Abstract
AIM: To evaluate the efficacy and safety of the addition of vildagliptin to low-dose metformin and compare it to an uptitration of metformin in type 2 diabetes mellitus (T2DM) patients who have inadequate control with metformin monotherapy.
METHODS: Eligible patients were randomized to receive vildagliptin 100 mg qd or metformin (500 mg qd for 2 wk and then 500 mg bid) added to open label metformin 500 mg bid for the 24 wk. The primary endpoint was baseline to endpoint hemoglobin A1c (HbA1c) change.
RESULTS: The adjusted mean change from baseline in HbA1c at the 24th wk was -0.51% in the vildagliptin/metformin group (mean baseline HbA1c: 7.4%) and -0.37% in the metformin monotherapy group (mean baseline HbA1c: 7.3%). The mean difference was -0.14% with 95% Confidence Interval (-0.24%, -0.05%). As non-inferiority (margin of 0.4%) was achieved, a test for superiority was performed. This test showed statistically significant superiority of the combination over monotherapy group (P = 0.002). Gastrointestinal (GI) adverse events were significantly more frequent in the metformin group than the combination group (21.0% vs 15.4%, P = 0.032).
CONCLUSION: In patients with T2DM inadequately controlled with metformin up to 1000 mg daily, the addition of vildagliptin 100 mg daily achieved larger HbA1c reduction with fewer GI events than with increasing the metformin dose.
Keywords: Vildagliptin, Metformin, Dipeptidyl peptidase-4, Hemoglobin A1c, Glucagon-like peptide-1, Gastrointestinal side effects
INTRODUCTION
Metformin is the gold standard first-line treatment for type 2 diabetes mellitus (T2DM)[1]. This recommendation is based on its therapeutic efficacy, a relatively low incidence of hypoglycemia and no weight gain. In addition, data from the UK Prospective Diabetes Study showed that metformin can improve cardiovascular outcomes in overweight patients with T2DM[2]. However, gastrointestinal (GI) symptoms such as nausea, diarrhea and abdominal pain are common and often lead to discontinuation. The incidence of GI disturbances has been reported to be dose-related and may remit if the dose is reduced[3].
Vildagliptin is a potent and selective dipeptidyl peptidase-4 (DPP-4) inhibitor that improves pancreatic islet function as evidenced from the improved ability of the α-cell and β-cell to sense and response to glucose following treatment[4]. In addition, vildagliptin inhibits hepatic glucose production during meals as well as during the overnight post-absorptive period[5]. Furthermore, vildagliptin has been shown to improve insulin resistance[6]. Targeting multiple defects using agents with synergistic or complementary mechanisms of action may be beneficial in achieving glycemic targets. Mechanistic studies have suggested that vildagliptin may be particularly effective when used in combination with metformin with a potential synergistic effect. The effect of vildagliptin to increase plasma levels of intact glucagon-like peptide-1 (GLP-1) was enhanced in patients receiving concomitant metformin while there was no evidence of an effect of metformin on plasma DPP-4 activity or on GLP-1 or GIP in the absence of vildagliptin[7]. When glucose levels are above normal fasting levels, enhanced GLP-1 levels stimulate insulin secretion and inhibit glucagon secretion[8,9]. In addition, enhanced GLP-1 levels may increase islet cell mass. In prior clinical studies, vildagliptin added to ongoing metformin monotherapy significantly improved fasting plasma glucose (FPG) and glycosylated hemoglobin (HbA1c). These effects were associated with an improvement in measures of β-cell function, no weight gain and no increase in the incidence of hypoglycemia[10,11].
The present study was designed to compare the glycemic efficacy and safety of the addition of vildagliptin (100 mg qd) to low-dose metformin (500 mg bid) with that of an upward titration of metformin in patients with T2DM with inadequate glycemic control on metformin monotherapy.
MATERIALS AND METHODS
Study population
Male and female (non-fertile or using a medically approved birth control method) patients aged 18-78 years with HbA1c 6.5%-9.0%, FPG < 270 mg/dL (15 mmol/L) and a body mass index (BMI) of 22-45 kg/m2 who received metformin 850-1000 mg daily for at least 2 mo prior to screening were eligible to participate in the study.
Patients were excluded if they had a history of type 1 or secondary forms of diabetes, evidence of significant diabetic complications, acute infections, myocardial infarction, unstable angina or coronary artery bypass surgery within the previous 6 mo. Congestive heart failure requiring pharmacological treatment, malignancy (not including basal cell skin cancer) and liver disease, such as cirrhosis or chronic active hepatitis, also precluded participation. Patients with electrocardiogram (ECG) abnormalities, such as Torsades de pointes, sustained and clinically relevant ventricular tachycardia or ventricular fibrillation, second-degree atrioventricular (AV) block (Mobitz 1 and 2), third-degree AV block, and prolonged QTc (> 500 ms) were also excluded. Patients with any of the following laboratory abnormalities were also excluded: Alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) greater than two times the upper limit of the normal range at visit 1, confirmed by repeat measurement within 3 working days; total bilirubin greater than two times the upper limit of the normal range and/or direct bilirubin greater than the upper limit of the normal range at visit 1, confirmed by repeat measurement within 3 working days; clinically significant renal dysfunction as indicated by serum creatinine levels ≥ 1.5 mg/dL (132 μmol/L) in males, ≥ 1.4 mg/dL (123 μmol/L) in females, or a history of abnormal creatinine clearance; clinically significant TSH values outside normal range at visit 1; fasting triglycerides > 700 mg/dL (7.9 mmol/L) at visit 1. Patients were also excluded if they were taking any of the following medications/treatments: chronic insulin treatment (> 4 wk of treatment in the absence of an intercurrent illness) within the past 6 mo and/or any oral antidiabetic drug other than metformin within 3 mo prior to visit 1; chronic oral or parenteral corticosteroid treatment (> 7 consecutive days of treatment) within 8 wk prior to visit 1; treatment with growth hormone or similar drugs; treatment with class Ia, Ib and Ic or III antiarrhythmics; treatment with any drug with a known and frequent toxicity to a major organ system within the past 3 mo (i.e. cytostatic drugs). Finally, contraindications and warnings according to the country-specific label for metformin, history of active substance abuse (including alcohol) within the past 2 years and participation in previous vildagliptin studies also precluded participation.
A patient’s treatment was discontinued if one or more of the following pertained: unsatisfactory therapeutic effect [defined as FPG > 240 mg/dL (13.3 mmol/L) after 12 wk of treatment confirmed by a repeated measurement in the absence of an intercurrent illness]; symptoms of worsening hyperglycemia in the absence of any intercurrent illness or other incidental circumstances potentially causing deterioration of glucose control; the occurrence of an adverse event (AE) including GI side effects or clinically significant laboratory change or an abnormality that, in the judgment of the investigator, warranted discontinuation of the treatment; pregnancy; severe or frequent hypoglycemia (i.e. unexplained hypoglycemic events requiring the assistance of another person to treat or > 3 hypoglycemic events per week); treatment with prohibited concomitant medications.
All patients provided written informed consent to participate and the study protocol was reviewed and approved by the appropriate committees and authorities for each study site. The study was performed in accordance with the Declaration of Helsinki.
Study design
The overall study design of this 24-wk, randomized, double-blind trial is presented in Figure 1. Each patient attended a screening visit (Week -4) to assess the inclusion/exclusion criteria. All patients received open-label metformin 500 mg bid at visit 1 for a period of 4 wk. Eligible patients were then randomized to receive either vildagliptin 100 mg qd or metformin 500 mg qd (double-dummy design) for 2 wk and then metformin 500 mg bid. All patients continued with the open-label metformin 500 mg bid for the 24 wk. Dose adjustments of vildagliptin or open-label metformin were not allowed at any time after randomization.
Figure 1.
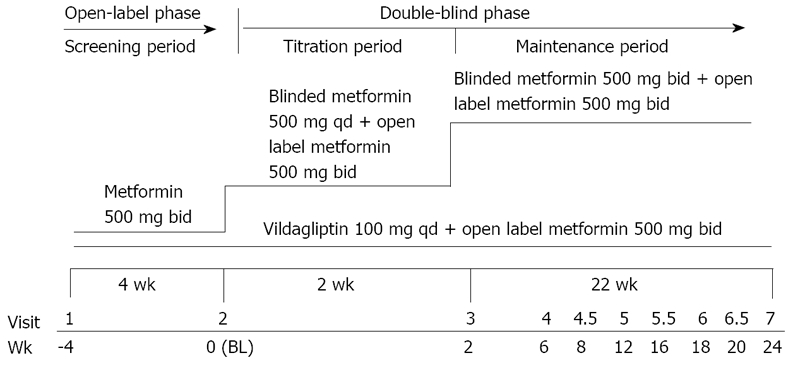
Study design.
If the patients were unable to tolerate the study drug due to GI symptoms following the uptitration, metformin could be reduced by one tablet only until the GI symptoms had improved. The dose had to be then restored gradually over 1-2 wk based on the patient's ability to tolerate the study drug. No rescue medication (additional oral antidiabetic drugs or insulin to control glycemia) was permitted in this study; patients with unsatisfactory therapeutic effect were discontinued from the study. Patients who were prematurely withdrawn from the study were not replaced. Efficacy and tolerability were assessed in eight visits over the 24 wk.
Study assessments
The primary efficacy assessment was change in HbA1c from baseline. Secondary assessments included FPG, body weight and GI tolerability.
HbA1c, FPG, body weight and vital signs were assessed at screening, baseline and Week 2, 6, 12, 18 and 24. Liver function (AST, ALT, direct and total bilirubin and alkaline phosphatase) was monitored by taking blood samples at Week 2, 6, 8, 16 and 20. Standard hematology and biochemistry laboratory assessments were made at screening, baseline and Week 12 and 24. Fasting lipid levels [triglyceride, total/calculated low-density lipoprotein, high-density lipoprotein (HDL), non-HDL cholesterol and calculated very-low-density lipoprotein cholesterol] were measured at baseline and at the end of the study. ECG was performed at screening and at Week 0 and 24. All AEs were recorded. Patients were provided with glucose-monitoring devices and supplies and instructed on their use. Patients were educated on hypoglycemic symptoms and their treatment.
All laboratory assessments were made by a central laboratory (Covance-US, Indianapolis, IN, USA). HbA1c was measured with an ion exchange high-performance liquid chromatography method and all assays were performed with standardized and validated procedures according to Good Laboratory Practice.
An independent Cardiovascular and Cerebrovascular Adjudication Committee reviewed all occurrences of selected cardiovascular AEs, while an independent Internal Medicine Adjudication Committee reviewed occurrence of selected GI disorders (GI hemorrhage), general system disorders (generalized edema/anasarca), renal failure, skin and subcutaneous tissue disorders (angio-edema, generalized urticaria), and deaths (non-cardiovascular or cerebrovascular cause).
Statistical analyses
The primary efficacy analysis assessed whether (margin of 0.4%) the study treatments were non-inferior with regard to the HbA1c at Week 24 or at the final visit (for patients who did not have HbA1c measurement at Week 24, the last observation carried forward approach was adopted). An analysis of covariance (ANCOVA) model was fitted including terms for treatment and baseline HbA1c as the covariate. When non-inferiority was achieved, a test for superiority was performed. The analysis of the primary efficacy variable using the intention to treat (ITT) population (received at least one dose of each study drug and had at least one post-baseline HbA1c assessment) was the primary basis of conclusion. An analysis based on the per protocol (PP) population was also performed to assess the robustness of the conclusion. The per protocol population included ITT patients who completed at least 22 wk of treatment and those who discontinued the study due to unsatisfactory therapeutic effect (FPG > 240 mg/dL) after 12 wk of treatment, provided they had no major protocol deviations and had a valid assessment of HbA1c within 7 d after the last dose of study drug. The change from baseline in FPG and body weight at the end of the study was analyzed using the ANCOVA model at the statistical significance level of 0.05.
The assessment of safety was based mainly on the frequency of treatment-emergent AEs, on the number of post-baseline laboratory values that fell outside pre-determined ranges and on the frequency and severity of hypoglycemic events. The incidence of patients with at least a single GI event was compared between treatment groups using a χ2-test.
RESULTS
A total of 914 patients (Figure 2) were randomized to receive either vildagliptin 100 mg qd/metformin 500 mg bid (n = 456) or metformin monotherapy (n = 458 patients) up to 1000 mg bid (final mean metformin dose after uptitration at visit 4 was 1984 mg). Of these patients, 798 (87.3%) completed the study. Discontinuation was higher in patients from the metformin group (14.4%) than in the vildagliptin/metformin group (11.0%). The most frequent reason for discontinuation was withdrawal of consent which was more frequent in the metformin than in the vildagliptin/metformin group (5.5% vs 3.7%).
Figure 2.
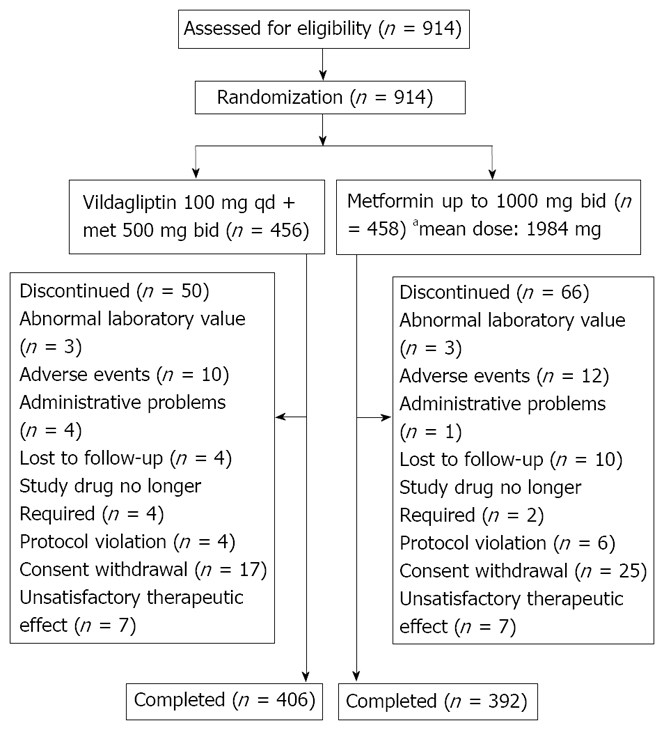
Patient flow diagram. aDose after titration at Visit 4 (Week 6).
The patient baseline demographics were comparable between the treatment groups (Table 1). There was no imbalance observed in any concomitant therapies across both groups. The primary efficacy endpoint was the change from baseline to endpoint in HbA1c in the ITT population. The adjusted mean change from baseline in HbA1c at 24 wk was -0.51% in the vildagliptin/metformin group (mean baseline HbA1c: 7.4%), and -0.37% in the metformin monotherapy group (mean baseline HbA1c: 7.3%). The mean difference was -0.14% with 95% Confidence Interval (CI) (-0.24%, -0.05%). As non-inferiority (margin of 0.4%) was achieved, a test for superiority was performed. This test showed statistically significant superiority of the combination over monotherapy group (P = 0.002). Consistent results were observed in the PP population.
Table 1.
Demographic and baseline characteristics (randomized population)
| Demographic variable | Vilda 100 mg qd/metformin 500 mg bid N = 456 | Metformin up to 1000 mg bid N = 458 | Total N = 914 |
| Gender; female, n (%) | 226 (49.6) | 252 (55) | 478 (52.3) |
| Age; mean ± SD (years) | 56.9 ± 9.76 | 57.0 ± 10.02 | 57.0 ± 9.89 |
| Age group (≥ 65 years), n (%) | 106 (23.2%) | 114 (24.9%) | 220 (24.1%) |
| Race | |||
| Caucasian | 242 (53.1%) | 237 (51.7%) | 479 (52.4%) |
| Asian (non-indian subcontinent) | 44 (9.6%) | 44 (9.6%) | 88 (9.6%) |
| Hispanic or latino | 147 (32.2%) | 145 (31.7%) | 292 (31.9%) |
| Black | 9 (2.0%) | 12 (2.6%) | 21 (2.3%) |
| Asian (indian subcontinent) | 3 (0.7%) | 5 (1.1%) | 8 (0.9%) |
| Native american | 1 (0.2%) | 2 (0.4%) | 3 (0.3%) |
| Other | 10 (2.2%) | 13 (2.8%) | 23 (2.5%) |
| Body weight; mean ± SD (kg) | 84.6 ± 17.01 | 84.4 ± 18.94 | 84.5 ± 17.99 |
| BMI (kg/m2); mean ± SD | 31.1 ± 5.11 | 31.2 ± 5.47 | 31.1 ± 5.29 |
| HbA1c (%); mean ± SD | 7.4 ± 0.78 | 7.3 ± 0.79a | 7.3 ± 0.79 |
| FPG (mmol/L); mean ± SD | 8.7 ± 2.28 | 8.5 ± 2.25 | 8.6 ± 2.27 |
| Duration of type 2 diabetes; mean ± SD (years) | 4.6 ± 4.91 | 4.7 ± 4.94 | 4.7 ± 4.92 |
Demographic information is collected on the day of the screening measurement (Week-4, Visit 1).
Hemoglobin A1c (HbA1c) measurements at Visit 1 not available for one patient (n = 457). FPG: Fasting plasma glucose; BMI: Body mass index.
Mean changes in HbA1c (%) over time are depicted in Figure 3. Starting with similar baseline values, HbA1c in the vildagliptin/metformin group was consistently lower than in the metformin group at all study visits. For all baseline HbA1c pre-defined categories, changes were larger in the vildagliptin/metformin than in the monotherapy group. In both treatment groups, patients with a higher baseline value (HbA1c > 8%) showed the greatest reduction in HbA1c from baseline at endpoint. In patients with HbA1c > 8% at baseline, the mean reductions in HbA1c from baseline to endpoint were -0.65% ± 0.13% and -0.51 ± 0.12 in the combination and monotherapy groups and -0.46% ± 0.03% and -0.31 ± 0.03 respectively in patients with HbA1c ≤ 8%. In both treatment groups, patients with a BMI < 30 kg/m2 at baseline showed a greater reduction (vildagliptin/metformin -0.51% ± 0.05%; metformin monotherapy -0.41% ± 0.05%) in HbA1c compared to the changes in the more obese patients (vildagliptin/metformin -0.49 ± 0.05; metformin monotherapy -0.29 ± 0.05). HbA1c changes were similar in older patients (≥ 65 years) and patients < 65 years of age.
Figure 3.
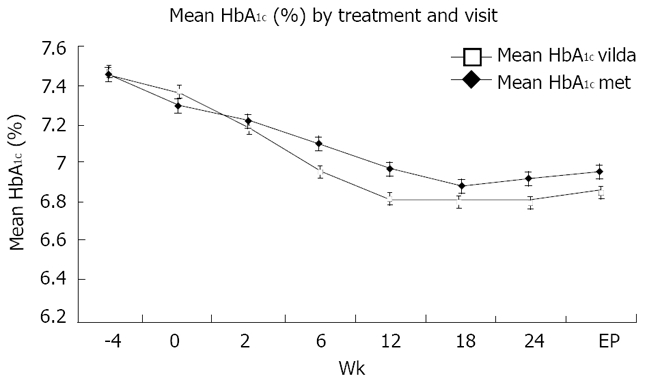
Mean HbA1c (%) by treatment and visit. Data are presented as mean ± SEM. vilda: vildagliptin/metformin; met: metformin.
The proportion of patients reaching HbA1c < 7% was 49.5% in the vildagliptin/metformin group versus 43.5% in the metformin monotherapy group but the difference did not reach statistical significance (P = 0.154). The proportion of patients achieving HbA1c ≤ 6.5% was significantly higher in the combination group compared with the metformin monotherapy group (53.8% vs 41.2%).
The adjusted mean change from baseline in FPG was numerically higher in the vildagliptin/metformin than in the metformin group [-0.77 mmol/L and -0.59 mmol/L respectively; 95% CI for difference in the mean change between treatments (-0.38, 0.02), P = 0.070]. A reduction from baseline in body weight was observed at endpoint in both treatment groups. The change was significantly higher in the metformin group than in the combination group (-1.35 kg vs -0.62 kg, P < 0.001).
Safety and tolerability
The overall AE rate was comparable in patients receiving vildagliptin/metformin and metformin (48.2% and 51.7% respectively). The most frequently reported AEs (with an incidence of ≥ 2%) in both groups (Table 2) were diarrhea and headache. The incidence rates of AEs by primary system organ class were generally comparable between the two treatment groups except for GI AEs which were significantly more frequent in the metformin monotherapy treatment group than in the vildagliptin group (21.0% vs 15.4%, P = 0.032).
Table 2.
Number (%) of patients reporting common AEs (≥ 2% in any group) and biochemistry abnormalities (safety population)
| Preferred term | Vilda 100 mg qd/metformin 500 mg bid N = 456 n (%) | Metformin up to 1000 mg bid N = 458 n (%) |
| Any preferred term | 220 (48.2) | 237 (51.7) |
| Diarrhea | 21 (4.6) | 39 (8.5) |
| Headache | 18 (3.9) | 28 (6.1) |
| Nasopharyngitis | 15 (3.3) | 13 (2.8) |
| Back pain | 14 (3.1) | 18 (3.9) |
| Dizziness | 14 (3.1) | 16 (3.5) |
| Flatulence | 13 (2.9) | 8 (1.7) |
| Upper respiratory tract infection | 13 (2.9) | 10 (2.2) |
| Arthralgia | 11 (2.4) | 6 (1.3) |
| Hypertension | 11 (2.4) | 12 (2.6) |
| Nausea | 11 (2.4) | 22 (4.8) |
| Pharyngitis | 10 (2.2) | 12 (2.6) |
| Pain in extremity | 8 (1.8) | 10 (2.2) |
| Urinary tract infection | 8 (1.8) | 24 (5.2) |
| Dyspepsia | 7 (1.5) | 11 (2.4) |
| Influenza | 7 (1.5) | 11 (2.4) |
| Abdominal pain | 5 (1.1) | 12 (2.6) |
| Laboratory evaluation | ||
| Any notable abnormalitya | 14 (3.2) | 14 (3.2) |
| Alkaline phosphatase ≥ 3 × ULN | 0 (0.0) | 0 (0.0) |
| ALT (Alanine aminotransferase) ≥ 3 × ULN | 1 (0.2) | 1 (0.2) |
| AST (aspartate aminotransferase) ≥ 3 × ULN | 1 (0.2) | 2 (0.5) |
| Bilirubin (direct/conjugated) ≥ 3 × ULN | 0 (0.0) | 0 (0.0) |
| Blood Urea Nitrogen (BUN) ≥ 9.99 mmol/L | 9 (2.0) | 6 (1.4)b |
| Creatine phosphokinase (CPK) ≥ 5 × ULN | 1 (0.2) | 0 (0.0)b |
| Creatinine ≥ 176.8 μmol/L | 0 (0.0) | 0 (0.0)b |
| Potassium ≤ 3.0 or ≥ 6.0 mmol/Ll | 3 (0.7)c | 5 (1.1)b |
| Sodium ≤ 125 or ≥ 160 mmol/L | 1 (0.2) | 0 (0.0)b |
No. of patients with evaluable criterion in both categories is 441.
No. of patients with evaluable criterion is 437.
No. of patients with evaluable criterion is 440. AEs: Adverse events; ULN: Upper limits of normal.
The incidence of serious adverse events and AEs leading to discontinuation or causing study drug interruption were low and comparable between the treatment groups. Serious abdominal pain (n = 2) and diarrhea leading to discontinuation (n = 2) were reported in patients in the metformin group whereas no such cases were reported in the combination group.
The proportion of patients with clinically significant AEs confirmed by the Cardiovascular and Cerebrovascular Adjudication Committee were similar between both the treatment groups (1.3% and 1.1% in the combination and the metformin monotherapy respectively). Clinically significant AEs confirmed by the Internal Medicine Adjudication Committee were infrequent and were reported in three patients in the combination group (two patients with GI hemorrhage and one patient with renal failure) and one patient in the metformin monotherapy group reported angio-edema.
The incidence of edema (0.4% vs 0.4%), hepatic disorders (0.4% vs 0.4%), muscle-event (3.2% vs 4.6%) and paresthesia- (1.1% vs 1.1%), skin- (0.4% vs 0.2%) and vascular-related AEs (0.0% vs 0.2%) were similar or lower in the combination group compared with the metformin monotherapy group. One patient in each treatment group experienced one hypoglycemic event (grade 1). Both events were of mild severity and did not lead to discontinuation.
No major changes from baseline to endpoint were observed for any hematological, biochemical (Table 2), urinalysis parameter or vital signs. The frequency and nature of ECG changes from baseline to endpoint were comparable in the two treatment groups.
DISCUSSION
Previous studies have shown that addition of vildagliptin to patients inadequately treated with maximum tolerated doses of metformin leads to reductions in HbA1c (~1%) relative to metformin alone[7] and to equivalent reductions in HbA1c (~1%) relative to metformin plus a TZD[12]. Both of these studies were from baseline HbA1c levels of ~8.5%. On the other hand, in a previous study, the combination of vildagliptin with low-dose metformin (1000 mg daily) achieved superior efficacy to high doses of metformin in patients with HbA1c levels of ~8.7% without any associated GI tolerability issues[13]. The evidence suggests, however, that earlier, more aggressive treatment is needed[14]. The present study was designed to compare the benefits of more aggressive treatment by either escalating the dose of metformin or by adding vildagliptin to a lower dose of metformin in patients with lower baseline HbA1c levels. The data showed that an addition of vildagliptin (100 mg daily) to a low-dose metformin (500mg bid) provided larger reductions in HbA1c of 0.51% from a baseline HbA1c of -7.3%-7.4% than reductions in HbA1c of 0.37% seen in patients where the metformin dose was increased to 2000 mg. Furthermore, a significantly higher proportion of patients achieved HbA1c ≤ 6.5% (53.8% vs 41.2%) with no increase in the hypoglycemic event rate in the combination group compared with the metformin monotherapy group.
The present study also showed that GI events (diarrhea, nausea and abdominal pain) were less frequent in patients receiving vildagliptin added to low doses of metformin than in the patients treated with metformin monotherapy 2000 mg daily. This is important since, in clinical practice, metformin is uptitrated up to 2-3 g daily with many patients having GI AEs, which may lead to low compliance and treatment discontinuation. A dose-related incidence of GI adverse effects with metformin has been reported by some[3] although not all studies[15]. It has been suggested that the use of gradual dose escalation might explain the lack of a linear dose relationship for GI AEs[15]. In any case, in the present study, even though metformin was increased gradually and the mean incidence of total GI disturbances was lower than the one reported in previous studies[15], the incidences of diarrhea and nausea were twice as high in the metformin monotherapy group compared with the vildagliptin/metformin group (8.5 vs 4.5% and 4.8 vs 2.4% respectively). Although the total numbers of patients who discontinued due to adverse events was low in this 24-wk study, serious abdominal pain and diarrhea led four patients to discontinuation in the metformin monotherapy group but only one patient from the combination treatment group reported discontinuation.
The HbA1c changes observed in this study with vildagliptin are consistent with previously reported data utilizing vildagliptin in patients with comparable glycemic control at baseline[16]. Furthermore, the results are consistent with a similarly designed study in which the efficacy and safety of adding rosiglitazone to low dose of metformin was compared to increasing the dose of metformin in patients with T2DM[17]. The rosiglitazone study with a comparable baseline glycemic control to the present vildagliptin study showed similar efficacy of the rosiglitazone/metformin combination group (mean change from baseline: -0.33%) compared with the uptitrated metformin monotherapy group (mean change from baseline: -0.13%). Similar to the present vildagliptin study, there were fewer episodes of diarrhea, abdominal pain or dyspepsia and the instances of withdrawal from the study due to GI disturbances were fewer in the combination group compared with the metformin group. In contrast, in this previous study, body weight increased by 1.79 kg after 24 wk of treatment in the metformin/rosiglitazone group relative to the high dose metformin group, whereas in the present study, a reduction from baseline in the body weight was observed at endpoint in both the treatment groups (-1.35 kg vs -0.62 kg, P < 0.001 in the metformin and metformin/vildagliptin group, respectively).
In summary, in patients with T2DM inadequately controlled with metformin up to 1000 mg daily, the addition of vildagliptin 100 mg daily achieved larger HbA1c reduction with fewer GI events. These data suggest that an early addition of vildagliptin to submaximal doses of metformin has the potential to offer benefits over uptitration of metformin with superior efficacy, lower incidence of GI AE, no increase in the hypoglycemic event rate and no weight gain.
CONFLICT OF INTEREST
Claudia Filozof and James Foley are employees of Novartis Pharmaceuticals Corporation and hold stocks in the company. Sherwyn Schwartz was involved in the conduct of the study as a clinical trial investigator. No additional known conflict of interest exists and no honoraria were offered or received for coauthor participation in the writing of this manuscript.
COMMENTS
Background
Metformin is an established gold standard and the first option for the treatment of type 2 diabetes. Previous clinical studies demonstrate that the addition of vildagliptin to metformin monotherapy in patients not reaching therapeutic targets HbA1c ≅ 8.5% with maximum tolerated doses of metformin significantly improved fasting plasma glucose and glycosylated hemoglobin by 1% with an efficacy similar to a TZD (with no weight gain). The evidence suggests, however, that earlier more aggressive treatment is needed. In clinical practice, metformin is uptitrated from 850 mg to 2-3 g or more before adding any second antidiabetic. High doses of metformin are associated with more Gastrointestinal (GI) events, potentially leading to lower compliance. The present study was designed to compare efficacy and safety of either adding vildagliptin to a low-dose of metformin (1g) or escalating the dose of metformin in patients with HbA1c levels < between 6.5%-9%.
Research frontiers
In the present study, early add-on of vildagliptin to metformin, over uptitration of metformin doses achieve larger HbA1c drops and lower incidence of GI adverse events with no increase in hypoglycemic events and no weight gain.
Innovations and breakthroughs
The evidence suggests that earlier more aggressive treatment with low risk of hypoglycemic events is needed to prevent long-term complications. This study showed that an early (HbA1c 6.5%-9%) addition of vildagliptin to a low-dose metformin provided larger reductions in HbA1c relative to increasing the metformin dose. Furthermore, a significantly higher proportion of patients achieved HbA1c ≤ 6.5% (53.8% vs 41.2%) with no increase in the hypoglycemic event rate in the combination group compared with the metformin monotherapy group.
Applications
Evidence suggests that patients with type 2 diabetes mellitus (T2DM) increasingly require multiple pharmacological combinations to reach treatment goals. In clinical practice, metformin is uptitrated up to maximum doses before adding a second antidiabetic. An early combination, using two oral anti-diabetic drugs with complementary mechanisms of action is an alternative approach that may provide better or more sustained glycemic control with better tolerability. Our approach to earlier aggressive treatment with vildagliptin add on to metformin in patients with T2DM could be of interest in patients like elderly populations (> 60 years of age), as well as patients susceptible to GI events.
Terminology
Glucagon-like peptide-1 (GLP-1) has been shown to increase insulin secretion and suppress glucagon release in a glucose-dependent manner. However, active circulating GLP-1 has a half-life of approximately 1 to 2 min and is rapidly degraded by dipeptidyl peptidase-4 (DPP-4) to an inactive state. Inhibition of DPP-4 with vildagliptin, a selective DPP-4 inhibitor, results in enhanced active GLP-1 levels in vivo.
Peer review
The manuscript was well prepared. The study was well designed and performed. The data were solid to support the hypothesis and conclusion.
Acknowledgments
Claudia Filozof and James E Foley wrote this paper on behalf of many individuals at Novartis who contributed to the design, implementation, analysis and reporting of the data including Michele Valentin, Laurence Colin, David Holmes, Thomas Thuren, Florence Buccheit, Juergen Degen and Wolfgang Kothny. Sherwyn Schwartz wrote this paper as the principal investigator on behalf of the study investigators. This study was funded by Novartis Pharmaceuticals Corporation.
Footnotes
Supported by Novartis Pharmaceuticals Corporation, NCT00396357
Peer reviewer: Lu Cai, PhD, Associate Professor, Department of Pediatrics, University of Louisville School of Medicine, 570 South Preston Street, Suite 304F, Louisville, KY 40202, United States
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Goodarzi MO, Bryer-Ash M. Metformin revisited: re-evaluation of its properties and role in the pharmacopoeia of modern antidiabetic agents. Diabetes Obes Metab. 2005;7:654–665. doi: 10.1111/j.1463-1326.2004.00448.x. [DOI] [PubMed] [Google Scholar]
- 2.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 3.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 4.He YL, Wang Y, Bullock JM, Deacon CF, Holst JJ, Dunning BE, Ligueros-Saylan M, Foley JE. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J Clin Pharmacol. 2007;47:633–641. doi: 10.1177/0091270006299137. [DOI] [PubMed] [Google Scholar]
- 5.Balas B, Baig MR, Watson C, Dunning BE, Ligueros-Saylan M, Wang Y, He YL, Darland C, Holst JJ, Deacon CF, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249–1255. doi: 10.1210/jc.2006-1882. [DOI] [PubMed] [Google Scholar]
- 6.Azuma K, Rádiková Z, Mancino J, Toledo FG, Thomas E, Kangani C, Dalla Man C, Cobelli C, Holst JJ, Deacon CF, et al. Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:459–464. doi: 10.1210/jc.2007-1369. [DOI] [PubMed] [Google Scholar]
- 7.Dunning BE, Ligueros-Saylan M, D’Alessio DA, Balas B, Kelley DE, Deacon CF. Differential effects of DPP-4 inhibition on incretin hormone levels in drug-naive and metformin-treated patients with type 2 diabetes. Diabetologia. 2006;49 Suppl 1:110–111. [Google Scholar]
- 8.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 9.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hüfner M, Schmiegel WH. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 10.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 11.Ahrén B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–2880. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
- 12.Bolli G, Dotta F, Rochotte E, Cohen SE. Efficacy and tolerability of vildagliptin vs. pioglitazone when added to metformin: a 24-week, randomized, double-blind study. Diabetes Obes Metab. 2008;10:82–90. doi: 10.1111/j.1463-1326.2007.00820.x. [DOI] [PubMed] [Google Scholar]
- 13.Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11:506–515. doi: 10.1111/j.1463-1326.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 15.Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med. 1997;103:491–497. doi: 10.1016/s0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 16.Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahrén B, Byiers S, Shao Q, Dejager S. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157–166. doi: 10.1111/j.1463-1326.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 17.Bailey CJ, Bagdonas A, Rubes J, McMorn SO, Donaldson J, Biswas N, Stewart MW. Rosiglitazone/metformin fixed-dose combination compared with uptitrated metformin alone in type 2 diabetes mellitus: a 24-week, multicenter, randomized, double-blind, parallel-group study. Clin Ther. 2005;27:1548–1561. doi: 10.1016/j.clinthera.2005.10.012. [DOI] [PubMed] [Google Scholar]


