Abstract
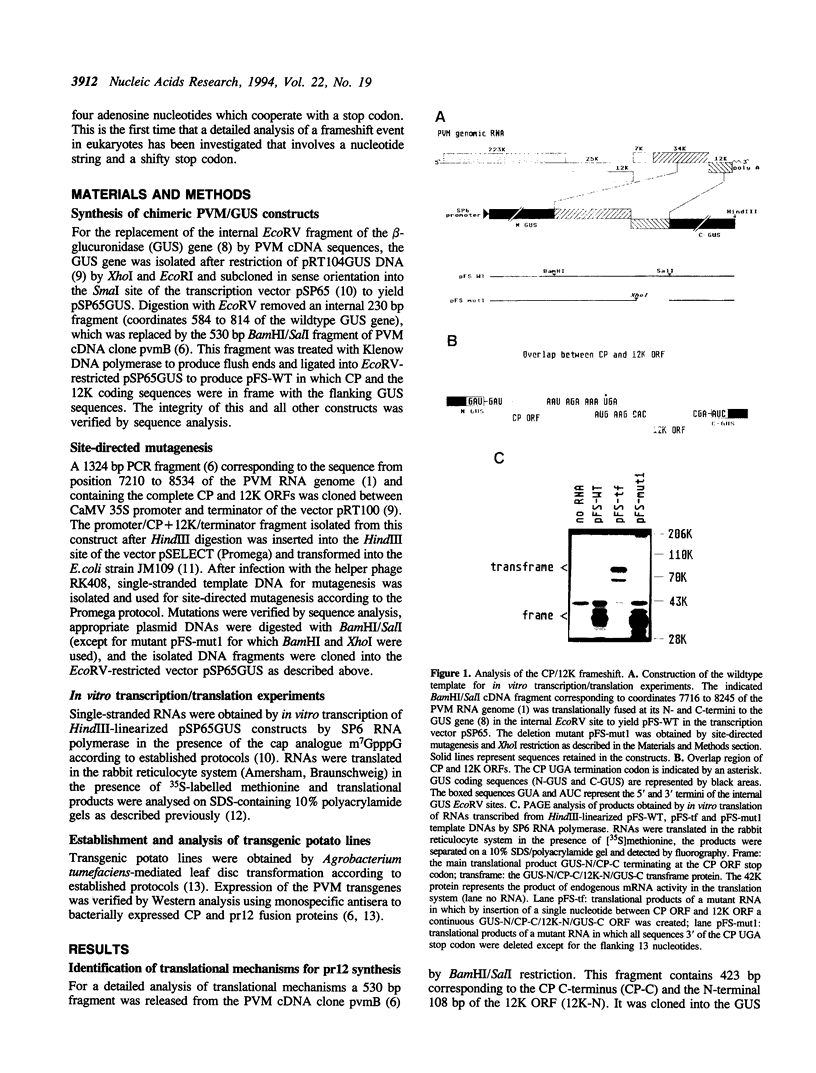
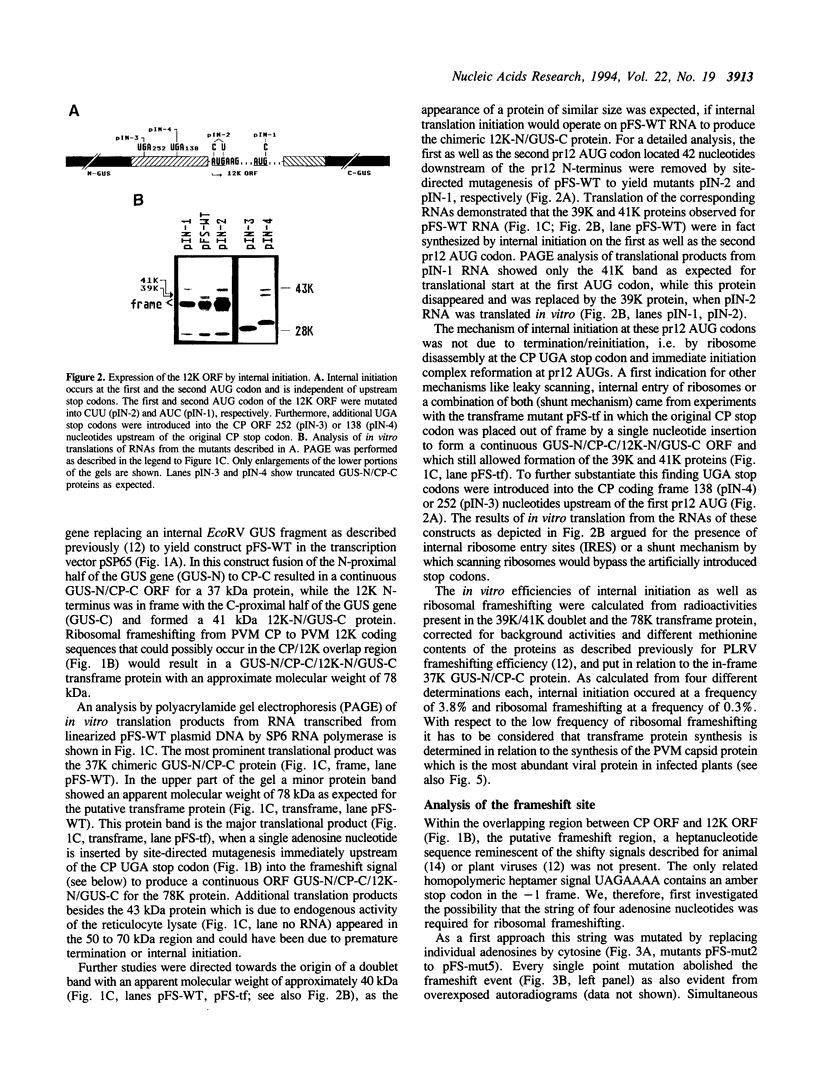
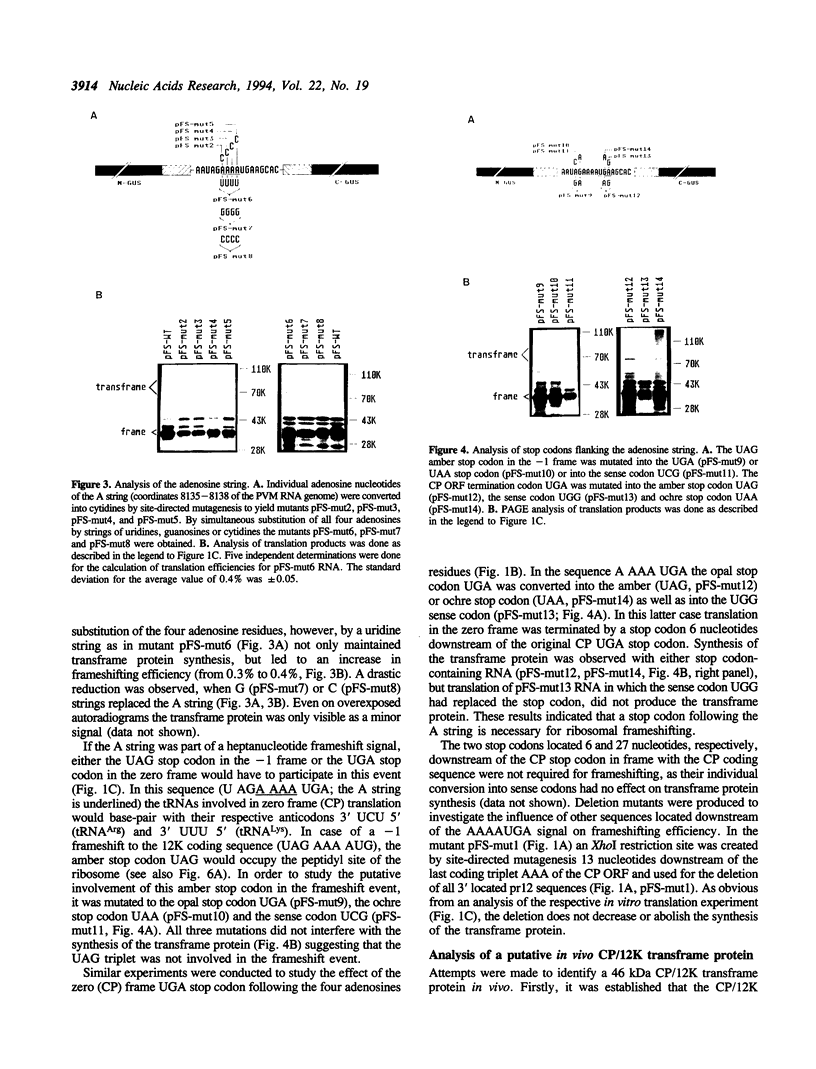
The genes for the capsid protein CP and the nucleic acid-binding 12K protein (pr12) of potato virus M (PVM) constitute the 3' terminal gene cluster of the PVM RNA genome. Both proteins are presumably translated from a single subgenomic RNA. We have identified two translational strategies operating in pr12 gene expression. Internal initiation at the first and the second AUG codon of the pr12 coding sequence results in the synthesis of the 12K protein. In addition the protein is produced as a CP/12K transframe protein by ribosomal frameshifting. For these studies parts of the CP and pr12 coding sequences including the putative frameshift region were introduced into an internal position of the beta-glucuronidase gene. Mutational analyses in conjunction with in vitro translation experiments identified a homopolymeric string of four adenosine nucleotides which together with a 3' flanking UGA stop codon were required for efficient frameshifting. The signal AAAAUGA is the first frameshift signal with a shifty stop codon to be analyzed in the eukaryotic system. Substitution of the four consecutive adenosine nucleotides by UUUU increased the efficiency of frameshifting, while substitution by GGGG or CCCC dramatically reduced the synthesis of the transframe protein. Also, UAA and UAG could replace the opal stop codon without effect on the frameshifting event, but mutation of UGA to the sense codon UGG inhibited transframe protein formation. These findings suggest that the mechanism of ribosomal frameshifting at the PVM signal is different from the one described by the 'simultaneous slippage' model in that only the string of four adenosine nucleotides represents the slippery sequence involved in a -1 P-site slippage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Weiss R. B., Gesteland R. F. Ribosome gymnastics--degree of difficulty 9.5, style 10.0. Cell. 1990 Aug 10;62(3):413–423. doi: 10.1016/0092-8674(90)90007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. L., Guilford P. J., Voot D. M., Andersen M. T., Forster R. L. Triple gene block proteins of white clover mosaic potexvirus are required for transport. Virology. 1991 Aug;183(2):695–702. doi: 10.1016/0042-6822(91)90998-q. [DOI] [PubMed] [Google Scholar]
- Bol J. F., van Vloten-Doting L., Jaspars E. M. A functional equivalence of top component a RNA and coat protein in the initiation of infection by alfalfa mosaic virus. Virology. 1971 Oct;46(1):73–85. doi: 10.1016/0042-6822(71)90007-9. [DOI] [PubMed] [Google Scholar]
- Brault V., Miller W. A. Translational frameshifting mediated by a viral sequence in plant cells. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2262–2266. doi: 10.1073/pnas.89.6.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I., Digard P., Inglis S. C. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 1989 May 19;57(4):537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster G. D., Mills P. R. Evidence for the role of subgenomic RNAs in the production of potato virus S coat protein during in vitro translation. J Gen Virol. 1990 May;71(Pt 5):1247–1249. doi: 10.1099/0022-1317-71-5-1247. [DOI] [PubMed] [Google Scholar]
- Gramstat A., Courtpozanis A., Rohde W. The 12 kDa protein of potato virus M displays properties of a nucleic acid-binding regulatory protein. FEBS Lett. 1990 Dec 10;276(1-2):34–38. doi: 10.1016/0014-5793(90)80500-i. [DOI] [PubMed] [Google Scholar]
- Hatfield D. L., Levin J. G., Rein A., Oroszlan S. Translational suppression in retroviral gene expression. Adv Virus Res. 1992;41:193–239. doi: 10.1016/S0065-3527(08)60037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D. L., Smith D. W., Lee B. J., Worland P. J., Oroszlan S. Structure and function of suppressor tRNAs in higher eukaryotes. Crit Rev Biochem Mol Biol. 1990;25(2):71–96. doi: 10.3109/10409239009090606. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Oroszlan S. The where, what and how of ribosomal frameshifting in retroviral protein synthesis. Trends Biochem Sci. 1990 May;15(5):186–190. doi: 10.1016/0968-0004(90)90159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Jacks T., Townsley K., Varmus H. E., Majors J. Two efficient ribosomal frameshifting events are required for synthesis of mouse mammary tumor virus gag-related polyproteins. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4298–4302. doi: 10.1073/pnas.84.12.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Lommel S. A. Identification and analysis of the site of -1 ribosomal frameshifting in red clover necrotic mosaic virus. Virology. 1994 May 1;200(2):574–582. doi: 10.1006/viro.1994.1220. [DOI] [PubMed] [Google Scholar]
- Kujawa A. B., Drugeon G., Hulanicka D., Haenni A. L. Structural requirements for efficient translational frameshifting in the synthesis of the putative viral RNA-dependent RNA polymerase of potato leafroll virus. Nucleic Acids Res. 1993 May 11;21(9):2165–2171. doi: 10.1093/nar/21.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov SYu, Dolja V. V., Atabekov J. G. Probable reassortment of genomic elements among elongated RNA-containing plant viruses. J Mol Evol. 1989 Jul;29(1):52–62. doi: 10.1007/BF02106181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty I. T., French R., Jones R. W., Jackson A. O. Identification of barley stripe mosaic virus genes involved in viral RNA replication and systemic movement. EMBO J. 1990 Nov;9(11):3453–3457. doi: 10.1002/j.1460-2075.1990.tb07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüfer D., Tacke E., Schmitz J., Kull B., Kaufmann A., Rohde W. Ribosomal frameshifting in plants: a novel signal directs the -1 frameshift in the synthesis of the putative viral replicase of potato leafroll luteovirus. EMBO J. 1992 Mar;11(3):1111–1117. doi: 10.1002/j.1460-2075.1992.tb05151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randles J. W., Rohde W. Nicotiana velutina mosaic virus: evidence for a bipartite genome comprising 3 kb and 8 kb RNAs. J Gen Virol. 1990 May;71(Pt 5):1019–1027. doi: 10.1099/0022-1317-71-5-1019. [DOI] [PubMed] [Google Scholar]
- Saigo K., Kugimiya W., Matsuo Y., Inouye S., Yoshioka K., Yuki S. Identification of the coding sequence for a reverse transcriptase-like enzyme in a transposable genetic element in Drosophila melanogaster. Nature. 1984 Dec 13;312(5995):659–661. doi: 10.1038/312659a0. [DOI] [PubMed] [Google Scholar]
- Sehnke P. C., Mason A. M., Hood S. J., Lister R. M., Johnson J. E. A "zinc-finger"-type binding domain in tobacco streak virus coat protein. Virology. 1989 Jan;168(1):48–56. doi: 10.1016/0042-6822(89)90402-9. [DOI] [PubMed] [Google Scholar]
- Tacke E., Schmitz J., Prüfer D., Rohde W. Mutational analysis of the nucleic acid-binding 17 kDa phosphoprotein of potato leafroll luteovirus identifies an amphipathic alpha-helix as the domain for protein/protein interactions. Virology. 1993 Nov;197(1):274–282. doi: 10.1006/viro.1993.1588. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi Z., Brown P. O. Sequence requirements for efficient translational frameshifting in the Escherichia coli dnaX gene and the role of an unstable interaction between tRNA(Lys) and an AAG lysine codon. Genes Dev. 1992 Mar;6(3):511–519. doi: 10.1101/gad.6.3.511. [DOI] [PubMed] [Google Scholar]
- Valle R. P., Morch M. D. Stop making sense: or Regulation at the level of termination in eukaryotic protein synthesis. FEBS Lett. 1988 Aug 1;235(1-2):1–15. doi: 10.1016/0014-5793(88)81225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verver J., Le Gall O., van Kammen A., Wellink J. The sequence between nucleotides 161 and 512 of cowpea mosaic virus M RNA is able to support internal initiation of translation in vitro. J Gen Virol. 1991 Oct;72(Pt 10):2339–2345. doi: 10.1099/0022-1317-72-10-2339. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Atkins J. F., Gesteland R. F. Ribosomal frameshifting from -2 to +50 nucleotides. Prog Nucleic Acid Res Mol Biol. 1990;39:159–183. doi: 10.1016/s0079-6603(08)60626-1. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Shuh M., Atkins J. F., Gesteland R. F. E. coli ribosomes re-phase on retroviral frameshift signals at rates ranging from 2 to 50 percent. New Biol. 1989 Nov;1(2):159–169. [PubMed] [Google Scholar]
- Xiong Z., Kim K. H., Kendall T. L., Lommel S. A. Synthesis of the putative red clover necrotic mosaic virus RNA polymerase by ribosomal frameshifting in vitro. Virology. 1993 Mar;193(1):213–221. doi: 10.1006/viro.1993.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Lommel S. A. The complete nucleotide sequence and genome organization of red clover necrotic mosaic virus RNA-1. Virology. 1989 Aug;171(2):543–554. doi: 10.1016/0042-6822(89)90624-7. [DOI] [PubMed] [Google Scholar]
- Zavriev S. K., Kanyuka K. V., Levay K. E. The genome organization of potato virus M RNA. J Gen Virol. 1991 Jan;72(Pt 1):9–14. doi: 10.1099/0022-1317-72-1-9. [DOI] [PubMed] [Google Scholar]
- van Vloten-Doting L. Coat protein is required for infectivity of tobacco streak virus: biological equivalence of the coat proteins of tobacco streak and alfalfa mosaic viruses. Virology. 1975 May;65(1):215–225. doi: 10.1016/0042-6822(75)90022-7. [DOI] [PubMed] [Google Scholar]