Abstract
Purpose
Among postmenopausal women with endocrine-responsive breast cancer, the aromatase inhibitor letrozole, when compared with tamoxifen, has been shown to significantly improve disease-free survival (DFS) and time to distant recurrence (TDR). We investigated whether letrozole monotherapy prolonged overall survival (OS) compared with tamoxifen monotherapy.
Patients and Methods
Of 8,010 postmenopausal women with hormone receptor–positive, early breast cancer enrolled on the Breast International Group (BIG) 1-98 study, 4,922 were randomly assigned to 5 years of continuous adjuvant therapy with either letrozole or tamoxifen. Of 2,459 patients enrolled in the tamoxifen treatment arm, 619 (25.2%) selectively crossed over to either adjuvant or extended letrozole after initial trial results were presented in January 2005. To gain better estimates of relative treatment effects in the presence of selective crossover, we used inverse probability of censoring weighted (IPCW) modeling.
Results
Weighted Cox models, by using IPCW, estimated a statistically significant, 18% reduction in the hazard of an OS event with letrozole treatment (hazard ratio [HR], 0.82; 95% CI, 0.70 to 0.95). Estimates of 5-year OS on the basis of IPCW were 91.8% and 90.4% for letrozole and tamoxifen, respectively. The HRs of DFS and TDR events by using IPCW modeling were 0.83 (95% CI, 0.74 to 0.94) and 0.80 (95% CI, 0.67 to 0.94), respectively (P < .05 for DFS, OS, and TDR). Median follow-up was 74 months.
Conclusion
Adjuvant treatment with letrozole, compared with tamoxifen, significantly reduces the risk of death, the risk of recurrent disease, and the risk of recurrence at distant sites in postmenopausal women with hormone receptor–positive breast cancer.
INTRODUCTION
Several recent reports of large breast cancer trials confirm the value of aromatase inhibitors as adjuvant systemic therapy in postmenopausal women with endocrine-responsive early breast cancer,1–7 and therapy with aromatase inhibitors has been recommended as part of the standard of care for these patients.8–9 Studies have shown that 5 years of adjuvant therapy with an aromatase inhibitor alone improved disease-free survival (DFS) and time to distant recurrence (TDR) compared with 5 years of tamoxifen in this population2,10; however, no trial has yet demonstrated an overall survival difference.
The Breast International Group (BIG) 1-98 study is a four-arm trial comparing 5 years of monotherapy with tamoxifen or with letrozole or with sequences of 2 years of one of these agents followed by 3 years of the other. Initial results of the BIG 1-98 primary core analysis,1 which showed statistically significant reductions in both distant recurrences and DFS events with letrozole compared with tamoxifen, were presented in January 2005, and led to the unblinding of patients in the tamoxifen-alone treatment arm. Women enrolled in the tamoxifen-alone arm of the trial, who were disease free, were eligible to receive treatment with either adjuvant or extended letrozole. Of 2,459 patients enrolled in the tamoxifen-alone treatment arm, 619 (25.2%) selectively crossed over to letrozole.
The estimates of the magnitude of letrozole benefit in the intention-to-treat (ITT) analyses of the monotherapy comparison in a recently published report of a protocol-specified 2009 update10 are likely to be attenuated by the selective crossover of patients randomly assigned to receive tamoxifen. Evidence from large, phase III studies has shown that patients who switched to an aromatase inhibitor after 2 to 3 years of tamoxifen had a survival benefit compared with patients who remained on tamoxifen monotherapy.3–7 Therefore, the 25.2% of the patients in the BIG 1-98 tamoxifen monotherapy arm who selectively crossed over to letrozole actually received a sequential endocrine therapy regimen that is superior to tamoxifen alone. As a result, in analyses updated since 2005, the estimated outcomes of the tamoxifen arm in the ITT analysis are likely to be better than if all patients randomly assigned to tamoxifen continued to receive the drug for 5 years.
To account for selective crossover and improve on the ITT analysis, we considered various approaches to adjust for potential bias caused by this specific nonadherence with the randomized treatment assignment. Inverse probability weighted modeling is a well-established approach in randomized and observational studies to overcome such estimation biases. This method analyzes only the available data under conditions of informative missing data. Specifically, we employed inverse probability of censoring weighted (IPCW) Cox modeling.11 In the setting of selective crossover, IPCW modeling artificially creates a scenario of missing follow-up data by censoring the follow-up of each woman at the time she crossed over (informative censoring). However, the truncated follow-up is re-created by applying weighting to the follow-up of women with similar demographic and disease characteristics who did not cross over. In this way, the follow-up of women who remain on tamoxifen accounts not only for themselves in the analysis but also for comparable women whose experience remaining on tamoxifen cannot be observed because they selectively changed treatments.
For this report, we analyzed the data set of the protocol-specified update at 10 years after trial initiation,10 and we characterized the efficacy and final safety data of letrozole versus tamoxifen monotherapy, taking into consideration the selective crossover. After follow-up was truncated, the median follow-up was reduced to 74 months (IPCW) from 76 months (ITT).
PATIENTS AND METHODS
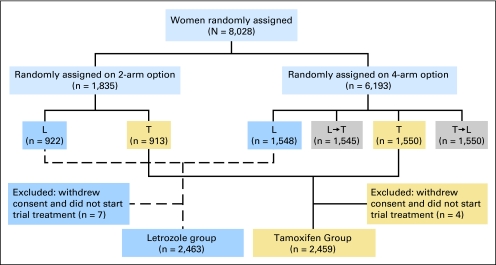
The design and conduct of the trial have been described elsewhere1,10,12,13 (Fig 1). Briefly, BIG 1-98 is a randomized, phase III, double-blind trial involving postmenopausal women with hormone receptor–positive, early invasive breast cancer. From March 1998 through March 2000, women were randomly assigned to receive only letrozole (2.5 mg daily) or only tamoxifen (20 mg daily) for 5 years. From April 1999 through May 2003, women were randomly assigned to one of four study treatments: tamoxifen monotherapy for 5 years, letrozole monotherapy for 5 years, letrozole for 2 years followed by tamoxifen for 3 years, or tamoxifen for 2 years followed by letrozole for 3 years. The ITT population included 8,010 women, of whom 4,922 were allocated to 5 years of either letrozole or tamoxifen monotherapy.
Fig 1.
Consort diagram of Breast International Group 1-98 trial. The shaded boxes, which denote the sequential therapy groups, are not included in this analysis. L, letrozole; T, tamoxifen.
The 2005 results,1 which showed statistically significant reductions in both distant recurrences and DFS events with letrozole compared with tamoxifen, led to the recommendation by the International Breast Cancer Study Group Data and Safety Monitoring Committee and a decision by the BIG 1-98 Steering Committee to inform women in the tamoxifen-alone arm of their treatment, thereby allowing informed decisions to be made about their future care. An amendment of the protocol in April 2005 allowed for the provision of letrozole to any patient assigned to tamoxifen monotherapy who was free of disease, who was still receiving or recently (within 6 months) stopped tamoxifen, and who wished to cross over to letrozole (selective crossover). The double-blind nature of the study was maintained for the three remaining treatment arms.
For the assessment of adverse events, the safety population included 4,895 of 4,922 patients, excluding 27 who did not receive any trial treatment. Adverse events that occurred more than 30 days after selective crossover from tamoxifen to letrozole were collected but excluded from data summaries.
The primary trial end point was DFS, defined as the time from random assignment to the earliest occurrence of one of the following: invasive recurrence in local, regional, or distant sites; new invasive contralateral breast cancer; any second nonbreast malignancy; or death as a result of any cause. Other end points included overall survival (OS), defined as the time from random assignment to death as a result of any cause, and time to distant recurrence, defined as the time from random assignment to recurrence at a distant site.
Statistical Analyses
The BIG 1-98 trial primary analytic approach was ITT; if an event was not observed, then follow-up was censored at the date of last disease assessment. To account for selective crossover in the estimation of relative treatment effects, IPCW analyses provided valid estimates, assuming no unmeasured confounders of an end point and selective crossover.11 In these analyses, among women assigned to tamoxifen monotherapy who selectively crossed over to letrozole, an end point was censored at the date of selective crossover. The IPCW estimators correct for bias as a result of this dependent censoring at selective crossover, when most of the selective crossover can be explained by measured time-independent and/or time-dependent prognostic factors and a nearly correct time-dependent Cox model for the cause-specific hazard of censoring at selective crossover.11
For each end point, a set of time-varying weights was estimated among patients assigned to tamoxifen monotherapy, as the inverse of the conditional probability of remaining on tamoxifen.11 These probabilities were estimated with Cox proportional hazards models of the time to selective crossover, stratified by random assignment option (two- or four-arm option) and chemotherapy use, and adjusted for demographic and disease characteristics (including age, local treatment, nodal status, locally assessed estrogen receptor/progesterone receptor status, tumor grade) and time-varying performance status. The model covariates were those confounding factors that were associated both with the time-to-event end point and with time to selective crossover. For patients assigned to letrozole, the time-varying weights were set to one throughout follow-up. A stratified Cox proportional hazards model with time-varying weights was used to obtain IPCW estimates of hazard ratios with robust standard errors used for 95% confidence intervals and Wald χ2 test statistics.11,14,15 IPCW Kaplan-Meier estimates of 5-year time to event were also estimated.
Adverse event incidence was compared between treatment arms by using Fisher's exact test. Kaplan-Meier cumulative incidence estimates summarized the onset of adverse events; between-treatment comparisons used log-rank tests. The distribution of time until stopping trial treatment as a result of adverse events was estimated and compared by using competing risks analysis,16 with cessation of treatment as a result of competing causes of DFS event, selective crossover, completion of 5 years of trial treatment, or other (ie, protocol violation, withdrawn consent, loss to follow-up, administrative issues).
All reported P values were two sided. The analysis used SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.6.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Role of the Coordinating Group, Trial Steering Committee and Funding Source
The International Breast Cancer Study Group was responsible for study design and coordination, data collection and management, medical review, data analysis, and reporting (including the decision to publish). The ethics committees and required health authorities of each participating institution approved the study protocol, and all patients gave written informed consent. Novartis (Basel, Switzerland), the manufacturer of letrozole, provided financial support for study conduct but imposed no restrictions on the investigators with respect to trial data. The manuscript was prepared by the authors, who had full access to the data and who made final decisions on content, whereas the Steering Committee (including a minority membership of Novartis employees) reviewed the paper and offered changes.
RESULTS
Characteristics of the 4,922 patients in the monotherapy cohort have been summarized previously.12 At the time of this analysis, all patients had reached the end of the 5-year trial treatment period. Seventy-one percent of the 2,463 patients assigned to letrozole completed 5 years of protocol therapy. Of the 2,459 patients randomly assigned to tamoxifen monotherapy, 44% completed 5 years of protocol therapy, and 619 (25.2%) patients crossed over to letrozole. These patients who crossed over had been receiving treatment for at least 2.5 years before crossover, with the majority between 3 and 5 years, and the median durations of letrozole therapy and of follow-up after starting letrozole were 18 months and 21 months (range, 0 to 36 months), respectively.
Efficacy
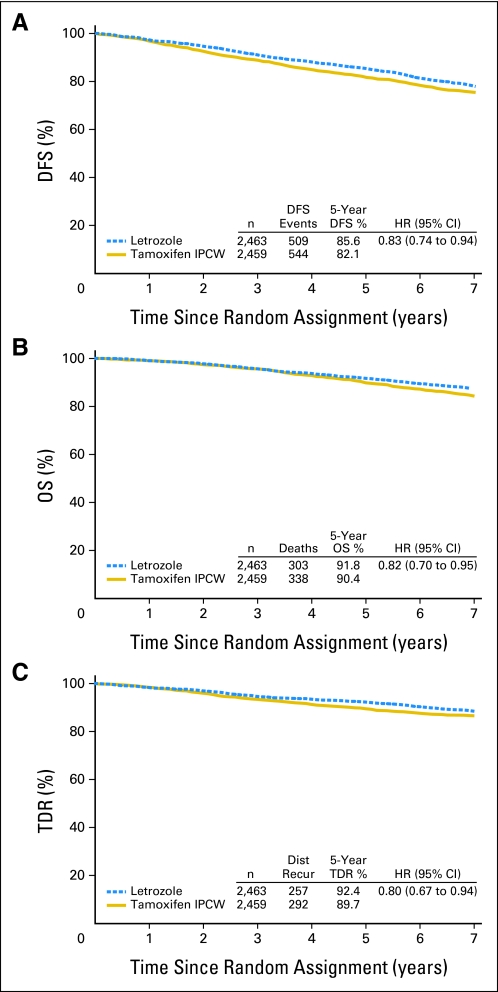
The IPCW estimate of 5-year DFS was 82.1% for tamoxifen compared with 85.6% for letrozole (Fig 2A), and the estimate of the hazard ratio for DFS was 0.83 (95% CI, 0.74 to 0.94). Details of DFS events are summarized in Table 1. The estimate of the hazard ratio for OS was 0.82 (95% CI, 0.70 to 0.95), with 5-year overall survival estimates of 90.4% for tamoxifen versus 91.8% for letrozole (Fig 2B). The estimate of the hazard ratio for TDR was 0.80 (95% CI, 0.67 to 0.94), and 5-year distant recurrence-free estimates were 89.7% for tamoxifen versus 92.4% for letrozole (Fig 2C). The differences for all three end points were statistically significant (P < .05).
Fig 2.
Weighted inverse probability of censoring weighted (IPCW) Kaplan-Meier estimates of (A) disease-free survival (DFS), (B) overall survival (OS), and (C) time to distant recurrence (TDR) comparing letrozole with tamoxifen. The median follow-up time was 74 months, and all patients completed the protocol treatment period. HR, hazard ratio; Dist Recur, distant recurrence.
Table 1.
Sites of First Disease Failure According to Random Treatment Assignment and Accounting for Selective Crossover
| Disease Failure* | Treatment |
|||||
|---|---|---|---|---|---|---|
| Letrozole (n = 2,463) |
Tamoxifen (n = 2,459) |
Total (N = 4,922) |
||||
| No. | % | No. | % | No. | % | |
| Overall | 509 | 20.7 | 544 | 22.1 | 1,053 | 21.4 |
| Local | 30 | 1.2 | 44 | 1.8 | 74 | 1.5 |
| Contralateral breast | 30 | 1.2 | 34 | 1.4 | 64 | 1.3 |
| Regional | 19 | 0.8 | 16 | 0.7 | 35 | 0.7 |
| Distant | 239 | 9.7 | 254 | 10.3 | 493 | 10.0 |
| Soft tissue | 15 | 0.6 | 21 | 0.9 | 36 | 0.7 |
| Bone | 109 | 4.4 | 111 | 4.5 | 220 | 4.5 |
| Viscera | 115 | 4.7 | 122 | 5.0 | 237 | 4.8 |
| Second (nonbreast) malignancy | 101 | 4.1 | 106 | 4.3 | 207 | 4.2 |
| Head and neck | 3 | — | 3 | — | 6 | — |
| Thyroid | 4 | — | 5 | — | 9 | — |
| Lung | 8 | — | 11 | — | 19 | — |
| Lung carcinoid | 1 | — | — | — | 1 | — |
| Esophageal | 3 | — | — | — | 3 | — |
| Gastric | 6 | — | 4 | — | 10 | — |
| Pancreatic | 3 | — | 4 | — | 7 | — |
| Pancreatic and renal | 1 | — | — | — | 1 | — |
| Hepatic/Biliary | 1 | — | 3 | — | 4 | — |
| Colorectal | 20 | — | 23 | — | 53 | — |
| Gastrointestinal NOS | — | — | 1 | — | 1 | — |
| Renal | 3 | — | 9 | — | 12 | — |
| Urothelial | 2 | — | 2 | — | 4 | — |
| Ovarian | 7 | — | 7 | — | 14 | — |
| Endometrial | 6 | — | 19 | — | 25 | — |
| Mixed Muellerian tumor | — | — | 2 | — | 2 | — |
| Uterine sarcoma | — | — | 2 | — | 2 | — |
| Cervical | 4 | — | 1 | — | 5 | — |
| Vaginal/vulvar | 4 | — | — | — | 4 | — |
| CNS glioblastoma | 1 | — | 1 | — | 2 | — |
| Melanoma | 11 | — | 2 | — | 13 | — |
| Melanoma and lung | 1 | — | — | — | 1 | — |
| Leukemia | 4 | — | 4 | — | 8 | — |
| Lymphoma NHL | 4 | — | 3 | — | 7 | — |
| Multiple myeloma | 3 | — | — | — | 3 | — |
| Unknown† | 1 | — | — | — | 1 | — |
| Death without prior cancer event | 87 | 3.5 | 86 | 3.5 | 173 | 3.5 |
| Unknown | 3 | 0.1 | 4 | 0.2 | 7 | 0.1 |
NOTE. Follow-up censored among 619 tamoxifen-assigned women at time of selective crossover to letrozole. Disease-free survival events that occurred after selective crossover were not included (n = 21 DFS events; n = 5 deaths; and n = 6 distant recurrences ignored relative to intention-to-treat analysis).
Abbreviations: NOS, not otherwise specified; NHL, non-Hodgkin's lymphoma.
Disease-free survival events.
Data requested but not received.
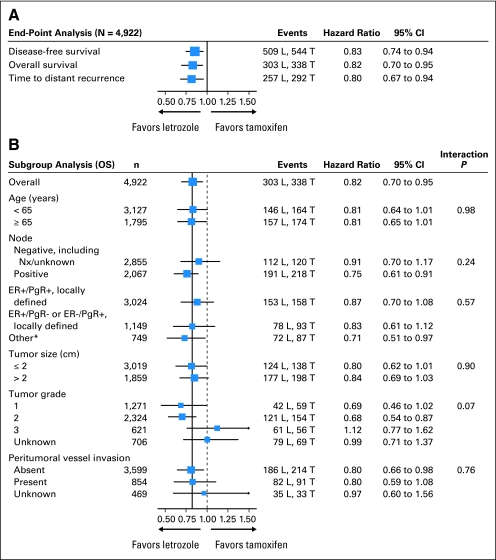
We explored protocol-defined subgroups to identify whether there was any apparent difference in the relative efficacy of letrozole on OS compared with the overall benefit observed (Fig 3). IPCW hazard ratio estimates in nearly all subgroups favored letrozole over tamoxifen. Heterogeneity in the relative treatment efficacy was suggested only for tumor grade.
Fig 3.
Inverse probability of censoring weighted Cox model results of (A) primary and secondary end points and (B) subgroups with primary end point of overall survival (OS). The size of the boxes was inversely proportional to the standard error of the hazard ratio. The solid vertical line was placed at 0.82, which was the hazard ratio estimate for the overall analysis of the OS end point. DFS, disease-free survival; L, letrozole; T, tamoxifen; ER, estrogen receptor; PgR, progesterone receptor; Nx, regional lymph nodes. TDR, time to distant recurrence. (*) Other includes ER+/PgR unknown, ER unknown/PgR+, ER−/PgR− (ineligible), ER−/PgR unknown, and ER unknown/PgR unknown.
Safety
Among patients on letrozole, 13.6% discontinued trial treatment early as a result of an adverse event, compared with 11.9% of patients on tamoxifen (Gray's test P = .08 accounting for competing causes of discontinuation). The incidence of treatment discontinuation as a result of an adverse event was greatest during the first 2 years of treatment (8.6% among patients assigned to letrozole and 7.4% among those assigned to tamoxifen) and stabilized to an additional 1% to 2% per year for the remainder of the 5-year protocol therapy period.
Table 2 summarizes the worst grade of adverse events among the 4,895 patients in the safety population. Patients on tamoxifen experienced significantly more thromboembolic events, vaginal bleeding, hot flushes, and night sweating. Patients taking letrozole experienced significantly more bone fractures, osteoporosis, arthralgia, vaginal dryness, carpal tunnel syndrome, and low-grade cholesterol elevation. There were trends toward greater incidences of ischemic heart disease, other cardiovascular events (although overall cardiac events were similar), myalgia, and subjective nervous system or psychiatric events on letrozole. Among patients who did not have a prior hysterectomy, a greater number on tamoxifen had endometrial biopsies performed (59 [3.1%] of 1,909 on letrozole and 268 [13.8%] of 1,943 on tamoxifen), resulting in a diagnosis of endometrial cancer during treatment in four patients (0.2%) taking letrozole and 11 (0.6%) taking tamoxifen.
Table 2.
Worst Grade of Adverse Events
| Event | Treatment |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Letrozole (n = 2,448) |
Tamoxifen (n = 2,447)* |
P | |||||||||||||
| Grade |
Grade |
||||||||||||||
| 1 No. | 2 No. | 3 No. | 4 No. | 5 No. | Overall |
1 No. | 2 No. | 3 No. | 4 No. | 5 No. | Overall |
||||
| No. | % | No. | % | ||||||||||||
| CVA/TIA | 0 | 0 | 13 | 25 | 7 | 45 | 1.8 | 0 | 0 | 7 | 28 | 3 | 38 | 1.6 | .51 |
| Thromboembolic event | 5 | 27 | 16 | 10 | 5 | 63 | 2.6 | 3 | 48 | 31 | 18 | 4 | 104 | 4.3 | .001 |
| Cardiac event† | 52 | 24 | 60 | 23 | 10 | 169 | 6.9 | 73 | 28 | 34 | 10 | 7 | 152 | 6.2 | .36 |
| Ischemic heart disease | 12 | 9 | 25 | 18 | 5 | 69 | 2.8 | 13 | 11 | 15 | 8 | 2 | 49 | 2.0 | .08 |
| Cardiac failure‡ | 6 | 3 | 14 | 2 | 5 | 30 | 1.2 | 10 | 3 | 6 | 2 | 4 | 25 | 1.0 | .59 |
| Other cardiovascular event‡ | 6 | 15 | 3 | 0 | 0 | 24 | 1.0 | 10 | 2 | 1 | 0 | 0 | 13 | 0.5 | .10 |
| Hypertension‡ | 53 | 36 | 41 | 2 | 0 | 132 | 5.4 | 62 | 27 | 39 | 2 | 0 | 130 | 5.3 | .95 |
| Hypotension‡ | 11 | 2 | 1 | 0 | 0 | 14 | 0.6 | 3 | 4 | 2 | 0 | 0 | 9 | 0.4 | .40 |
| Hypercholesterolemia | 1021 | 259 | 11 | 0 | 0 | 1291 | 52.7 | 564 | 63 | 4 | 2 | 0 | 633 | 25.9 | < .001 |
| Vaginal bleeding | 89 | 14 | 1 | 0 | 0 | 104 | 4.2 | 167 | 49 | 6 | 0 | 0 | 222 | 9.1 | < .001 |
| Vaginal dryness‡ | 44 | 42 | 2 | 0 | 0 | 88 | 3.6 | 27 | 10 | 0 | 0 | 0 | 37 | 1.5 | < .001 |
| Nausea | 183 | 69 | 6 | 0 | 0 | 258 | 10.5 | 195 | 39 | 8 | 0 | 0 | 242 | 9.9 | .48 |
| Vomiting | 50 | 29 | 3 | 0 | 0 | 82 | 3.3 | 62 | 11 | 5 | 0 | 0 | 78 | 3.2 | .81 |
| Hot flushes | 408 | 412 | 0 | 0 | 0 | 820 | 33.5 | 426 | 499 | 0 | 0 | 0 | 925 | 37.8 | .002 |
| Night sweating | 186 | 168 | 0 | 0 | 0 | 354 | 14.5 | 195 | 223 | 0 | 0 | 0 | 418 | 17.1 | .01 |
| Bone fractures | 0 | 160 | 84 | 0 | 0 | 244 | 10.0 | 0 | 122 | 43 | 0 | 0 | 165 | 6.7 | < .001 |
| Osteoporosis‡ | 35 | 79 | 10 | 0 | 0 | 124 | 5.1 | 20 | 29 | 5 | 0 | 0 | 54 | 2.2 | < .001 |
| Arthralgia‡ | 304 | 197 | 48 | 2 | 0 | 551 | 22.5 | 244 | 131 | 31 | 0 | 0 | 406 | 16.6 | < .001 |
| Myalgia‡ | 136 | 53 | 16 | 1 | 0 | 206 | 8.4 | 120 | 39 | 13 | 0 | 0 | 172 | 7.0 | .08 |
| Carpal tunnel‡ | 7 | 7 | 6 | 0 | 0 | 20 | 0.8 | 3 | 1 | 1 | 0 | 0 | 5 | 0.2 | .004 |
| Nausea and vomiting | 196 | 78 | 6 | 0 | 0 | 280 | 11.4 | 210 | 45 | 8 | 0 | 0 | 263 | 10.7 | .47 |
| Hot flushes and night sweating | 455 | 447 | 0 | 0 | 0 | 902 | 36.8 | 450 | 547 | 0 | 0 | 0 | 997 | 40.7 | .005 |
| Arthralgia and myalgia‡ | 379 | 230 | 58 | 2 | 0 | 669 | 27.3 | 309 | 159 | 40 | 0 | 0 | 508 | 20.8 | < .001 |
| Osteoporosis (grade 3) or bone fracture‡ | 0 | 157 | 93 | 0 | 0 | 250 | 10.2 | 0 | 121 | 46 | 0 | 0 | 167 | 6.8 | < .001 |
| Subjective nervous system/psychiatricठ| 234 | 144 | 33 | 2 | 0 | 413 | 16.9 | 200 | 132 | 34 | 0 | 0 | 366 | 15.0 | .07 |
NOTE. P values were derived by using Fisher's exact test and compared the incidence of grades 1 through 5 events between letrozole and tamoxifen.
Abbreviations: CVA, cerebrovascular accident; TIA, transient ischemic attack.
Prespecified adverse events were collected on case-report forms every 6 months while on trial treatment until 30 days after treatment completion and were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0, if available, and otherwise according to protocol-defined criteria. Nonspecified adverse events that were recorded as other were coded by an independent agency according to Medical Dictionary for Regulatory Activities without knowledge of treatment assignment. All cardiovascular adverse events were medically reviewed by the International Breast Cancer Study Group. Adverse events that occurred more than 30 days after selective crossover were collected but were excluded from this summary.
Cardiac events included ischemic cardiovascular disease, arrhythmia, heart failure, cardiopathy, valvular disease, ECG changes, sudden cardiac death, and cardiac not otherwise specified. It did not include hypertension or hypotension.
Not a prespecified adverse event; obtained from Medical Dictionary for Regulatory Activities coding of reported other adverse events.
Subjective nervous system/psychiatric adverse events were cognition disorders (eg, blackout, memory loss, impatience), perception disorders (eg, burning sensation, dysphasia, migraine), or other related disorders (eg, anxiety, melancholia, irritability, nightmares). Adverse events that were not included were nervous system disorders, such as CVA, TIA, Alzheimer's disease, Parkinson's disease, Bell's palsy, stenosis, diabetic coma, epilepsy/seizure, tremors, aneurism, cranial bleeding, sciatica, neuropathy; and psychiatric disorders, such as delirium and loss of libido.
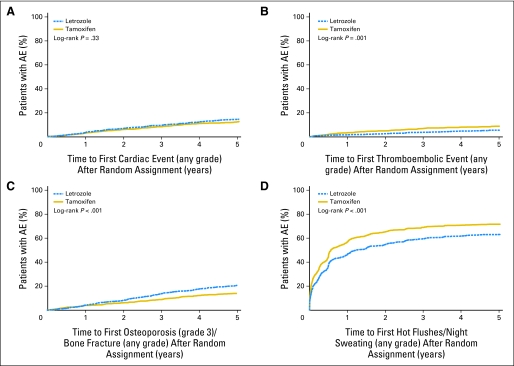
Kaplan-Meier cumulative incidence estimates of cardiac and thromboembolic adverse events (any grade), osteoporosis (grade 3) or bone fracture (any grade), and hot flushes or night sweating (any grade) are summarized in Figure 4. The incidence rates of cardiac events, thromboembolic events, and bone fractures or severe osteoporosis (Figs 4A to 4C) were relatively constant throughout follow-up. In contrast, 24% of women taking letrozole and 29% taking tamoxifen reported hot flushes/night sweating within the first year of therapy, with the incidence rates gradually decreasing over time (Fig 4D). There were 207 second (nonbreast) malignancies (n = 101, letrozole; n = 106, tamoxifen) and 173 deaths without prior cancer events (n = 87, letrozole; n = 86, tamoxifen) considered as sites of first disease failure (Table 1).
Fig 4.
Kaplan-Meier cumulative incidence of (A) cardiac events (any grade), (B) thromboembolic events (any grade), (C) osteoporosis (grade 3) or bone fractures (any grade), and (D) hot flushes or night sweats (any grade) comparing letrozole with tamoxifen. AE, adverse event.
DISCUSSION
Recently published results of well-conducted, randomized, phase III trials involving tamoxifen and aromatase inhibitors support several treatment options for postmenopausal women who require endocrine therapy.8,9 Although optimal adjuvant hormonal therapy for a postmenopausal woman with hormone receptor–positive breast cancer should include an aromatase inhibitor either as initial therapy or after treatment with tamoxifen in the majority of cases, women with hormone receptor–positive, early breast cancer and their physicians must weigh the risks and benefits of these therapeutic options. For example, aromatase inhibitors as up-front endocrine treatment may be preferable in patients at higher risk of early relapse,17 but there is limited information on whether to switch from tamoxifen to an aromatase inhibitor after 2 to 3 years of tamoxifen or to start the adjuvant therapy with an aromatase inhibitor.
The results of this study indicate that adjuvant treatment with letrozole, compared with tamoxifen, significantly reduces the risk of death in postmenopausal women with hormone receptor–positive breast cancer. Previously published long-term updates from the BIG 1-98 study showed that 5 years of treatment with letrozole significantly improved DFS, but not OS, compared with 5 years of tamoxifen alone according to the ITT analysis approach.10 The long-term update of the BIG 1-98 ITT analysis estimated a 13% reduction in the hazard of an OS event with letrozole treatment (P = .08), a 12% reduction in the hazard of a DFS event (P = .03) and a 15% reduction in the hazard of a distant recurrence (P = .05). However, in the BIG 1-98 ITT analysis, follow-up in the tamoxifen-alone treatment group included the follow-up of women after selective crossover from tamoxifen to letrozole, biasing the estimation of relative treatment effects against letrozole. The IPCW analysis showed that a significant survival benefit in the BIG 1-98 study would have been observed had there been no selective crossover.
This result is not unexpected, as the switch from tamoxifen to letrozole after crossover would have improved the outcome for these patients, as evidenced in the recently published meta-analysis of randomized trials of aromatase inhibitors compared with tamoxifen.7 Cohort 2 of the meta-analysis (studies investigating the switch strategy), at a mean of 3.7 women-years of follow-up from the time of the switch, showed that switching to an aromatase inhibitor after 2 to 3 years of tamoxifen statistically significantly reduced the risk of recurrence by 29%, the risk of distant recurrence by 23%, and the risk of death by 21%. Thus, the 25.2% of patients assigned to tamoxifen who selectively crossed over to letrozole actually received a more effective treatment than tamoxifen alone and would be expected to have better OS than would have been observed in the absence of selective crossover. As a consequence, the estimates of letrozole benefit in the recently published10 ITT analyses of the monotherapy comparison in BIG 1-98 were likely to be attenuated by the selective crossover in the tamoxifen treatment group. Conversely, a censored analysis of OS showed a statistically significant advantage of letrozole over tamoxifen.10 However, this simple censoring may yield an overestimate of the difference, mainly because women who had recurrent disease were not candidates for crossover.
The IPCW analysis of the 10-year update of BIG 1-98 was implemented in an attempt to remove the impact of the selective crossover from the treatment comparison and to estimate the magnitude of the treatment effect had 25.2% of patients randomly assigned to tamoxifen not selectively crossed over to receive letrozole. IPCW estimation is a method that has been used in large, randomized, phase III trials and in observational studies to improve on ITT analyses by adjusting for potential bias caused by nonadherence with the randomization treatment assignment.18–20 The IPCW analysis estimated a statistically significant 18% reduction in the hazard of an OS event with letrozole treatment as well as a 17% reduction in the hazard of a DFS event and a 20% reduction in the hazard of a distant recurrence; each of these estimates was greater than the corresponding estimate of risk reduction from ITT analysis.10
The results of the BIG 1-98 trial showed that tamoxifen and letrozole have different safety profiles that continue to be consistent in the current analysis with those previously reported.1,10,12 Overall, a slightly higher percentage of patients stopped letrozole early because of adverse events, but this should be weighed against the improvements in disease control and survival. Moreover, the limited numbers of deaths as a result of causes other than cancer and life-threatening adverse events in the letrozole group suggest a relatively favorable safety profile.
In conclusion, previous analyses of the BIG 1-98 trial have shown that letrozole monotherapy significantly reduced the risks of recurrent disease and recurrence at distant sites when compared with tamoxifen alone.1,10,12 The IPCW analysis reported here confirmed these observations and also indicated a statistically significant improvement in OS with letrozole treatment compared with tamoxifen, probably reflecting the early reduction in the risk of distant metastases. The absolute differences in survival probability are relatively small, and the different adverse event profiles of the two agents (Table 2) may therefore help inform the selection of therapy for individual patients. Although uncertainty persists about the optimal time to introduce aromatase inhibitors and the optimal duration of their use as adjuvant therapy, this analysis adds information to support a role for up-front use of letrozole in the adjuvant treatment of postmenopausal women with steroid hormone receptor–positive early breast cancer.
Supplementary Material
Acknowledgment
Supported in part by Novartis and the International Breast Cancer Study Group (funded by the Swedish Cancer Society, the Cancer Council Australia, Australia New Zealand Breast Cancer Trials Group, Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, National Cancer Institute [CA-75362], and the Foundation for Clinical Cancer Research of Eastern Switzerland).
Presented in part at the Cancer Therapy & Research Center-American Association for Cancer Research San Antonio Breast Cancer Symposium, December 10-13, 2009, San Antonio, TX.
Footnotes
See accompanying editorial on page 1093
Written on behalf of the BIG 1-98 Collaborative and International Breast Cancer Study Groups.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00004205.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTSOF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jacquie Chirgwin, Novartis (C), AstraZeneca (C); Andrew Wardley, Novartis (C) Stock Ownership: Beat Thürlimann, Novartis Honoraria: Marco Colleoni, Novartis; Henning Mouridsen, Novartis; John F. Forbes, AstraZeneca; Jacquie Chirgwin, Novartis, AstraZeneca; Tadeusz Pienkowski, Novartis; Andrew Wardley, Novartis; Aron Goldhirsch, Novartis, Pfizer Research Funding: Jacquie Chirgwin, Novartis, AstraZeneca, Pfizer; Tadeusz Pienkowski, Novartis; Richard D. Gelber, Novartis Expert Testimony: None Other Remuneration: Jacquie Chirgwin, Novartis, AstraZeneca; Aron Goldhirsch, Novartis, Pfizer
AUTHOR CONTRIBUTIONS
Conception and design: Marco Colleoni, Anita Giobbie-Hurder, Meredith M. Regan, Beat Thürlimann, Henning Mouridsen, Louis Mauriac, John F. Forbes, Ian Smith, Richard D. Gelber, Alan S. Coates, Aron Goldhirsch
Provision of study materials or patients: Marco Colleoni, Beat Thürlimann, Henning Mouridsen, Louis Mauriac, John F. Forbes, Robert Paridaens, István Láng, Ian Smith, Jacquie Chirgwin, Tadeusz Pienkowski, Andrew Wardley, Karen N. Price, Aron Goldhirsch
Collection and assembly of data: Marco Colleoni, Anita Giobbie-Hurder, Meredith M. Regan, Beat Thürlimann, Henning Mouridsen, Louis Mauriac, John F. Forbes, Robert Paridaens, István Láng, Ian Smith, Jacquie Chirgwin, Tadeusz Pienkowski, Andrew Wardley, Karen N. Price, Richard D. Gelber, Aron Goldhirsch
Data analysis and interpretation: Marco Colleoni, Anita Giobbie-Hurder, Meredith M. Regan, Henning Mouridsen, Louis Mauriac, Andrew Wardley, Richard D. Gelber, Alan S. Coates,Aron Goldhirsch
Manuscript writing: Marco Colleoni, Anita Giobbie-Hurder, Meredith M. Regan, Beat Thürlimann, Henning Mouridsen, Louis Mauriac, John F. Forbes, Robert Paridaens, István Láng, Ian Smith, Jacquie Chirgwin, Tadeusz Pienkowski, Andrew Wardley, Karen N. Price, Richard D. Gelber, Alan S. Coates, Aron Goldhirsch
Final approval of manuscript: Marco Colleoni, Anita Giobbie-Hurder, Meredith M. Regan, Beat Thürlimann, Henning Mouridsen, Louis Mauriac, John F. Forbes, Robert Paridaens, István Láng, Ian Smith, Jacquie Chirgwin, Tadeusz Pienkowski, Andrew Wardley, Karen N. Price, Richard D. Gelber, Alan S. Coates, Aron Goldhirsch
REFERENCES
- 1.Breast International Group 1-98 Collaborative Group. Thürlimann B, Keshaviah A, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 2.Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 3.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: The ARNO 95 study. J Clin Oncol. 2007;25:2664–2670. doi: 10.1200/JCO.2006.08.8054. [DOI] [PubMed] [Google Scholar]
- 5.Boccardo F. Switching to anastrozole after tamoxifen improves survival in postmenopausal women with breast cancer. Nat Clin Pract Oncol. 2008;5:76–77. doi: 10.1038/ncponc1015. [DOI] [PubMed] [Google Scholar]
- 6.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [Erratum, Lancet 369:906, 2007] [DOI] [PubMed] [Google Scholar]
- 7.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 8.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: Status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 9.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The BIG 1-98 Collaborative Group. Mouridsen H, Giobbie-Hurder A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 12.Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine- responsive early breast cancer: Update of study BIG 1-98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 13.Giobbie-Hurder A, Price KN, Gelber RD. Design, conduct, and analyses of Breast International Group (BIG) 1-98: A randomized, double-blind, phase-III study comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Clinical Trials. 2009;6:272–287. doi: 10.1177/1740774509105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Xiao Y, Abrahamowicz M, Moodie EEM. Accuracy of conventional and marginal structural Cox model estimators: A simulation study. Int J Biostat. 2010;6 doi: 10.2202/1557-4679.1208. http://www.bepress.com/ijb/vol6/iss2/ [DOI] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Mauriac L, Keshaviah A, Debled M, et al. Predictors of early relapse in postmenopausal women with hormone receptor-positive breast cancer in the BIG 1-98 trial. Ann Oncol. 2007;18:859–867. doi: 10.1093/annonc/mdm001. [DOI] [PubMed] [Google Scholar]
- 18.Cain LE, Cole SR. Inverse probability-of-censoring weights for the correction of time-varying noncompliance in the effect of randomized highly active antiretroviral therapy on incident AIDS or death. Stat Med. 2009;28:1725–1738. doi: 10.1002/sim.3585. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 20.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: The Women's Health Initiative randomized controlled dietary modification trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.