reviewed and edited the manuscript, and contributed to discussion.
Parts of this study were presented orally at the American Diabetes Association 69th Scientific Sessions, New Orleans, Louisiana, 5–9 June 2009, and in poster form at the Wellcome Trust/Nature Genetics “Genomics of Common Diseases” meeting, Hinxton, U.K., 23–26 September 2009.
We acknowledge the role of the field centers and investigators who participated in this genetics study: Belfast, U.K. (D. McCance); Manchester, U.K. (K. Cruickshank); Brisbane, Australia (D. McIntyre); and Newcastle, Australia (J. Lowe).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 2. Watanabe RM, Valle T, Hauser ER, Ghosh S, Eriksson J, Kohtamaki K, Ehnholm C, Tuomilehto J, Collins FS, Bergman RN, Boehnke M. the Finland-United States Investigation of NIDDM Genetics (FUSION) Study investigators Familiality of quantitative metabolic traits in Finnish families with non-insulin-dependent diabetes mellitus. Hum Hered 1999;49:159–168 [DOI] [PubMed] [Google Scholar]
- 3. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chevre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jorgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Levy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 2009;41:89–94 [DOI] [PubMed] [Google Scholar]
- 4. Weedon MN, Clark VJ, Qian Y, Ben-Shlomo Y, Timpson N, Ebrahim S, Lawlor DA, Pembrey ME, Ring S, Wilkin TJ, Voss LD, Jeffery AN, Metcalf B, Ferrucci L, Corsi AM, Murray A, Melzer D, Knight B, Shields B, Smith GD, Hattersley AT, Di Rienzo A, Frayling TM. A common haplotype of the glucokinase gene alters fasting glucose and birth weight: association in six studies and population-genetics analyses. Am J Hum Genet 2006;79:991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen WM, Erdos MR, Jackson AU, Saxena R, Sanna S, Silver KD, Timpson NJ, Hansen T, Orru M, Grazia Piras M, Bonnycastle LL, Willer CJ, Lyssenko V, Shen H, Kuusisto J, Ebrahim S, Sestu N, Duren WL, Spada MC, Stringham HM, Scott LJ, Olla N, Swift AJ, Najjar S, Mitchell BD, Lawlor DA, Smith GD, Ben-Shlomo Y, Andersen G, Borch-Johnsen K, Jorgensen T, Saramies J, Valle TT, Buchanan TA, Shuldiner AR, Lakatta E, Bergman RN, Uda M, Tuomilehto J, Pedersen O, Cao A, Groop L, Mohlke KL, Laakso M, Schlessinger D, Collins FS, Altshuler D, Abecasis GR, Boehnke M, Scuteri A, Watanabe RM. Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest 2008;118:2620–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouatia-Naji N, Rocheleau G, Van Lommel L, Lemaire K, Schuit F, Cavalcanti-Proenca C, Marchand M, Hartikainen AL, Sovio U, De Graeve F, Rung J, Vaxillaire M, Tichet J, Marre M, Balkau B, Weill J, Elliott P, Jarvelin MR, Meyre D, Polychronakos C, Dina C, Sladek R, Froguel P. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 2008;320:1085–1088 [DOI] [PubMed] [Google Scholar]
- 7. Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orru M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR. Variants in MTNR1B influence fasting glucose levels. Nat Genet 2009;41:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 2009;41:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orho-Melander M, Melander O, Guiducci C, Perez-Martinez P, Corella D, Roos C, Tewhey R, Rieder MJ, Hall J, Abecasis G, Tai ES, Welch C, Arnett DK, Lyssenko V, Lindholm E, Saxena R, de Bakker PI, Burtt N, Voight BF, Hirschhorn JN, Tucker KL, Hedner T, Tuomi T, Isomaa B, Eriksson KF, Taskinen MR, Wahlstrand B, Hughes TE, Parnell LD, Lai CQ, Berglund G, Peltonen L, Vartiainen E, Jousilahti P, Havulinna AS, Salomaa V, Nilsson P, Groop L, Altshuler D, Ordovas JM, Kathiresan S. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes 2008;57:3112–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martinez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Rios M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rung J, Cauchi S, Albrechtsen A, Shen L, Rocheleau G, Cavalcanti-Proenca C, Bacot F, Balkau B, Belisle A, Borch-Johnsen K, Charpentier G, Dina C, Durand E, Elliott P, Hadjadj S, Jarvelin MR, Laitinen J, Lauritzen T, Marre M, Mazur A, Meyre D, Montpetit A, Pisinger C, Posner B, Poulsen P, Pouta A, Prentki M, Ribel-Madsen R, Ruokonen A, Sandbaek A, Serre D, Tichet J, Vaxillaire M, Wojtaszewski JF, Vaag A, Hansen T, Polychronakos C, Pedersen O, Froguel P, Sladek R. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet 2009;41:1110–1115 [DOI] [PubMed] [Google Scholar]
- 12. Prokopenko I, McCarthy MI, Lindgren CM. Type 2 diabetes: new genes, new understanding. Trends Genet 2008;24:613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjogren M, Ling C, Eriksson KF, Lethagen UL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del Prato S, Groop L. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007;117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pascoe L, Tura A, Patel SK, Ibrahim IM, Ferrannini E, Zeggini E, Weedon MN, Mari A, Hattersley AT, McCarthy MI, Frayling TM, Walker M. Common variants of the novel type 2 diabetes genes CDKAL1 and HHEX/IDE are associated with decreased pancreatic β-cell function. Diabetes 2007;56:3101–3104 [DOI] [PubMed] [Google Scholar]
- 15. Grarup N, Rose CS, Andersson EA, Andersen G, Nielsen AL, Albrechtsen A, Clausen JO, Rasmussen SS, Jorgensen T, Sandbaek A, Lauritzen T, Schmitz O, Hansen T, Pedersen O. Studies of association of variants near the HHEX, CDKN2A/B, and IGF2BP2 genes with type 2 diabetes and impaired insulin release in 10,705 Danish subjects: validation and extension of genome-wide association studies. Diabetes 2007;56:3105–3111 [DOI] [PubMed] [Google Scholar]
- 16. Grarup N, Andersen G, Krarup NT, Albrechtsen A, Schmitz O, Jorgensen T, Borch-Johnsen K, Hansen T, Pedersen O. Association testing of novel type 2 diabetes risk alleles in the JAZF1, CDC123/CAMK1D, TSPAN8, THADA, ADAMTS9, and NOTCH2 loci with insulin release, insulin sensitivity, and obesity in a population-based sample of 4,516 glucose-tolerant middle-aged Danes. Diabetes 2008;57:2534–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kong A, Steinthorsdottir V, Masson G, Thorleifsson G, Sulem P, Besenbacher S, Jonasdottir A, Sigurdsson A, Kristinsson KT, Jonasdottir A, Frigge ML, Gylfason A, Olason PI, Gudjonsson SA, Sverrisson S, Stacey SN, Sigurgeirsson B, Benediktsdottir KR, Sigurdsson H, Jonsson T, Benediktsson R, Olafsson JH, Johannsson OT, Hreidarsson AB, Sigurdsson G, Ferguson-Smith AC, Gudbjartsson DF, Thorsteinsdottir U, Stefansson K. Parental origin of sequence variants associated with complex diseases. Nature 2009;462:868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU, Kao WH, Li M, Glazer NL, Manning AK, Luan J, Stringham HM, Prokopenko I, Johnson T, Grarup N, Boesgaard TW, Lecoeur C, Shrader P, O'Connell J, Ingelsson E, Couper DJ, Rice K, Song K, Andreasen CH, Dina C, Kottgen A, Le Bacquer O, Pattou F, Taneera J, Steinthorsdottir V, Rybin D, Ardlie K, Sampson M, Qi L, van Hoek M, Weedon MN, Aulchenko YS, Voight BF, Grallert H, Balkau B, Bergman RN, Bielinski SJ, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Buchanan TA, Bumpstead SJ, Cavalcanti-Proenca C, Charpentier G, Chen YD, Chines PS, Collins FS, Cornelis M, J Crawford G, Delplanque J, Doney A, Egan JM, Erdos MR, Firmann M, Forouhi NG, Fox CS, Goodarzi MO, Graessler J, Hingorani A, Isomaa B, Jorgensen T, Kivimaki M, Kovacs P, Krohn K, Kumari M, Lauritzen T, Levy-Marchal C, Mayor V, McAteer JB, Meyre D, Mitchell BD, Mohlke KL, Morken MA, Narisu N, Palmer CN, Pakyz R, Pascoe L, Payne F, Pearson D, Rathmann W, Sandbaek A, Sayer AA, Scott LJ, Sharp SJ, Sijbrands E, Singleton A, Siscovick DS, Smith NL, Sparso T, Swift AJ, Syddall H, Thorleifsson G, Tonjes A, Tuomi T, Tuomilehto J, Valle TT, Waeber G, Walley A, Waterworth DM, Zeggini E, Zhao JH, Illig T, Wichmann HE, Wilson JF, van Duijn C, Hu FB, Morris AD, Frayling TM, Hattersley AT, Thorsteinsdottir U, Stefansson K, Nilsson P, Syvanen AC, Shuldiner AR, Walker M, Bornstein SR, Schwarz P, Williams GH, Nathan DM, Kuusisto J, Laakso M, Cooper C, Marmot M, Ferrucci L, Mooser V, Stumvoll M, Loos RJ, Altshuler D, Psaty BM, Rotter JI, Boerwinkle E, Hansen T, Pedersen O, Florez JC, McCarthy MI, Boehnke M, Barroso I, Sladek R, Froguel P, Meigs JB, Groop L, Wareham NJ, Watanabe RM. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 42:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006;38:320–323 [DOI] [PubMed] [Google Scholar]
- 20. Freathy RM, Weedon MN, Bennett A, Hypponen E, Relton CL, Knight B, Shields B, Parnell KS, Groves CJ, Ring SM, Pembrey ME, Ben-Shlomo Y, Strachan DP, Power C, Jarvelin MR, McCarthy MI, Davey Smith G, Hattersley AT, Frayling TM. Type 2 diabetes TCF7L2 risk genotypes alter birth weight: a study of 24,053 individuals. Am J Hum Genet 2007;80:1150–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954;16:330–342 [DOI] [PubMed] [Google Scholar]
- 22. Weedon MN, Frayling TM, Shields B, Knight B, Turner T, Metcalf BS, Voss L, Wilkin TJ, McCarthy A, Ben-Shlomo Y, Davey Smith G, Ring S, Jones R, Golding J, Byberg L, Mann V, Axelsson T, Syvanen AC, Leon D, Hattersley AT. Genetic regulation of birth weight and fasting glucose by a common polymorphism in the islet cell promoter of the glucokinase gene. Diabetes 2005;54:576–581 [DOI] [PubMed] [Google Scholar]
- 23. Shaat N, Karlsson E, Lernmark A, Ivarsson S, Lynch K, Parikh H, Almgren P, Berntorp K, Groop L. Common variants in MODY genes increase the risk of gestational diabetes mellitus. Diabetologia 2006;49:1545–1551 [DOI] [PubMed] [Google Scholar]
- 24. Shaat N, Lernmark A, Karlsson E, Ivarsson S, Parikh H, Berntorp K, Groop L. A variant in the transcription factor 7-like 2 (TCF7L2) gene is associated with an increased risk of gestational diabetes mellitus. Diabetologia 2007;50:972–979 [DOI] [PubMed] [Google Scholar]
- 25. Lauenborg J, Grarup N, Damm P, Borch-Johnsen K, Jorgensen T, Pedersen O, Hansen T. Common type 2 diabetes risk gene variants associate with gestational diabetes. J Clin Endocrinol Metab 2009;94:145–150 [DOI] [PubMed] [Google Scholar]
- 26. Cho YM, Kim TH, Lim S, Choi SH, Shin HD, Lee HK, Park KS, Jang HC. Type 2 diabetes-associated genetic variants discovered in the recent genome-wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia 2009;52:253–261 [DOI] [PubMed] [Google Scholar]
- 27. Watanabe RM, Allayee H, Xiang AH, Trigo E, Hartiala J, Lawrence JM, Buchanan TA. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes 2007;56:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Association of Diabetes and Pregnancy Study Groups Consensus Panel: International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Int J Gynaecol Obstet 2002;78:69–77 [DOI] [PubMed] [Google Scholar]
- 30. Nesbitt GS, Smye M, Sheridan B, Lappin TR, Trimble ER. Integration of local and central laboratory functions in a worldwide multicentre study: experience from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Clin Trials 2006;3:397–407 [DOI] [PubMed] [Google Scholar]
- 31. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 2009;58:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol 1995;173:1176–1181 [DOI] [PubMed] [Google Scholar]
- 33. Chandak GR, Janipalli CS, Bhaskar S, Kulkarni SR, Mohankrishna P, Hattersley AT, Frayling TM, Yajnik CS. Common variants in the TCF7L2 gene are strongly associated with type 2 diabetes mellitus in the Indian population. Diabetologia 2007;50:63–67 [DOI] [PubMed] [Google Scholar]
- 34. Ng MC, Park KS, Oh B, Tam CH, Cho YM, Shin HD, Lam VK, Ma RC, So WY, Cho YS, Kim HL, Lee HK, Chan JC, Cho NH. Implication of genetic variants near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in type 2 diabetes and obesity in 6,719 Asians. Diabetes 2008;57:2226–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo Y, Wang H, Han X, Ren Q, Wang F, Zhang X, Sun X, Zhou X, Ji L. Meta-analysis of the association between SNPs in TCF7L2 and type 2 diabetes in East Asian population. Diabetes Res Clin Pract 2009;85:139–146 [DOI] [PubMed] [Google Scholar]
- 36. Chambers JC, Zhang W, Zabaneh D, Sehmi J, Jain P, McCarthy MI, Froguel P, Ruokonen A, Balding D, Jarvelin MR, Scott J, Elliott P, Kooner JS. Common genetic variation near melatonin receptor MTNR1B contributes to raised plasma glucose and increased risk of type 2 diabetes among Indian Asians and European Caucasians. Diabetes 2009;58:2703–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tam CH, Ma RC, So WY, Wang Y, Lam VK, Germer S, Martin M, Chan JC, Ng MC. Interaction effect of genetic polymorphisms in glucokinase (GCK) and glucokinase regulatory protein (GCKR) on metabolic traits in healthy Chinese adults and adolescents. Diabetes 2009;58:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang YC, Chang TJ, Jiang YD, Kuo SS, Lee KC, Chiu KC, Chuang LM. Association study of the genetic polymorphisms of the transcription factor 7-like 2 (TCF7L2) gene and type 2 diabetes in the Chinese population. Diabetes 2007;56:2631–2637 [DOI] [PubMed] [Google Scholar]
- 39. Ren Q, Han XY, Wang F, Zhang XY, Han LC, Luo YY, Zhou XH, Ji LN. Exon sequencing and association analysis of polymorphisms in TCF7L2 with type 2 diabetes in a Chinese population. Diabetologia 2008;51:1146–1152 [DOI] [PubMed] [Google Scholar]
- 40. Ng MC, Tam CH, Lam VK, So WY, Ma RC, Chan JC. Replication and identification of novel variants at TCF7L2 associated with type 2 diabetes in Hong Kong Chinese. J Clin Endocrinol Metab 2007;92:3733–3737 [DOI] [PubMed] [Google Scholar]
- 41. Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet 2006;2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris R, Bradburn M, Deeks J, Altman D, Harbord R, Steichen T, Sterne J. METAN: Stata module for fixed and random effects meta-analysis [article online], 2006. Boston College Department of Economics. Available from http://ideas.repec.org/c/boc/bocode/s456798.html Accessed 29 January 2010
- 43. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gauderman WJ, Morrison JM: QUANTO 1.1: a computer program for power and sample size calculations for genetic epidemiology studies [article online], 2006. Aavailable from http://hydra.usc.edu/gxe Accessed 29 January 2010
- 45. Stride A, Vaxillaire M, Tuomi T, Barbetti F, Njolstad PR, Hansen T, Costa A, Conget I, Pedersen O, Sovik O, Lorini R, Groop L, Froguel P, Hattersley AT. The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia 2002;45:427–435 [DOI] [PubMed] [Google Scholar]
- 46. Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov 2009;8:399–416 [DOI] [PubMed] [Google Scholar]
- 47. Byrne MM, Sturis J, Clement K, Vionnet N, Pueyo ME, Stoffel M, Takeda J, Passa P, Cohen D, Bell GI, et al. Insulin secretory abnormalities in subjects with hyperglycemia due to glucokinase mutations. J Clin Invest 1994;93:1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murphy R, Tura A, Clark PM, Holst JJ, Mari A, Hattersley AT. Glucokinase, the pancreatic glucose sensor, is not the gut glucose sensor. Diabetologia 2009;52:154–159 [DOI] [PubMed] [Google Scholar]
- 49. Rose CS, Ek J, Urhammer SA, Glumer C, Borch-Johnsen K, Jorgensen T, Pedersen O, Hansen T. A −30G>A polymorphism of the β-cell–specific glucokinase promoter associates with hyperglycemia in the general population of whites. Diabetes 2005;54:3026–3031 [DOI] [PubMed] [Google Scholar]
- 50. Hattersley AT, Beards F, Ballantyne E, Appleton M, Harvey R, Ellard S. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet 1998;19:268–270 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.