Abstract
Purpose
Pain can hinder immunotherapy with anti-GD2 monoclonal antibodies (MoAbs) like 3F8. Heat-modified 3F8 (HM3F8) lacks effector functions and could mask GD2 or cross-reactive epitopes on nerves, thereby preventing a subsequent dose of unmodified 3F8 from activating pain fibers. We hypothesized that 3F8 dose escalation is possible without increased analgesic requirements in patients pretreated with HM3F8.
Patients and Methods
Thirty patients with resistant neuroblastoma (NB) received one to two cycles of 3F8 plus granulocyte-macrophage colony-stimulating factor. 3F8 dosing began at 20 mg/m2/d and increased by 20 mg/m2/d in the absence of dose-limiting toxicity (DLT). Premedication included analgesics, antihistamines, and 5-minute infusions of HM3F8. On the basis of experience with 3F8 10 mg/m2/d in prior protocols, the DLT of pain was defined as more than seven doses of opioids administered within 2 hours. Opioid use was compared with a contemporary control group treated with 3F8 20 mg/m2/d but no HM3F8. Disease response was assessed.
Results
Treatment was administered in the outpatient setting. Dose escalation stopped at 160 mg/m2/d because of drug supply limitations; even through this dosage level, analgesic requirements were similar to historical controls, and there were no DLTs. Analgesic requirements at 3F8 dosage levels through 80 mg/m2/d were significantly less compared with controls. Anti-NB activity occurred at all dosages.
Conclusion
Multifold dose escalation of 3F8 is feasible. The findings can be interpreted as compatible with the possibility that HM3F8 can modify toxicity without blunting anti-NB activity. This pain control strategy may help achieve dose escalation with other anti-GD2 MoAbs.
INTRODUCTION
The murine 3F8 monoclonal antibody (MoAb) and other anti-GD2 MoAbs achieved clinical responses in phase I1–4 and II5 trials of patients with neuroblastoma (NB) and, as adjuvant therapy, produced encouraging results in a Memorial Sloan-Kettering Cancer Center (MSKCC) study,6 although not in a German cooperative group study.7 Adding granulocyte-macrophage colony-stimulating factor (GM-CSF)8,9 or interleukin-210 promised greater anti-NB activity. Indeed, a Children's Oncology Group randomized trial found a significantly superior outcome with anti-GD2 MoAb ch14.18 plus GM-CSF/interleukin-2,11 as developed in a phase I study.12
Generalized pain and pain-associated hypertension were dose-limiting toxicities (DLTs) in phase I studies of 3F8 and other anti-GD2 MoAbs.2–4 The adverse effects were attributed to inflammatory effects (eg, via complement activation13) on GD2 (+) nerves. A standardized analgesic regimen was developed with improved control of 3F8 adverse effects.5,6,9 The advance eventuated in the routine outpatient treatment of up to 12 patients per day and a shortening of 3F8 infusions to 30 minutes (in contrast to 8 hours1 or 90 minutes5,6,9 in prior studies).
The impetus for a new phase I study of 3F8 became evident. Higher dosing was appealing, given the dose-response relationship between 3F8 and antibody-dependent cellular cytotoxicity (ADCC)14,15 and complement-mediated cytotoxicity (CMC).16 In addition, a further decrease in pain, without affecting antitumor activity, seemed possible through use of heat-modified 3F8 (HM3F8). This concept was based on the following observations: HM3F8 retained GD2 immunoreactivity but lost the effector functions causative of the adverse effects of anti-GD2 MoAbs; a pretreatment dose of HM3F8 in animal models did not alter 3F8 localization to NB, reduce anti-NB effects of 3F8, or cause pain; and 3F8 localization in tumor peaked at 24 hours, whereas pain occurred within minutes of 3F8 administration. The data suggested that HM3F8 might block GD2 or cross-reactive epitopes on nerves, thereby reducing nerve-related adverse effects of a subsequent (treatment) dose of native 3F8, and, at low doses, would have little effect on 3F8 targeting to NB in patients. In the new phase I study reported herein, HM3F8 seemed to help achieve multifold dose escalation of 3F8 without impairing anti-NB activity.
PATIENTS AND METHODS
MSKCC protocol 05015 (ClinicalTrials.gov identifier: NCT00450307) was prospectively designed to find the maximum-tolerated dosage (MTD) of native 3F8 when administered after GM-CSF and HM3F8. The study was open to patients who had resistant NB by the International Neuroblastoma Response Criteria17 documented more than 3 weeks after prior therapy and who were ineligible for other MSKCC immunotherapy protocols. There were no eligibility restrictions regarding prior therapy, including stem-cell transplantation and MoAb-based treatments. Major organ toxicity was required to be grade ≤ 2 (National Cancer Institute Common Terminology Criteria for Adverse Events version 3); however, neutrophil count ≥ 500/μL and platelet count ≥ 10,000/μL were acceptable. Patients could not be taking antihypertensive medication. Informed written consents were obtained according to institutional review board rules.
Preparation of Antibodies
3F8 was prepared as described.18 HM3F8 was prepared by incubating 3F8 in a water bath at 56°C for 30 minutes and was diluted in 10 mL of 5% human serum albumin and millipore (0.22 μm) filtered before clinical use. In vitro properties of HM3F8 were assessed using previously described methods14–16,19 (Appendix Tables A1 and A2, online only). Binding to GD2 was 62.4% to 79.6% of the GD2 binding of unmodified 3F8. HM3F8 displayed near complete loss of effector functions (0.2% to 1.6% of 3F8 activity) in ADCC with human leukocytes and CMC with human complement.
Treatment
The protocol allowed two cycles. Each cycle comprised 5 days of yeast-derived human recombinant GM-CSF (sargramostim, Leukine; Immunex, Seattle, WA), followed by GM-CSF/HM3F8/3F8 for 5 days a week for 2 weeks (days 0 to 4 of week 1 and days 7 to 11 of week 2; Table 1). As in other 3F8 trials,5,6,9 analgesics at standard dosages (hydromorphone 0.0075 to 0.015 mg/kg or morphine sulfate 0.05 to 0.1 mg/kg) and antihistamines at standard dosages (diphenhydramine 1 mg/kg, hydroxyzine 1 mg/kg, and/or loratadine 5 mg for patients age 2 to 5 years or 10 mg for patients age ≥ 6 years) were administered before initiation of the daily 3F8 infusion (in this protocol, before the daily infusion of HM3F8) and then as needed. Hence, patients were often sedated when the treatment began.
Table 1.
One Cycle of Treatment
| Treatment Days | Therapy |
|---|---|
| Days −5 to −1 (Wednesday-Sunday) | GM-CSF 250 μg/m2/d subcutaneously |
| Days 0 to +4(Monday-Friday) | GM-CSF 500 μg/m2/d subcutaneously; heat-modified 3F8 (2 mg/m2/d) by 5-minute IV infusion; 10-15 minutes later, 3F8 by 30-minute IV infusion |
| Days +5 and +6(Saturday, Sunday) | GM-CSF 500 μg/m2/d subcutaneously |
| Days +7 to +11(Monday-Friday) | GM-CSF 500 μg/m2/d subcutaneously; heat-modified 3F8 (2 mg/m2/d) by 5-minute IV infusion; 10-15 minutes later, 3F8 by 30-minute IV infusion |
NOTE. The daily GM-CSF was not administered if the absolute neutrophil count was > 20,000/μL. The protocol allowed two cycles, with 2 to 4 weeks between the end of the first cycle and the start of the second cycle.
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IV, intravenous.
The first level of 3F8 dosage was 20 mg/m2/d per cycle and was increased by increments of 20 mg/m2/d per cycle if zero of three patients or ≤ one of six patients at a given dosage experienced DLT. As mandated by the protocol for safety reasons, six patients were treated at the first level and at 100 mg/m2/d. Serum levels of 3F8 were measured as described.1 In the absence of DLT, progressive disease (PD), and elevated human antimouse antibody (HAMA) titers (measured as previously described20), patients could start a second cycle of therapy 2 to 4 weeks after completion of the first cycle.
Definition of DLT
Toxicity was scored for 14 days after each cycle. DLT was defined as grade 4 toxicity (National Cancer Institute Common Terminology Criteria for Adverse Events version 3) attributable to 3F8. Vital signs and blood pressure were regularly assessed, including before and after HM3F8, before and after 3F8, and before discharge from the clinic.
Additional DLT definitions were used for the pain and hypertension expected with anti-GD2 MoAbs.1–12 DLT of hypertension was defined as medicinal intervention for 24 hours. DLT of pain was defined using historical experience. Thus, in an unselected cohort of 50 patients who, in earlier studies, received 435 administrations of 3F8/GM-CSF, the proportion of patients treated with ≥ seven doses of opioids in 1 day was 0.06. Therefore, when this study was designed, DLT of pain was defined as ≥ seven doses of opioids administered within 2 hours. One dose of analgesic was defined as hydromorphone 0.015 mg/kg or an equianalgesic dose of morphine sulfate.
Pain Assessment
The same outpatient clinic personnel treated all patients in all concurrent 3F8 protocols. As in prior5,6,9 and concurrent 3F8 studies, clinical research nurses determined when to give rescue doses of opioids to control pain; physicians were not involved in bedside decisions. Self-administration of medications and questionnaires about pain were judged to be impractical (and were not used in phase I studies of other anti-GD2 MoAbs2–4,8,10,12) because most patients are too young, remain sedated for more than 1 hour after 3F8 treatment, and, when they awake, remember the treatment experience (including pain) vaguely, if at all.
Definition of Disease Response
Pre- and postprotocol disease status was defined by the International Neuroblastoma Response Criteria,17 as expanded to include metaiodobenzylguanidine (MIBG) findings and to encompass anterior iliac crests (not just posterior sites) for bone marrow (BM) aspirates and biopsies, yielding a total of six to eight specimens. Complete response (CR) was defined as no evidence of NB. Very good partial response was defined as a more than 90% decrease in all disease parameters, except unchanged or improved bone scan, and BM histology and MIBG scan free of NB. Partial response was defined as a more than 50% decrease in all disease parameters, except unchanged or improved bone scan; ≤ one positive BM site by histology; and MIBG scan improved in all lesions. Mixed response was defined as a more than 50% decrease in ≥ one but not all disease markers and MIBG scan improved in some but not all sites. No response was defined as a less than 50% decrease in all tumor markers and unchanged MIBG findings. PD was defined as a new lesion or more than 25% increase in an existing lesion.
Statistical Analysis
In addition to the study's primary objective regarding identification of the MTD, a retrospective analysis was undertaken to compare the analgesic requirements of study patients at each dosage level with the analgesics received by a contemporary control cohort. The control cohort comprised all 33 patients treated on MSKCC study 03077 (ClinicalTrials.gov identifier: NCT00072358) for primary refractory NB over the same 43-month period that this MSKCC 05015 study accrued patients. Controls and study patients were comparable for age, race, sex, BM metastases, bone metastases, and MYCN amplification (Table 2). GM-CSF use and the 30-minute infusion of 3F8 were the same in both cohorts. The controls received 3F8 20 mg/m2/d, without HM3F8. The number of daily opioid rescues was recorded for each control and study patient on days 1 through 5 of cycle 1 of protocol therapy; the mean number of rescues was calculated for each patient. On the present 05015 study, 146 of 150 days had complete analgesic records; among controls, all 165 days of treatment were available to tabulate analgesic use. Both Mann-Whitney and repeated measures analysis of variance tests were used for statistical analyses.
Table 2.
Demographics and Clinical Characteristics of Controls and Study Patients
| Characteristic | Controls(n = 33) | Study Patients (n = 29)* |
|---|---|---|
| Female, No. | 14 | 12 |
| Race, No. | ||
| White | 27 | 25 |
| Black | 4 | 2 |
| Asian | 2 | 1 |
| Unknown | 0 | 1 |
| Age at diagnosis, years | ||
| Mean | 3.9 | 3.3 |
| Range | 1.4-14.9 | 0.5-16.8 |
| Median | 5.3 | 4.0 |
| Age at protocol, years | ||
| Mean | 4.7 | 5.3 |
| Range | 2.3-16.1 | 1.4-17.9 |
| Median | 6.1 | 6.5 |
| BM metastases,† No. | 30 | 28 |
| Bone metastases,† No. | 30 | 25 |
| MYCN amplification, No. | 6 | 5 |
| Stage 4, No. | 33 | 29 |
Abbreviation: BM, bone marrow.
One patient was treated at the 60 mg/m2/d and 140 mg/m2/d dosage levels.
Documented at some point in the patient's clinical course.
RESULTS
Patient Characteristics
The study patients were accrued from August 2005 through March 2009 (Table 2). Twenty-four patients had resistant but nonprogressing NB, including 16 patients with incomplete responses to initial induction therapy (ie, primary refractory disease) and eight patients with incomplete responses to salvage therapy for a first or subsequent relapse (ie, secondary or later refractory disease). The other patients were enrolled to treat PD: one patient had never achieved remission, and five patients experienced PD during treatment of a third or later relapse. One of the latter patients had previously been enrolled and treated at a lower dosage level.
Toxicity
All patients completed protocol therapy as planned. There were no DLTs, including absence of hypertension. Dose escalation stopped at 160 mg/m2/d as a result of drug supply limitations. Acute toxicities were tolerable and manageable in all patients (including the five adolescents), which allowed outpatient treatment. There have been no late-onset toxicities in the 17 survivors, with follow-up ranging from 18+ to 60+ months (median, 40+ months).
No cycle had to be deferred as a result of persistent or delayed-onset toxicities from the first cycle. Thus, 19 patients received the protocol's two cycles and had similar adverse effects with each cycle: 17 of these patients received cycle 2 after the protocol-mandated 2-week (n = 8), 3-week (n = 6), or 4-week (n = 3) interval; one patient was removed after cycle 1 as a result of nonprotocol issues but subsequently received cycle 2: and one patient developed HAMA after cycle 1 and received cycle 2 when HAMA became negative. The other patients did not receive cycle 2: four patients developed HAMA after cycle 1, and seven patients developed PD after cycle 1 (including one patient who received two cycles when treated at a lower dosage).
Serum 3F8 levels were proportional to dosages (Table 3), which are data relevant to possible toxicity. Regarding pain, using both Mann-Whitney and repeated measures analysis of variance tests, the amount of analgesics received by the control cohort was compared with the amount received by the entire study cohort (n = 146 days) and the amount received by study patients grouped according to 3F8 dosage (72 days for lower dosage and 74 days for dosage ≥ 100 mg/m2/d). HM3F8 use was associated with a significant decrease in analgesics received (F1,57 = 10.36, P = .002). Patients treated with 20 to 80 mg/m2/d received significantly less analgesics than patients treated with ≥ 100 mg/m2/d (F2,56 = 6.2, P = .004; Table 3).
Table 3.
Analgesic Requirements and Peak Serum Levels
| No. of Rescues*per Day |
Peak Serum Level (μg/mL) |
||||||
|---|---|---|---|---|---|---|---|
| 3F8 Dosage | No. of Patients | No. of Days Evaluable for Pain Control | Mean | SD | P (repeated measures ANOVA) | Mean | SD |
| Control (20 mg/m2) | 33 | 165 | 2.9 | 1.0 | — | — | — |
| Dose level, mg/m2 | .002 | ||||||
| 20 | 6 | 30 | 2.1† | 0.9 | .004‡§ | 6.3 | 2.2 |
| 40 | 3 | 13 | 2.1 | 0.5 | 18.0 | 7.2 | |
| 60 | 3 | 14 | 1.7† | 0.5 | 26.4 | 6.5 | |
| 80 | 3 | 15 | 1.5† | 0.1 | 30.6 | 6.5 | |
| 100 | 6 | 29 | 2.5 | 0.7 | .15‖ | 32.2 | 13.0 |
| 120 | 3 | 15 | 2.2 | 0.5 | 39.3 | 13.1 | |
| 140 | 3 | 15 | 3.3 | 0.8 | 49.4 | 7.8 | |
| 160 | 3 | 15 | 1.7 | 0.4 | 68.3 | 13.8 | |
NOTE. Controls were on the standard 3F8/granulocyte-macrophage colony-stimulating factor protocol, which did not use heat-modified 3F8.
Abbreviations: SD, standard deviation; ANOVA, analysis of variance.
One rescue was defined as hydromorphone 0.015 mg/kg or an equianalgesic dose of morphine sulfate.
Mann-Whitney U/Wilcoxon rank sum test was significant (P ≤ .05).
Significant with Bonferroni or Games-Howell corrections.
For dosage levels 20 to 80 mg/m2.
For dosage levels 100 to 160 mg/m2.
HM3F8 did not cause any symptoms or adverse reactions, including no pain or hives (the classic symptoms of treatment with unmodified 3F8). This absence of symptomatology and the presence of stable vital signs allowed all HM3F8 infusions to be completed, as planned, in 5 minutes and to be followed 10 to 15 minutes later, as planned, by initiation of treatment with unmodified 3F8.
In all patients, pulse quickened as grade 2 pain (usually in chest and/or abdomen) developed toward the middle or end of the infusion of unmodified 3F8. Vital signs normalized before discharge from the clinic. The most common other adverse effects (Table 4), as expected from prior 3F8 experience, were urticaria during or shortly after the 30-minute infusion and fever shortly after the infusion. No differences in toxicities were seen with higher dosages (Table 4). Hypokalemia, hyponatremia, and hypoalbuminemia were generally of modest, if any, clinical import. Abnormalities in liver enzymes were transient. GM-CSF shots occasionally caused local erythema.
Table 4.
Toxicity Other Than Pain (National Cancer Institute Common Terminology Criteria for Adverse Events version 3)
| No. of Patients With Toxicity |
|||||||
|---|---|---|---|---|---|---|---|
| 3F8Dosage | Total No. of Patients | Elevated AST/ALT | Hypokalemia | Hyponatremia | Hypoalbuminemia | Urticaria | Fever* |
| 20 mg/m2 | 6† | ||||||
| Grade 1 | 2 | ||||||
| Grade 2 | 3 | ||||||
| Grade 3 | 1 | ||||||
| 40 mg/m2 | 3 | ||||||
| Grade 1 | 1 | 1 | 3 | 1 | |||
| Grade 2 | 1 | 1 | 3 | ||||
| 60 mg/m2 | 3 | ||||||
| Grade 1 | 1 | 2 | 1 | 2 | |||
| Grade 2 | 3 | 1 | |||||
| 80 mg/m2 | 3 | ||||||
| Grade 1 | 2 | 3 | |||||
| Grade 2 | 3 | ||||||
| 100 mg/m2 | 6† | ||||||
| Grade 1 | 1 | 1 | 5 | ||||
| Grade 2 | 1 | 6 | |||||
| Grade 3 | 1 | 1 | |||||
| 120 mg/m2 | 3 | ||||||
| Grade 1 | 1 | 1 | 2 | ||||
| Grade 2 | 3 | ||||||
| Grade 3 | 2 | ||||||
| 140 mg/m2 | 3 | ||||||
| Grade 1 | 1 | 2 | |||||
| Grade 2 | 2 | 3 | |||||
| Grade 3 | 1 | ||||||
| 160 mg/m2 | 3 | ||||||
| Grade 1 | 1 | 2 | 1 | 2 | 1 | ||
| Grade 2 | 3 | ||||||
| Grade 4 | 1 | ||||||
NOTE. All patients received the same dosage of heat-modified 3F8 (2 mg/m2) before start of 3F8 treatment.
No source of infection.
Protocol called for treating six patients at this dosage level.
HAMA
As shown in Table 5, in this trial, as in prior studies using standard-dose 3F8 treatments without HM3F8,21 early HAMA was uncommon if 3F8 treatment began less than 90 days after high-dose alkylator-based therapy (approximately 1% incidence after one cycle and approximately 15% incidence after two cycles of 3F8). In contrast, if the initial exposure to 3F8 occurred ≥ 90 days after high-dose alkylator-based therapy, HAMA developed in approximately 40% of patients after one cycle of 3F8.
Table 5.
Comparison of HAMA Results in Phase I and Phase II Trials
| HAMA After Cycle 1 |
HAMA After Cycle 2 |
|||
|---|---|---|---|---|
| Patient Group | No. of Patients/Total Patients | % | No. of Patients/Total Patients | % |
| Treated < 90 days after high-dose alkylators* | ||||
| Phase I (05015) cohort | 0/17 | 0 | 2/13 | 15 |
| Phase II (03077) cohort | 2/146 | 1 | 18/141 | 13 |
| Treated ≥ 90 days after high-dose alkylators* | ||||
| Phase I (05015) cohort | 6/13 | 46 | 2/6 | 33 |
| Phase II (03077) cohort | 28/70 | 40 | 18/42 | 43 |
NOTE. Phase II data are from same time period as the phase I trial.
Cyclophosphamide 4,200 mg/m2 (or 140 mg/kg) in a cycle or myeloablative doses of melphalan or thiotepa.
Disease Response
Anti-NB activity occurred at all dosages and mainly in patients treated for refractory disease rather than PD (Table 6). Five patients (all treated for primary or secondary refractory NB) had major responses; two partial responses and one CR involved CR in BM plus complete or near complete normalization of widely abnormal MIBG scans, and two CRs involved much less extensive disease (disappearance of supraclavicular node and normalization of urine catecholamine levels, respectively). Five patients had mixed responses, including four patients treated for refractory NB and one patient treated for PD; all five patients had improved MIBG findings, and two patients also had CR or partial response (decrease from five of eight to one of eight specimens involved) in BM.
Table 6.
Response to Antibody Treatment
| No. of Patients |
|||||
|---|---|---|---|---|---|
| Disease Status at Enrollment | Complete Response | Partial Response | Mixed Response | No Response | Progressive Disease |
| Primary refractory disease | 2 | 2 | 2 | 9* | 1 |
| Secondary or later refractory disease | 1 | 0 | 2 | 3† | 2 |
| Progressive disease | 0 | 0 | 1 | 0 | 5 |
Five patients had objective responses.
One patient had an objective response.
Eleven patients remain progression free 18+ to 60+ months (median, 39+ months) from enrollment: four of these patients proceeded to receive treatment on the MSKCC 03077 protocol, which used standard-dose 3F8/GM-CSF plus 13-cis-retinoic acid (CRA); three patients received CRA alone; two patients received CRA plus investigational agents; one patient received irinotecan/temozolomide; and one patient was treated with iodine-131 –MIBG. Eleven other patients developed PD at 2 to 50 months (median, 10 months) from enrollment, of whom six patients are alive at 21+ to 59+ months (median, 49+ months). All eight patients who experienced PD on this treatment died of NB at 3 to 20 months (median, 6 months).
DISCUSSION
This phase I study established that 3F8 dosing can be significantly escalated with acceptable toxicity; acute adverse effects resembled those in prior and concurrent phase II studies,5,6,9 and no delayed adverse effects were encountered. These observations eased concern that HM3F8 and/or high doses of 3F8 might have unexpected toxicities or that the acute neuropathic symptoms during 3F8 treatment might herald long-term nerve damage. Anti-NB activity occurred at all dosages. The findings support the hypothesis that anti-GD2 MoAbs devoid of ADCC and CMC functions, such as HM3F8, can modify pain adverse effects, without blunting anti-NB activity. A randomized trial would be needed to confirm this hypothesis.
The number of cycles was limited to two because that amount was deemed sufficient to answer the phase I study question regarding MTD. Prior experience with more than 200 patients showed that toxicities of 3F8 did not worsen with multiple cycles. Rather, pain and analgesic use were greatest on day 1 of a patient's initial cycle and diminished thereafter. This welcome finding was attributable in part to less anxiety of patients and parents as they became familiar with the symptoms.
A reluctance to dose escalate 3F8 was a result of pain-related hypertension in the initial phase I trial.1 However, a majority of patients were adults (most with melanoma, with age up to 56 years), an age group later identified as being especially susceptible to toxicity from anti-GD2 MoAbs.22,23 With subsequent experience, adverse effects proved more readily manageable. Treatment was safely transitioned to the outpatient setting. The 3F8 infusion was reduced to 30 minutes, without exacerbating toxicity. The shorter infusion time facilitated matters for patients and staff. The number of outpatients treated daily increased to 12. In the current study, despite the interference with general well-being for up to several hours per day of each cycle, no family stopped therapy for their child, and all five adolescents completed the treatment plan.
The goal of ever better control of adverse effects plus the promise of increased anti-NB activity with higher dosing converged when HM3F8 was found to have properties that might promote 3F8 dose escalation. Thus, we learned that HM3F8 did not cause pain; all six patients treated in a single day had no adverse effects when 3F8 was mistakenly thawed in hot water. This clinical mishap (never repeated) prompted laboratory investigations. In vitro, HM3F8 retained affinity to GD2 but lost the ADCC and CMC functions of unmodified 3F8 (see Patients and Methods). In animal studies using xenografts of human NB and prior administration of HM3F8, the latter did not affect the biodistribution of iodine-131–3F8 in mice (Appendix Fig A1, online only) or reduce the anti-NB activity of unmodified 3F8 in rats (Appendix Fig A2, online only). Rats pretreated with HM3F8 had less discomfort with a subsequent injection of native 3F8. (Mice remain asymptomatic after 3F8 injection irrespective of dose and are not a good model for pain adverse effects.)
All of the previously mentioned findings—the absence of pain in the clinical mishap, the retention by HM3F8 of GD2 binding despite obliteration of effector functions, and the excellent post-HM3F8 biodistribution and anti-NB activity of native 3F8 in animal models—were considered in the following context: the sharp contrast, in patients treated with 3F8, between the immediacy of pain and the much more delayed timing of tumor uptake (peaks at 24 hours).24 We hypothesized that HM3F8 could be used to block the pain fibers that quickly (within minutes) captured the first wave of MoAb as it was infused and thereby reduce pain adverse effects from a large treatment dose of 3F8 administered subsequently.
On the basis of the preclinical studies showing undiminished targeting of native 3F8 after HM3F8, we reasoned that a ratio of ≤ 1:10 (2 mg of HM3F8 v ≥ 20 mg of native 3F8) would have minimal negative effect on 3F8 localization to NB in patients and, therefore, would not reduce antitumor activity. Response is not usually a major aspect of a phase I study; for example, one notable phase I study of another anti-GD2 MoAb did not encompass antitumor responses.12 In our study, however, antitumor activity was of interest, given the possibility that HM3F8 might mask GD2 on NB and thereby reduce the anti-NB effects of unmodified 3F8. Disease regressions were, in fact, seen in this poor-prognosis patient population, although not in patients enrolled with PD (Table 6; similar to prior experience9). Nevertheless, it remains possible that HM3F8 masks GD2 on tumor. Furthermore, one must always consider that responses are delayed effects of prior therapies.
The protocol treatment had no evident impact on the development of HAMA, allaying concern that HM3F8 and/or high doses of 3F8 might increase the risk of HAMA. Thus, similar to the experience with standard-dose 3F8 and no HM3F8,21 HAMA after one cycle of HM3F8 plus high-dose 3F8 did not emerge when patients were less than 90 days from treatment with high doses of immunosuppressive alkylating agents (Table 5). Treatment before starting 3F8 was not standardized in the protocol because extensive prior multimodality therapy precludes safe use of strongly myelosuppressive regimens (eg, with high-dose cyclophosphamide) in many patients with resistant NB.
The critical message from this phase I study is the safe multifold escalation of 3F8 dosage in the outpatient setting. Although possibly attributable in part to pain-modifying effects of HM3F8, this clinical scenario is related to the improved management and alleviation of 3F8 adverse effects that emerged in the 1990s. The results of this phase I trial are serving as the basis for a new treatment program centered on HM3F8 and high dosing of 3F8.
Acknowledgment
We thank Irene Cheung, Yi Feng, and Xiao-Dong Huang for their assistance with human antimouse antibody and serum 3F8 assays; the clinical research nurses Catherine Enero, Lea Gregorio, and Jessica Merrill for administering the 3F8 infusions.
Appendix
Fig A1.
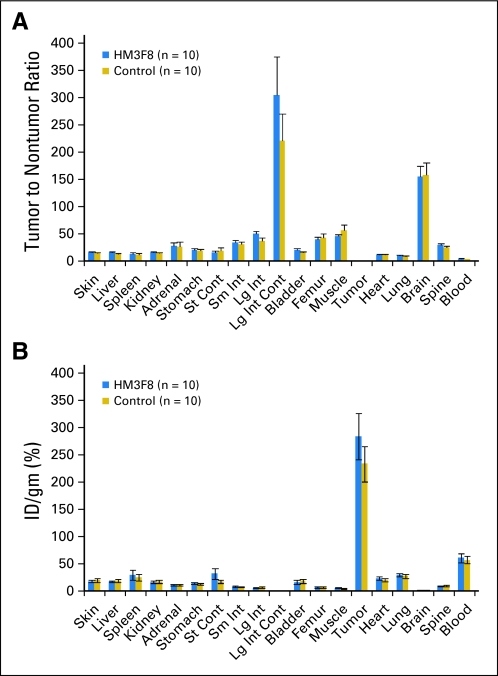
Results in mice xenografted with human neuroblastoma. The biodistribution of 25 mg/m2 (0.8 mg/kg) of 131I-3F8 was not altered by prior administration of 30 mg/m2 (0.9 mg/kg) of heat-modified 3F8 (HM3F8; a ratio of HM3F8:3F8 of approximately 1:1), as reflected in the (A) tumor-to-nontumor ratio and (B) percent injected dose (ID) per gram of tumor. st, stomach; cont, contents; sm int, small intestine; lg int, large intestine.
Fig A2.
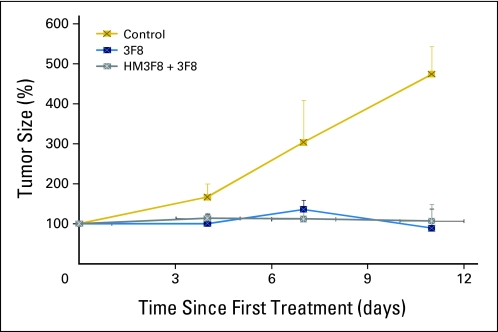
Results in rats xenografted with human neuroblastoma. Treatment with 3F8 (160 mg/m2, or 4.8 mg/kg) produced tumor shrinkage with or without prior administration of heat-modified 3F8 (HM3F8; 40 mg/m2, or 1.2 mg/kg; a heated-to-native ratio of 1:4).
Table A1.
Stability of HM3F8 in GD2-Binding and Effector Functions
| Antibody 3F8 | Binding Activity by ELISA (U/mg) | PBMC-ADCC (EC50) | PMN-ADCC (EC50) | CMC(EC50) | CD16-ADCC (EC50) | CD32-ADCC (EC50) | CMC(EC50) | % Purity(SDS gel) |
|---|---|---|---|---|---|---|---|---|
| Year 2003 | ||||||||
| No. of experiments | 8 | 2 | 2 | 3 | ||||
| PHB781, % | 100.00 | 100.00 | 100.00 | 100.00 | ||||
| PHB781-HM, % | 70.2 + 10.2 | 1.40 | 0 | 5.30 | ||||
| Year 2010 | ||||||||
| No. of experiments | 2 | 2 | 2 | 2 | 1 | |||
| PHB781, % | 100.00 | 100.00 | 100.00 | 100.00 | 96 | |||
| PHB781-HM, % | 65.90 | 0.20 | 1.60 | 0.20 | 97 |
NOTE. HM3F8 was prepared from the PHB781 lot of 3F8.
Abbreviations: HM, heat modified; ELISA, enzyme-linked immunosorbent assay; PBMC, peripheral-blood mononuclear cells; ADCC, antibody-dependent cellular cytotoxicity; EC50, half-maximal effective concentration; PMN, polymorphonuclear cells; CMC, complement-mediated cytotoxicity; CD16-ADCC, ADCC mediated by a standardized cloned NK92 cell line transfected with the human CD16 Fc receptor (NK92 effector cells were chosen to ensure reproducibility from day to day and over the years); CD32-ADCC, ADCC mediated by a standardized cloned NK92 cell line transfected with the human CD32 Fc receptor; SDS, sodium dodecyl sulfate.
Table A2.
GD2 Binding and Effector Functions of Current HM3F8
| Antibody 3F8 | Binding Activity by ELISA (U/mg) | CD16-ADCC (EC50) | CD32-ADCC (EC50) | CMC(EC50) | % Purity(SDS gel) |
|---|---|---|---|---|---|
| No. of experiments | 2 | 2 | 2 | 2 | 1 |
| PHB1500, % | 100.00 | 100.00 | 100.00 | 100.00 | 96 |
| PHB1500-HM 1, % | 62.40 | 0.20 | 2.70 | 1.50 | 96 |
| PHB1500-HM 2, % | 75.80 | 0.10 | 4.30 | 1.50 | 97 |
| PHB1500-HM 3, % | 79.60 | 0.30 | 2.00 | 1.70 | 96 |
| Average, % | |||||
| Mean | 71.2 | 0.2 | 3 | 1.6 | 96 |
| SD | 9.9 | 0.1 | 3.1 | 1.6 | 0.6 |
NOTE. HM3F8 was prepared from the PHB1500 lot of 3F8.
Abbreviations: HM, heat modified; ELISA, enzyme-linked immunosorbent assay; CD16-ADCC, antibody-dependent cellular cytotoxicity mediated by a standardized cloned NK92 cell line transfected with the human CD16 Fc receptor; EC50, half-maximal effective concentration; CD32-ADCC, antibody-dependent cellular cytotoxicity mediated by a standardized cloned NK92 cell line transfected with the human CD32 Fc-receptor; CMC, complement-mediated cytotoxicity; SDS, sodium dodecyl sulfate.
Footnotes
Supported in part by Grant No. CA10450 from the National Institutes of Health (Bethesda, MD) and grants from the Robert Steel Foundation (New York, NY) and Katie's Find a Cure Fund (New York, NY).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00450307.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: The antibody 3F8 was licensed by Memorial Sloan-Kettering Cancer Center to United Therapeutics in 2007, and Nai-Kong V. Cheung was named an inventor in the licensing agreement.
AUTHOR CONTRIBUTIONS
Conception and design: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Financial support: Nai-Kong V. Cheung
Administrative support: Nai-Kong V. Cheung
Provision of study materials or patients: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Collection and assembly of data: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Data analysis and interpretation: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Manuscript writing: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Final approval of manuscript: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
REFERENCES
- 1.Cheung NK, Lazarus H, Miraldi FD, et al. Ganglioside GD2 specific monoclonal antibody 3F8: A phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 2.Handgretinger R, Baader P, Dopfer R, et al. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14.G2a. Cancer Immunol Immunother. 1992;35:199–204. doi: 10.1007/BF01756188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handgretinger R, Anderson K, Lang P, et al. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur J Cancer. 1995;31A:261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 4.Yu AL, Uttenreuther-Fischer MM, Huang CS, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16:2169–2180. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 5.Cheung NK, Kushner BH, Yeh SD, et al. 3F8 monoclonal antibody treatment of patients with stage IV neuroblastoma: A phase II study. Int J Oncol. 1998;12:1299–1306. doi: 10.3892/ijo.12.6.1299. [DOI] [PubMed] [Google Scholar]
- 6.Cheung NK, Kushner BH, Cheung IY, et al. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16:3053–3060. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 7.Simon T, Hero B, Faldum A, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549–3557. doi: 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 8.Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: A Children's Cancer Group Study. J Clin Oncol. 2001;18:4077–4085. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 9.Kushner BH, Kramer K, Cheung NK. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 10.Frost JD, Hank JA, Reaman GH, et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: A report of the Children's Cancer Group. Cancer. 1997;80:317–333. doi: 10.1002/(sici)1097-0142(19970715)80:2<317::aid-cncr21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: A report from the Children's Oncology Group. J Clin Oncol. 2009;27:85–91. doi: 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorkin LS, Otto M, Baldwin WM, 3rd, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149:135–142. doi: 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munn DH, Cheung NK. Antibody-dependent antitumor cytotoxicity by human monocytes cultured with recombinant macrophage colony-stimulating factor: Induction of efficient antibody-mediated antitumor cytotoxicity not detected by isotope release assays. J Exp Med. 1989;170:511–526. doi: 10.1084/jem.170.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73:1936–1941. [PubMed] [Google Scholar]
- 16.Saarinen UM, Coccia PF, Gerson SL, et al. Eradication of neuroblastoma cells in vitro by monoclonal antibody and human complement: Method for purging autologous bone marrow. Cancer Res. 1985;45:5969–5975. [PubMed] [Google Scholar]
- 17.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 18.Yeh SD, Larson SM, Burch L, et al. Radioimmunodetection of neuroblastoma with iodine-131-3F8: Correlation with biopsy, iodine-131-metaiodobenzylguanidine and standard diagnostic modalities. J Nucl Med. 1991;32:769–776. [PubMed] [Google Scholar]
- 19.Cheung NK, Saarinen UM, Neely JE, et al. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45:2642–2649. [PubMed] [Google Scholar]
- 20.Cheung NK, Cheung IY, Canete A, et al. Antibody response to murine anti-GD2 monoclonal antibodies: Correlation with patient survival. Cancer Res. 1994;54:2228–2233. [PubMed] [Google Scholar]
- 21.Kushner BH, Cheung IY, Kramer K, et al. High-dose cyclophosphamide inhibition of humoral immune response to murine monoclonal antibody 3F8 in neuroblastoma patients: Broad implications for immunotherapy. Pediatr Blood Cancer. 2007;48:430–434. doi: 10.1002/pbc.20765. [DOI] [PubMed] [Google Scholar]
- 22.Saleh MN, Khazaeli MB, Wheeler RH, et al. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14.18 in patients with malignant melanoma. Hum Antibodies Hybridomas. 1992;3:19–24. [PubMed] [Google Scholar]
- 23.Murray JL, Cunningham JE, Brewer H, et al. Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. J Clin Oncol. 1994;12:184–193. doi: 10.1200/JCO.1994.12.1.184. [DOI] [PubMed] [Google Scholar]
- 24.Miraldi FD, Nelson AD, Kraly C, et al. Diagnostic imaging of human neuroblastoma with radiolabeled antibody. Radiology. 1986;161:413–418. doi: 10.1148/radiology.161.2.3763911. [DOI] [PubMed] [Google Scholar]