Abstract
Purpose
Premenopausal women with breast cancer receiving adjuvant chemotherapy are at risk for amenorrhea. The National Surgical Adjuvant Breast and Bowel Project B-30 trial included menstrual history (MH) and quality-of-life (QOL) studies to compare treatments on these outcomes.
Patients and Methods
Patients were randomly assigned to sequential doxorubicin (A) and cyclophosphamide (C) followed by docetaxel (T; AC→T), concurrent TAC, or AT, which varied in duration (24, 12, 12 weeks, respectively), and use of C. Endocrine therapy was prescribed for women with hormone receptor–positive tumors. MH and QOL were assessed with standardized questionnaires at baseline; cycle 4, day 1; and every 6 months through 24 months. Prespecified analyses examined rates of amenorrhea by treatment arm, the relationship between amenorrhea and QOL, and QOL by treatment arm.
Results
Amenorrhea 12 months after random assignment was significantly different between treatment groups: 69.8% for AC→T, 57.7% for TAC, and 37.9% for AT (P < .001). The AT group without tamoxifen had the lowest rate of amenorrhea. QOL was poorer for patients receiving AC→T at 6 months but similar to others by 12 months. Post-treatment symptoms were increased above baseline for all treatments. Multivariable repeated measures modeling demonstrated that treatment arm, time point, age, and tamoxifen use were significantly associated with symptom severity (all P values < .002).
Conclusion
Amenorrhea rates differed significantly by treatment arm, with the AT arm having the lowest rate. Patients treated with longer duration therapy (AC→T) had greater symptom severity and poorer QOL at 6 months, but did not differ from shorter duration treatments at 12 months.
INTRODUCTION
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-30 trial compared the efficacy of three adjuvant chemotherapy regimens in patients with early-stage, node-positive breast cancer.1 Treatments varied in duration (24 v 12 weeks) and composition (one arm without cyclophosphamide [C]), allowing protocol prespecified secondary objectives focused on assessment of comparative toxicity and amenorrhea risk for each regimen. Thus, the study included patient-reported outcome data collection focused on menstrual history (MH) and quality of life (QOL). B-30 trial results demonstrated that adjuvant therapy with sequential doxorubicin (A) and C followed by docetaxel (T; AC→T), compared with four cycles of AT or TAC, improved disease-free survival and overall survival, although the latter nonsignificantly for TAC. Amenorrhea was significantly associated with improved survival regardless of treatment.1
Ovarian dysfunction (transient or permanent amenorrhea; infertility; premature menopause) is one of the most feared complications of chemotherapy in premenopausal patients with breast cancer,2 with alkylating agents (eg, C), largely responsible for this problem.3–8 Few prospective data are available on risk for amenorrhea from contemporary adjuvant therapy regimens.8 A prespecified protocol hypothesis focused on the relationship between treatment-related amenorrhea and survival outcomes, and thus prospective collection of MH was a component of the main trial.1,9 This article provides results of the MH and QOL studies that were secondary outcomes in the B-30 trial.
PATIENTS AND METHODS
The B-30 trial was a three-arm multicenter study conducted in the United States and Canada with a target sample size of 5,300 women with node-positive breast cancer. A detailed description of the trial has been recently published by Swain et al.1 The treatment regimens were: AC every 3 weeks for four cycles followed by T every 3 weeks for four cycles (AC→T; 24 weeks); A plus T every 3 weeks for four cycles (AT; 12 weeks); and T plus A plus C every 3 weeks for four cycles (TAC; 12 weeks). Endocrine therapy was planned for all women with hormone receptor–positive tumors (initially concurrent tamoxifen, amended to sequential tamoxifen in 2002, and then amended to allow aromatase inhibitors in postmenopausal women in 2002). Paired statistical comparisons were made between the three arms for both disease-free survival and overall survival.1 The B-30 protocol had embedded MH and QOL studies; all were approved by local institutional review boards, and informed consent was obtained from each patient.
Patients for the MH and QOL Studies
We assessed menstrual status in all women at study entry before random assignment; women who were pre- or perimenopausal were included in the MH study. MH study enrollment was continuous throughout the trial; however, only the first 2,100 consecutively enrolled patients were entered in the QOL study, regardless of menstrual status.
Self-Report Questionnaires
Menstrual status was assessed at baseline and at follow-up visits with two similar questionnaires, adapted from previous breast cancer studies.6 Before chemotherapy initiation, all participants completed the baseline questionnaire that assessed surgical menopause and menstrual history in the previous 12 months. For women reporting menstrual bleeding in the past 12 months, additional questions queried the date of the most recent menstrual period, menstrual bleeding patterns, and recent use of hormone therapy or contraceptives. Women with surgical or natural menopause were excluded from the MH study after the baseline questionnaire. Follow-up questionnaires tracked changes in menstrual bleeding and possible surgical menopause only for those included in the MH study.
QOL and symptoms were measured with two established questionnaires previously used in breast cancer clinical trials.10,11 The Functional Assessment of Cancer Therapy (FACT) -B is a 44-item multidimensional, breast cancer–specific QOL questionnaire with excellent psychometric properties.12 Five subscales provide scores for physical well-being, social/family well-being, patient/physician relationship, emotional well-being, and functional well-being. Nine additional questions are breast-cancer specific. The generic FACT questionnaire has been widely used in clinical trials and is responsive to change over time.10,13 A 23-item trial outcome index (TOI) summarizes the physical and functional well-being scales, along with the disease-specific items. The FACT-TOI is currently used as a clinical trial outcome measure of physical and functional well-being.12 A 5-point difference between two treatment groups represents a clinically meaningful difference with a higher score being better.14
Symptoms were assessed using items from the Breast Cancer Prevention Trial symptom checklist,15–17 and other NSABP treatment trial toxicity questions. Patients rated each symptom during the past 7 days with a bother rating of 0 “not at all” to 4 “very much.” In B-30, 28 symptoms were rated ranging from nausea, vomiting, headaches, and skin problems, to vasomotor symptoms, vaginal symptoms, musculoskeletal aches and pains, body image concerns, and cognitive complaints. Total severity score (ie, symptom checklist summary score) ranged from 0 to 112, with higher being worse.
Assessment Schedule and Compliance Monitoring
MH and QOL questionnaires were administered at baseline (after consent, but before first treatment), and at office visits at the time of cycle 4, day 1 (during chemotherapy), and then at 6, 12, 18, and 24 months. Questionnaires were completed at an in-person visit, with mail and telephone collection as alternatives. A missing data form was completed if the woman declined to complete a scheduled questionnaire or for an administrative error in data collection. Compliance monitoring occurred through clinical site–specific updates on missing data, tailored letters, and telephone contacts from the NSABP Biostatistical Center. Patients received individualized calendars (in a monthly format, covering the entire time frame for assessments) to record menstrual cycles as an aid for completing the MH questionnaire. (Calendars were not submitted.)
Statistical Analysis Plans and Methods
The MH study included all eligible pre- and perimenopausal B-30 participants to maximize the number of subjects available for the survival end point analysis related to amenorrhea.1,9 Women with uncertain or perimenopausal status were included in the MH study; however, in this report we include only participants who were menstruating regularly (at least one cycle within 3 months) at the start of treatment. We define amenorrhea as not having a menstrual period for longer than 1 month (ascertained from date of the most recent menstrual cycle and reported changes in menstrual cycle in the past 12 months). We define prolonged amenorrhea as having at least 6 months without a menstrual cycle, which is the protocol-specified primary end point for the MH study reported here and for the survival end point analysis.1,9 The cumulative duration of amenorrhea is the total length of time during which a patient experienced amenorrhea. (For example, missed menses every other month for 2 years would be considered 1 year cumulative amenorrhea.) Patients who never experienced amenorrhea were assigned a duration of 0; those who remained amenorrheic at the time of their last measurement or who underwent hysterectomy or oophorectomy before amenorrhea were censored. A detailed description of this calculation is reported by Swain et al.9 We hypothesized that the lowest rate of amenorrhea would occur in the AT arm that excluded C.
At the time of protocol design, the FACT-B total score and total symptom severity score were designated primary outcomes, requiring a sample size of 2,100 to achieve 80% power for a mean change of 0.4 standard deviations in the FACT-B score, within subgroups based on age (≤ 49 v ≥ 50), and assuming a high rate of attrition. However, before initiating data analysis, the FACT-B TOI was selected as the primary QOL end point, primarily motivated by recent developments in the field of QOL assessment described in the methods section.12,14 We hypothesized that patients receiving AC→T would have poorer QOL at 6 months, which was at the end of the sequential treatment, in comparison to the two other regimens where treatment was completed at 12 weeks; however, we predicted that by 12 months there would be no significant difference in TOI among the treatment arms.
Compliance at 24 months, defined as percentage of expected MH and QOL forms, was compared between treatment groups and by demographic and medical characteristics using χ2 tests. Analyses included all available data. Due to the high, acceptable rate of compliance in NSABP trials and the observation that missing data in adjuvant trials are rarely related to the patient's health or emotional state,18 we did not conduct analyses to examine for potential missing data bias. Treatment groups were compared for demographic and medical characteristics, as well as prolonged amenorrhea at 12 months after the start of therapy, using the χ2 test. Kaplan-Meier and log-rank methods were used to compare the duration of amenorrhea by age and treatment group. Multivariable mixed models with repeated measures were used to analyze the change from baseline for each of the QOL outcomes by treatment group. Models also included time point, age, number of nodes, intended tamoxifen use, surgery/radiation, participation in MH study, and time-by-treatment interaction. Effect size (ES) reported is the absolute value of the estimated coefficient divided by the standard deviation of outcome at baseline. Intent-to-treat analyses were used including all participants with follow-up information available. Assignment to chemotherapy regimen or endocrine therapy (tamoxifen for women in MH study) was included on an intent-to-treat basis. Tests were two sided at a significance level of .05. SAS version 9.1 (SAS Institute Inc, Cary, NC) was used, with PROC MIXED for the repeated measures analyses.
RESULTS
Patient Characteristics
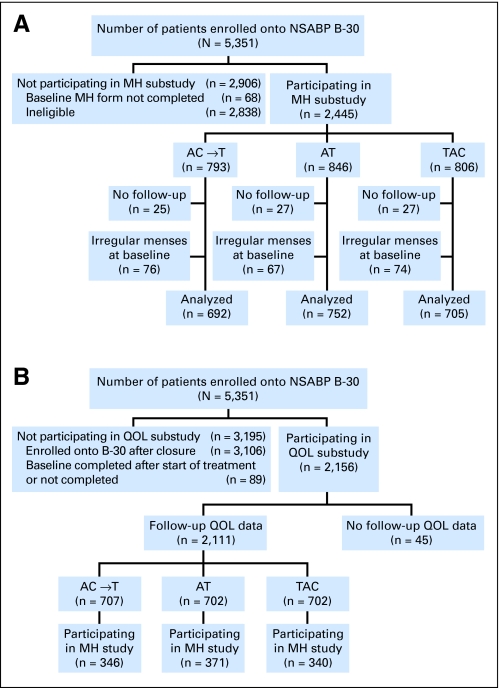
Between 1999 and 2004, 5,351 patients were randomly assigned to B-30, with 5,283 (99%) providing baseline MH information. Of these 5,283 patients, 2,445 (46%) were eligible for the MH follow-up study (793 AC → T, 846 AT, 806 TAC; Fig 1A), with available data after treatment initiation for 97% in each treatment group. Approximately 10% of patients had irregular menstrual periods at study entry (evenly distributed across the three treatment groups), yielding 2,149 patients for analysis. Demographic and medical characteristics of MH study participants are shown in Table 1. There were no significant differences by treatment group.
Fig 1.
(A) Flow diagram showing recruitment and participation of women from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-30 in the menstrual history (MH) substudy. (B) Flow diagram showing recruitment and participation of women from NSABP B-30 into the quality-of-life (QOL) and symptoms substudy. A, doxorubicin; C, cyclophosphamide; T, docetaxel.
Table 1.
Characteristics of Women Participating in the National Surgical Adjuvant Breast and Bowel Project B-30 Menstrual History Study
| Characteristic | Analyzed |
Enrolled Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC→T |
AT |
TAC |
Total |
P | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| No. of patients | 692 | 752 | 705 | 2,149 | 2,445 | ||||||
| Age, years | |||||||||||
| ≤ 40 | 217 | 31.4 | 216 | 28.7 | 209 | 29.6 | 642 | 29.9 | .76 | 681 | 27.9 |
| 41-50 | 411 | 59.4 | 456 | 60.6 | 421 | 59.7 | 1,288 | 59.9 | 1,405 | 57.5 | |
| ≥ 51 | 64 | 9.2 | 80 | 10.6 | 75 | 10.6 | 219 | 10.2 | 359 | 14.7 | |
| Ethnicity | |||||||||||
| White | 559 | 80.8 | 619 | 82.3 | 586 | 83.1 | 1,764 | 82.1 | .37 | 2,012 | 82.3 |
| Hispanic | 29 | 4.2 | 37 | 4.9 | 32 | 4.5 | 98 | 4.6 | 108 | 4.4 | |
| Black | 77 | 11.1 | 61 | 8.1 | 55 | 7.8 | 193 | 9.0 | 220 | 9.0 | |
| Other | 27 | 3.9 | 35 | 4.7 | 32 | 4.5 | 94 | 4.4 | 105 | 4.3 | |
| Mastectomy | 346 | 50.0 | 378 | 50.3 | 353 | 50.1 | 1,077 | 50.1 | .99 | 1,221 | 49.9 |
| Radiation therapy | 539 | 77.9 | 601 | 79.9 | 550 | 78.0 | 1,690 | 78.6 | .57 | 1,922 | 78.6 |
| Tamoxifen | 529 | 76.4 | 589 | 78.3 | 537 | 76.2 | 1,655 | 77.0 | .57 | 1,893 | 77.4 |
Abbreviations: A, doxorubicin; C, cyclophosphamide; T, docetaxel.
QOL study accrual was closed on July 20, 2001, at which point 2,245 patients had been enrolled in B-30. Figure 1B shows the flow of patients into the QOL study. At baseline, 2,156 patients submitted a QOL questionnaire. Follow-up QOL questionnaires were available for 2,111 patients (98%), evenly distributed across treatment arms. About half of the patients in the QOL study also participated in the MH study. Demographic and medical characteristics of patients in the QOL study are presented in Table 2. There were no significant differences according to treatment assignment.
Table 2.
Characteristics of Women Participating in the National Surgical Adjuvant Breast and Bowel Project B-30 Quality-of-Life Study
| Characteristic | Analyzed |
Enrolled Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC→T |
AT |
TAC |
Total |
P | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| No. of patients | 707 | 702 | 702 | 2,111 | 2,156 | ||||||
| Age, years | |||||||||||
| ≤ 40 | 118 | 16.7 | 118 | 16.8 | 109 | 15.5 | 345 | 16.3 | .40 | 356 | 16.5 |
| 41-50 | 256 | 36.2 | 276 | 39.3 | 249 | 35.5 | 781 | 37.0 | 794 | 36.8 | |
| ≥ 51 | 333 | 47.1 | 308 | 43.9 | 344 | 49.0 | 985 | 46.7 | 1,006 | 46.7 | |
| Ethnicity | |||||||||||
| White | 599 | 84.7 | 604 | 86.0 | 614 | 87.5 | 1,817 | 86.1 | .48 | 1,849 | 85.8 |
| Hispanic | 19 | 2.7 | 20 | 2.8 | 20 | 2.8 | 59 | 2.8 | 62 | 2.9 | |
| Black | 67 | 9.5 | 53 | 7.5 | 54 | 7.7 | 174 | 8.2 | 181 | 8.4 | |
| Other | 22 | 3.1 | 25 | 3.6 | 14 | 2.0 | 61 | 2.9 | 64 | 3.0 | |
| Mastectomy | 343 | 48.5 | 350 | 49.9 | 340 | 48.4 | 1,033 | 48.9 | .84 | 1,061 | 49.2 |
| Radiation therapy | 545 | 77.1 | 541 | 77.1 | 549 | 78.2 | 1,635 | 77.5 | .84 | 1,655 | 76.8 |
| Tamoxifen | 561 | 79.3 | 558 | 79.5 | 553 | 78.8 | 1,672 | 79.2 | .94 | 1,705 | 79.1 |
Abbreviations: A, doxorubicin; C, cyclophosphamide; T, docetaxel.
Compliance with expected assessments at 24 months was 77% for MH and 78% for QOL, with no significant differences according to treatment group, participant age, surgery type, or tamoxifen. There were significant differences by race (P = .017), with fewer Hispanic participants completing QOL assessments at 24 months, and by radiation therapy (P = .02), with higher MH compliance among those who received radiation. These differences are partly due to institution-level difficulties, as found in other NSABP behavioral outcomes studies.18
MH Outcomes
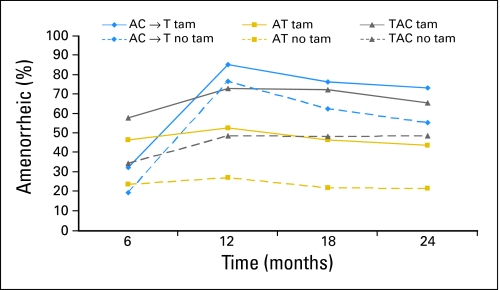
The rates of reported prolonged amenorrhea at 12 months after the start of therapy was significantly different between treatment groups: 69.8% for AC → T, 37.9% for AT, and 57.7% for TAC (P < .001). These percentages are conservative in that they include all participants in the denominator. If we instead exclude those women for whom menstrual status at 12 months was unknown, we arrive at the following results: 83.3% for AC → T, 47.1% for AT, and 67.2% for TAC (P < .001). Figure 2 shows rates of prolonged amenorrhea by chemotherapy treatment with and without tamoxifen. Women receiving AC→T had a higher rate of prolonged amenorrhea at 12, 18, and 24 months compared with AT, and the amenorrhea rates were higher with tamoxifen. Women who received AT without tamoxifen had the lowest rate of amenorrhea, hovering around 20% to 25% across the 24-month period of observation.
Fig 2.
Rate of prolonged amenorrhea at each time point for chemotherapy and tamoxifen intention-to-treat groups. Excludes those who experienced amenorrhea for 3 months at baseline and those with a hysterectomy/oophorectomy or unknown status at each time point. A, doxorubicin; C, cyclophosphamide; T, docetaxel; tam, tamoxifen.
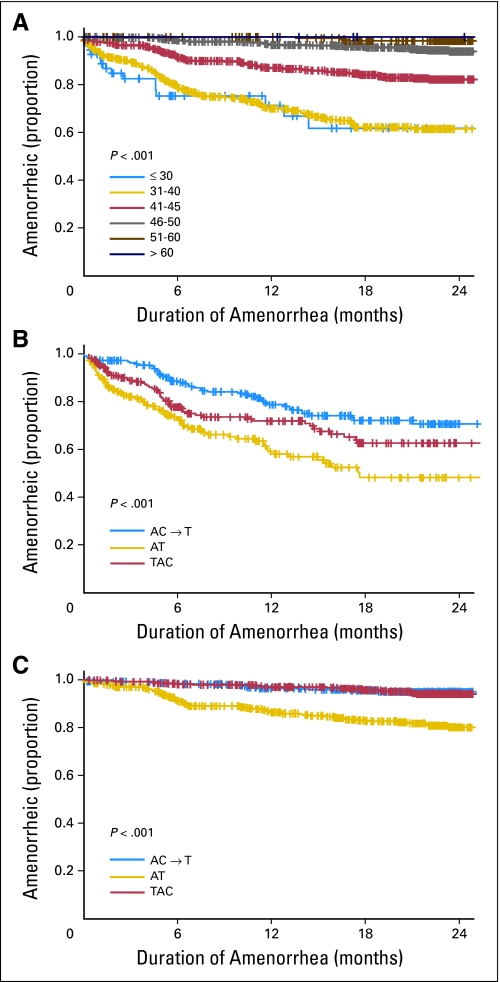
An estimated 61% of women ≤ 40 years old experienced at least 24 months of amenorrhea contrasting with nearly 100% among women older than 50 years (Fig 3A). Women treated with AT reported statistically significantly shorter duration of amenorrhea compared with the two other treatment groups (P < .001; Figs 3B and 3C).
Fig 3.
(A) Duration of amenorrhea in months according to age group. (B) Duration of amenorrhea in months by treatment group among those ≤ age 40 years. (C) Duration of amenorrhea in months by treatment group among those older than age 40 years. A, doxorubicin; C, cyclophosphamide; T, docetaxel.
QOL Outcomes
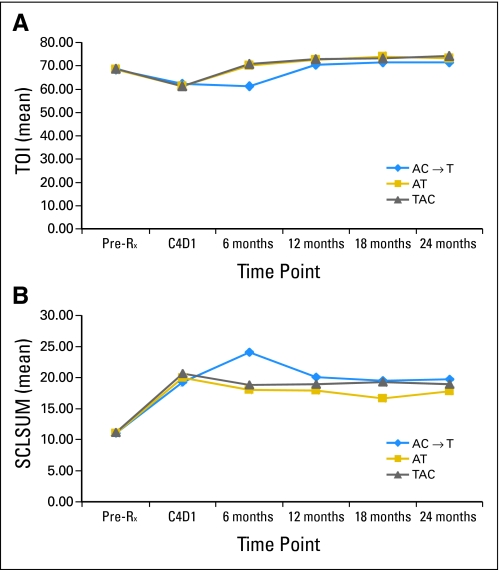
Figure 4A shows the FACT-B TOI by treatment arm and time point. Multivariable repeated measures regression analysis of the change from baseline in the FACT-B TOI over 24 months demonstrated that time (P < .001), chemotherapy regimen (P < .001), and surgery/radiation combination (P = .024) were statistically significant predictors. Relative to TAC, patients in the AC→T group experienced a mean change from baseline that was 2.4 points lower (ES, 0.2); AT patients' mean change was 1.0 point lower (ES, 0.1). The time-by-treatment interaction was significant because TAC and AT patients' TOI returned to baseline at 6 months, whereas AC→T patients' TOI returned to baseline at 12 months (P < .001; Fig 4A). Inclusion in the MH study, a proxy for baseline menopausal status, was not a significant predictor (P = .31).
Fig 4.
(A) Functional Assessment of Chronic Illness Therapy–B trial outcome index (TOI) by treatment group and time point. (B) Symptom severity by treatment group and time point. SCLSUM, symptom checklist summary score; Pre-Rx, pretreatment; C4D1, cycle 4, day 1; A, doxorubicin; C, cyclophosphamide; T, docetaxel.
Symptom severity (ie, symptom checklist summary score) also worsened during therapy, and remained bothersome throughout follow-up (Fig 4B). Multivariable repeated measures modeling demonstrated significant associations of severity with treatment arm and time point (illustrated in Fig 4B), age (greater severity among younger women), and tamoxifen use (all P values < .002). The time-by-treatment interaction was significant (P < .001) because, as with the TOI, symptoms remained severe beyond 6 months primarily for AC→T patients. ESs were modest, with a range of 0.14 to 0.47. Inclusion in the MH study was not a significant predictor (P = .76).
To examine the effect of prolonged amenorrhea on QOL and symptoms, we performed a longitudinal analysis over the first 12 months, restricted to those participants also in the MH study. Models included the significant variables from the primary analyses. The presence of prolonged amenorrhea at 12 months (indicating that the patient had not experienced a menstrual cycle during the interval from 6 to 12 months after therapy), did not significantly predict TOI over this time frame (P = .27; ES, 0.07). Chemotherapy assignment remained significant (P < .001; ES, 0.08 to 0.11). Prolonged amenorrhea did not significantly predict symptoms (P = .91; ES, 0.01), nor did tamoxifen predict symptoms in this subset (P = .26; ES, 0.10). Chemotherapy assignment remained significant (P < .001; ES, 0.19 to 0.26). The small ESs for prolonged amenorrhea and tamoxifen indicate that their nonsignificance should not be attributed to a lack of power due to the size of the subset.
DISCUSSION
Since the introduction of adjuvant chemotherapy treatment of early-stage breast cancer,19,20 differences in survival benefit have been noted according to age (< 50 years), with speculation that treatment-induced amenorrhea relating to alkylating agents was indicative of a biochemical oophorectomy. This therapeutic benefit was especially noted in women between 40 and 50 years, who coincidentally were more likely to become permanently amenorrheic. Despite these observations more than 30 years ago, there are few prospective data on the rate of amenorrhea (transient or permanent) with various chemotherapy regimens.7,8,21,22 In reporting of adverse events for TAC versus fluorouracil (F), A and C (FAC) chemotherapy, absence of menses for at least 3 months was reported for 61% of premenopausal patients treated with TAC and 52.4% treated with FAC.23 QOL assessed in that trial showed recovery of patients in both treatment arms in the 6 months post-treatment.23 To the best of our knowledge, no other clinical trial has assessed prospectively the influence of amenorrhea on symptoms, QOL, and survival.
Patient-reported MH data collection was feasible in the B-30 trial, and as hypothesized, the treatment regimen without C had the lowest rate of amenorrhea. As expected, treatment duration was a significant predictor of QOL and symptom severity at 6 months (worse in the AC→T group), and results of treatment arm differences resolved by 12 months. Post-treatment symptom severity exceeded baseline assessment out to 24 months among all patients, with younger women experiencing greater symptom severity, independent of treatment arm and amenorrhea status. These findings are consistent with other large observational studies in breast cancer survivors.4,6 Due to limitations of data collection in this trial, we could not establish whether these treatments impacted long-term fertility. This is an important survivorship concern24 and should be examined in future research studies. Of note, the lowest rate of amenorrhea occurred in women who received AT without tamoxifen. This information might be useful for younger women interested in preserving fertility, where treatment with AT (with or without tamoxifen) may offer a better chance of return of menses after adjuvant therapy.
The observed pattern of amenorrhea differed according to tamoxifen assignment, with a higher likelihood of amenorrhea when tamoxifen was added to chemotherapy. These findings are consistent with the report of Petrek et al8 in a prospective observational study of patients with breast cancer who recorded their menstrual bleeding prospectively on calendars collected post-treatment for a median of 45 months. That study also confirmed age- and treatment-related differences in development of amenorrhea.
As with any study, there are potential limitations of the data and their interpretation. The women who participated in the B-30 trial were volunteers, meeting the study eligibility requirements. Women with significant comorbid conditions were excluded, and the impact of the trial treatments on such women is unknown. However, the relative impacts of the three different regimens are likely to be similar. The rates of amenorrhea reported by age group are similar to those reported in other observational studies of younger women with breast cancer6–8,21; however, our limited follow-up (24 months) does not allow prediction of the permanence of amenorrhea. Without laboratory confirmation of menopausal status (eg, estradiol level), we cannot be certain if the amenorrhea reported at 24 months is permanent or transient, which can also be masked by concomitant tamoxifen. The NSABP B-36 trial, which is closed to accrual and in follow-up, includes a similar MH and QOL study with data collection through 36 months. That trial will permit confirmation of the B-30 trial findings related to amenorrhea, albeit with different adjuvant chemotherapy regimens.
In conclusion, the three chemotherapy regimens used in the B-30 trial (AC→T, TAC, and AT), induced different rates of amenorrhea that were also influenced by age and tamoxifen. QOL outcomes were directly related to the duration of treatment and returned to pretreatment levels by 12 months after random assignment; however, increased symptom severity persisted over the 2 years of follow-up and should be attended to as part of survivorship care. The detailed patient-reported outcome data collected as part of the B-30 trial provides new information that can be used by physicians and their patients in weighing the benefits and toxicities of each chemotherapy treatment regimen used in this trial.
Acknowledgment
Supported by Public Health Service Grants No. U10-CA-37377, U10-CA-69974, U10-CA-12027, U10-CA-69651, for the National Surgical Adjuvant Breast and Bowel Project; Grant No. CA07190 (J.K.E); Grant No. CA2115 for Eastern Cooperative Oncology Group; Grant No. CA-25224 for the North Central Cancer Treatment Group; Grants No. CA-32102, CA-13612, CA-58348, and CA-45808 for Southwest Oncology Group from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services; and auxiliary support from sanofi-aventis.
Presented in part at the 31st Annual San Antonio Breast Cancer Symposium, December 10-14, 2008, San Antonio, TX.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003782.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Eleftherios P. Mamounas, sanofi-aventis (C); Norman Wolmark, sanofi-aventis (U); Sandra M. Swain, sanofi-aventis (U), Rhone-Poulenc Rorer (U), BiPar Sciences (U) Stock Ownership: Eduardo R. Pajon, Bristol-Meyers Squibb, Abbott Honoraria: Victor G. Vogel, AstraZeneca; Eleftherios P. Mamounas, sanofi-aventis; Sandra M. Swain, Rhone-Poulenc Rorer Research Funding: Victor G. Vogel, AstraZeneca Expert Testimony: None Other Remuneration: Sandra M. Swain, sanofi-aventis
AUTHOR CONTRIBUTIONS
Conception and design: Patricia A. Ganz, Charles E. Geyer Jr, Joseph P. Costantino, Jonathan A. Polikoff, Eleftherios P. Mamounas, Sandra M. Swain
Administrative support: Stephanie R. Land, Charles E. Geyer Jr, Norman Wolmark
Provision of study materials or patients: Charles E. Geyer Jr, Eduardo R. Pajon, Louis Fehrenbacher, James N. Atkins, Jonathan A. Polikoff, Victor G. Vogel, John K. Erban, Robert B. Livingston, Edith A. Perez, Eleftherios P. Mamounas, Sandra M. Swain
Collection and assembly of data: Stephanie R. Land, Charles E. Geyer Jr, Joseph P. Costantino, Eduardo R. Pajon, Louis Fehrenbacher, Edith A. Perez, Sandra M. Swain
Data analysis and interpretation: Patricia A. Ganz, Stephanie R. Land, Charles E. Geyer Jr, Reena S. Cecchini, Joseph P. Costantino, Edith A. Perez, Eleftherios P. Mamounas, Sandra M. Swain
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 4.Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: Understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA. Menopause and breast cancer: Symptoms, late effects, and their management. Semin Oncol. 2001;28:274–283. doi: 10.1016/s0093-7754(01)90120-4. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Greendale GA, Petersen L, et al. Breast cancer in younger women: Reproductive and late health effects of treatment. J Clin Oncol. 2003;21:4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 7.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 8.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 9.Swain S, Land S, Ritter M, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113:315–320. doi: 10.1007/s10549-008-9937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Land SR, Kopec JA, Yothers G, et al. Health-related quality of life in axillary node-negative, estrogen receptor-negative breast cancer patients undergoing AC versus CMF chemotherapy: Findings from the National Surgical Adjuvant Breast and Bowel Project B-23. Breast Cancer Res Treat. 2004;86:153–164. doi: 10.1023/B:BREA.0000032983.87966.4e. [DOI] [PubMed] [Google Scholar]
- 11.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 12.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy- Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 13.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 14.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Ganz PA, Day R, Ware JE, Jr, et al. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention trial. J Natl Cancer Inst. 1995;87:1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 16.Cella D, Land S, Chang CH, et al. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): Psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat. 2008;109:515–526. doi: 10.1007/s10549-007-9682-9. [DOI] [PubMed] [Google Scholar]
- 17.Stanton AL, Bernaards CA, Ganz PA. The BCPT Symptom Scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 18.Land SR, Ritter MW, Costantino JP, et al. Compliance with patient-reported outcomes in multicenter clinical trials: Methodologic and practical approaches. J Clin Oncol. 2007;25:5113–5120. doi: 10.1200/JCO.2007.12.1749. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Carbone P, Economou SG, et al. 1-Phenylalanine mustard (L-PAM) in the management of primary breast cancer: A report of early findings. N Engl J Med. 1975;292:117–122. doi: 10.1056/NEJM197501162920301. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Fisher ER, Redmond C. Ten-year results from the National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trial evaluating the use of L-phenylalanine mustard (L-PAM) in the management of primary breast cancer. J Clin Oncol. 1986;4:929–941. doi: 10.1200/JCO.1986.4.6.929. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 22.International Breast Cancer Study Group (IBCSG) Castiglione-Gersch M, O'Neill A, et al. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: A randomized trial. J Natl Cancer Inst. 2003;95:1833–1846. doi: 10.1093/jnci/djg119. [DOI] [PubMed] [Google Scholar]
- 23.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]