Abstract
Chronic inflammatory diseases caused by obesity represent critical public health concerns worldwide. In these diseases such as metabolic syndrome, diabetes and atherosclerosis, adipose tissue acts as an endocrine organ that releases large quantities of inflammatory mediators into circulation. Besides classically recognized effectors on the development of obesity and resultant conditions, infection has attracted attention as an enhancer of chronic inflammatory diseases. Infectious diseases have long been associated with obesity, metabolic syndrome, diabetes and atherosclerosis. However, the infectious hypothesis for chronic inflammatory diseases has been challenged by inconclusive clinical trials. Nevertheless, the large body of evidence accumulated over decades on the association of infectious diseases with obesity, diabetes and cardiovascular disease should not be disregarded. Instead, re-formulation of hypotheses of the mechanisms by which microbes affect obesity-associated diseases may be required with an emphasis on the early events in the progression of such diseases and the multifactorial nature of pathogen-host interactions. This review focuses on pathogens that directly promote obesity and on pathogens that cause chronic infections and thereby enhance metabolic diseases in obese patients. A new perspective on the interaction between infections and obesity-related diseases may improve management of chronic inflammatory diseases that rank high among global threats to human health.
Keywords: Chronic inflammatory diseases, Obesity, Diabetes, Adenovirus-36, Chlamydia pneumoniae
INTRODUCTION
The incidence of obesity has dramatically increased during the recent decades worldwide. Currently, two-thirds of adults in the USA are overweight and around 32% are obese with obesity still trending upwards[1,2]. Worldwide, over 1 billion adults are overweight and more than 300 million are clinically obese (body mass index ≥ 30 kg/m2)[3]. Alarmingly, obesity has also increased markedly in children[2]. It has only been recognized over the last 15 years that obesity is an endocrine disease in which particularly white abdominal adipose tissue secretes large amounts of inflammatory mediators[4]. The chronic release of these mediators called adipo-cytokines in patients with high body mass index results in a combination of clinical symptoms characterized by high blood pressure, resistance to intracellular uptake of glucose such that glucose homeostasis requires increased insulin secretion (insulin resistance) and perturbation of the blood lipid profile (high total cholesterol and low-density lipoprotein). The cluster of these clinical symptoms is termed metabolic syndrome.
Obesity and its associated conditions such as metabolic syndrome, diabetes and atherosclerosis are now considered chronic inflammatory diseases[5]. Inflammation is the key characteristic of obesity and metabolic syndrome and production of pro-inflammatory cytokines such as TNF-α is essential to enhance the development of type 2 diabetes and atherosclerosis. For example, TNF-α induces insulin resistance by stimulating stress hormone production and decreasing tyrosine phosphorylation of insulin-induced insulin receptor substrate 1[6]. Similarly, inflammation is also a key characteristic of the host response to infectious agents[7].
While inflammation is a shared and key characteristic of both chronic inflammatory diseases and infections, infectious diseases have long been associated directly with obesity [i.e. Canine distemper virus (CDV), Rous-associated virus-7 (RAV-7), Borna disease virus (BDV), Scrapie agent and adenoviruses SMAM-1 and 36] as well as the consequences of obesity such as metabolic syndrome, diabetes and atherosclerosis [i.e. Helicobacter pylori (H. pylori), Chlamydia pneumoniae (C. pneumoniae), Porphyromonas gingivalis (P. gingivalis), hepatitis C virus (HCV) and human immunodeficiency virus (HIV)]. Clinical experience clearly shows that infectious diseases worsen glycemic control in diabetic patients[8-10]. Conversely, diabetic patients are also known to be at increased risk for infectious diseases and for higher severity of such diseases[8,11]. Obesity and exposure to infectious agents overlap in large population segments and therefore may mutually influence each other. Thus, profound practical medical benefits may result from a rational comprehensive approach to the management of chronic inflammatory diseases if therapy of either metabolic syndrome or infectious disease would also mitigate the respective other conditions.
This review focuses first on pathogens that directly promote obesity and, as a downstream consequence of obesity, the development of metabolic syndrome and its associated conditions. Subsequently, the review will focus on a second set of pathogens that cause chronic infections and subsequent release of inflammatory mediators and, via this mechanism, induce or exacerbate metabolic syndrome. A new perspective on the connection between infections and obesity-related diseases will better the management of these chronic inflammatory diseases that now rank very highly among the global threats to human health.
OBESITY-ENHANCING PATHOGENS
CDV
CDV is a lymphotropic and neurotropic negative-stranded RNA virus belonging to the genus Morbillivirus[12,13]. It affects mainly dogs and related mammals by invading the nervous system and replicating in neurons and glial cells of the white cell subgroup resulting in a frequently fatal disease[13]. Even though CDV is not considered a human pathogen, a suggestive association with human disease has been described[14]. CDV is antigenically closely related to human measles virus with both of them belonging to the same family of Paramyxoviridae viruses.
CDV was reported as the first obesity-promoting pathogen in 1982 when Lyons et al[15] published the landmark article in Science, “A virally induced obesity syndrome in mice” that reported that CDV infection induced obesity in Swiss Albino mice. CDV-inoculated mice showed increased body weight as well as an increased number and size of fat cells[15]. Anatomical damage and altered neurochemistry in the hypothalamus was subsequently demonstrated in CDV-infected mice[16-21]. The hypothalamus plays a well-documented role in appetite regulation, energy consumption and neuroendocrine function[18]. CDV-infected mice showed down-regulated leptin receptors in the hypothalamic area of the brain, explaining their inability to generate a proper response to leptin in the brain[18]. With lower number of leptin receptors, hunger may be induced despite high leptin plasma levels that signal satiety. In addition, CDV also down-regulates melanin-concentrating hormone[21], expression levels of neuropeptides and catecholamine[15,22] and production of proinflammatory cytokines[20]. Collectively, these data suggest that persistent CDV infection of the hypothalamus specifically alters satiety-signaling pathways and thereby induces excessive food consumption and eventually obesity.
RAV-7
RAV-7, an avian leukosis virus, was the second microbe reported to induce obesity. RAV-7 (avian leukosis virus subgroup C) is the most common poultry retrovirus associated with neoplastic disease[23]. RAV-7 causes obesity in chickens combined with growth stunting, hypertriglycemia, hypercholesterolemia as well as enlarged fatty liver, anemia and immunosuppression[23]. The lipid content of the diet did not influence the RAV-7-mediated induction of obesity[24]. By 20 d after hatching, infected chickens were smaller than uninfected hatch mates and developed ataxia and obesity over the next 30 d. These chickens also developed mild anemia and lipemia and had high levels of plasma uric acid. RAV-7 infection also induced a marked decrease in the weight of thymus and bursa of Fabricius[23]. The histological appearance of obesity is characterized in the liver by a diffuse panlobular accumulation of fat in microdroplets and by a lymphoblastoid cellular infiltrate in thyroid gland and pancreas. Carter et al[24] (1983) hypothesized that RAV-7 infection induces obesity by reducing thyroid hormone levels.
Carter et al[25] also investigated the specificity of obesity induction by RAV-7. Avian leucosis viruses of the subgroups A [RAV-1 and MAV-1 (O) causing osteopetrosis], B [MAV-2 (O) and MAV-2 (N) causing nephroblastoma], D (RAV-50) and F (RAV-61 and ring-necked pheasant virus) did not induce obesity[25].
BDV
Borna disease virus is an enveloped, non-segmented, negative-stranded RNA virus of the order Mononegavirales with replication and transcription inside the nucleus of the host cells[26-28]. BDV infection is found worldwide and induces fatal disease in numerous animals such as horse, sheep, cat and dog[29,30] and there is also evidence that BDV may affect humans[31,32]. Chalmers et al[32] (2005) reported between 0% to 48% BDV seropositivity and 0% to 82% BDV antigen prevalence in humans. Narayan et al[33] and Gosztonyi et al[34,35] described an obesity syndrome in rats apparently induced by BDV in an age-, genetic background- and virus strain-dependent manner. Infected obese rats showed massive visceral fat deposition with elevated serum glucose levels and hypertriglyceridemia[34]. Several investigators hypothesized that BDV infection induces obesity through inflammatory lesions and viral antigen expression in the brain, particularly in the hypothalamus, similar to CDV infection[36].
Scrapie agent
The causative agent of scrapie is thought to be a prion[37]. Scrapie agent causes a fatal neurodegenerative disease with a long incubation period in sheep and goats[38]. Scrapie agent is not known to infect humans. In the laboratory, many other animals such as hamster, mice, rats, voles, gerbils, mink, cattle and monkeys have been successfully infected with scrapie agent[37]. This disease is classified as transmissible spongiform encephalopathy, similar to human Creutzfeld-Jacob’s disease and related TSEs caused by prions[39-40].
Kim et al[41] reported that the ME-7 scrapie strain induced obesity and vacuolization in the forebrain of mice but other strains did not. Since adrenalectomy prevented obesity, it was suggested that this scrapie agent induces obesity via the hypothalamic-pituitary-adrenal axis[42]. Scrapie agent was also confirmed to cause hyperglycemia, hyperinsulinemia and diabetes by inducing pancreatic lesions and a significant decrease of the glucose transporter GLUT-1 in the brain[43,44].
Adenovirus-SMAM-1
Adenoviruses are non-enveloped DNA viruses with icosahedral symmetry and a diameter of 65-80 nm[45]. Adenoviruses were first isolated in 1953 during establishment of cell lines from pediatric adenoidal tissues obtained by tonsillectomy[46]. Adenoviruses infect a wide range of hosts such as birds, mammals and humans. Approximately 8% of the world-wide reported virus infections were caused by adenoviruses which can cause serious respiratory disease of epidemic proportions reported with a group of military recruits[47]. There are 5 major subgroups of human adenoviruses and each subgroup is also subdivided into several serotypes. The viral genome consists of 5 early transcription units (E1A, E1B, E2, E3, and E4), 2 delayed early units (IX and Iva2) and one major late unit to generate mRNAs (L1-L5)[38]. Adenoviruses produce a variety of serious diseases in people of all ages.
SMAM-1 is a strain of avian adenovirus responsible for a poultry epidemic in India during the 1980s[48] and is serologically related to another poultry adenovirus, chick embryo lethal orphan virus (CELO). SMAM-1-inoculated 3 wk-old chickens showed 53% greater visceral fat 3 wk post inoculation compared to uninfected controls. Paradoxically, the increased adiposity in SMAM-1 infected chickens was accompanied by reduced body weight and lower blood concentrations of cholesterol and triglycerides than in controls[49]. Livers of the SMAM-1 infected chickens were significantly heavier and showed severe congestion, fatty infiltration and presence of intranuclear inclusion bodies under histopathological examination. The infected chickens showed also atrophied bursae, spleen and thymus[49]. Dhurandhar et al[50] (1997) reported an association between SMAM-1 seropositivity and human obesity. SMAM-1 antibody-positive humans showed significantly higher BMI and significantly lower blood cholesterol and triglycerides compared to the antibody-negative subjects.
Adenovirus-36
The Adenovirus-36 strain of adenoviruses was first identified in 1978 in Germany from the feces of a 6-year-old girl with diabetes and enteritis[51]. Accumulated evidence from animal models, in vitro experiments and human epidemiology strongly suggest a positive association between adenovirus-36 and human obesity. Atkinson et al showed that 11%-30% of Americans are seropositive against adenovirus-36[52]. Dhurandhar et al[53] (2000) explored the influence of adenovirus-36 infection on the development of obesity in chickens. Adenovirus-36 challenged chickens showed 100% greater visceral fat and total body weight than the control group inoculated with sterile cell culture medium and these conclusions were confirmed by the subsequent studies[54]. Adenovirus-36 inoculated male marmoset monkeys showed an astonishing 3-fold weight gain compared to uninfected controls[55]. In vitro studies showed that adenovirus-36 promotes the proliferation, differentiation and lipid accumulation in 3T3-L1 preadipocytes[38]. Atkinson et al screened the sera of 360 obese (BMI ≥ 30 kg/m2) and 142 non obese (BMI ≤ 30 kg/m2) subjects in Wisconsin, Florida and New York for adenovirus-36 antibodies. Adenovirus-36 antibodies were 30% and 11% prevalent in obese and non-obese subjects respectively and obese and non obese subjects with adenovirus-36 antibodies had significantly greater BMI than their respective seronegative counterparts[52]. The authors concluded that the influence of adenovirus-36 seropositivity on obesity was highly significant independent of age, sex and origin of the human subjects[38,52]. In vitro studies shed light on the mechanisms of adenovirus-36-inducing obesity. Adenovirus-36 infected human preadipocytes showed increased replication, differentiation, lipid accumulation as well as reduced leptin secretion in fat cells[38,54,56]. This effect is specific for adenovirus-36 and is not observed with nonadipogenic adenovirus (Ad-2). It is likely that adenovirus-36 infection increases the number of fat cells, glucose uptake by adipocytes and promotes lipogenesis[56]. Na et al[57] and Atkinson et al[58] reported a positive association between human adenovirus-36 and obesity in children. However, two recent epidemiological surveys[59,60] indicated that human adenovirus-36 did not play a direct role in the development of obesity in both Western Europe and the US.
Adenovirus type 5 has been widely used for gene therapy because it is a safe and efficient vector and accommodates large antigen-encoding structures[61]. So et al[62] (2005) showed that adenovirus-5 infected mice attained significantly greater body weight and higher adiposity than control group 22-23 wk post inoculation. The same mechanisms as found in adenovirus-36, i.e. increased preadipocyte differentiation, also apply for adenovirus-5 infection[56]. Human adenovirus-37 was first isolated by de Jong et al[63]. Adenovirus-37 caused adiposity in chickens and the visceral fat pads were three times heavier in adenovirus-37-inoculated chickens than in controls. Different from adenovirus-36, adenovirus-37 infections did not induce reduced concentrations of serum cholesterol. Among over 50 strains of adenoviruses among five subgroups maintained by ATCC, strains adenovirus-36 and adenovirus-37, adenovirus-5 are adipogenic and adenovirus-2 and adenovirus-31 are not adipogenic. The potential of other human adenoviruses on the development of obesity remains unknown.
PATHOGENS ENHANCING HUMAN CHRONIC INFLAMMATORY DISEASES AND OBESITY
H. pylori
H. pylori is a spiral-shaped gram-negative flagellated bacterium and causes highly prevalent chronic infections worldwide[64]. H. pylori is the etiology of diseases such as gastritis and stomach cancer but most H. pylori infections are “silent” and produce no clinical symptoms and in particular are asymptomatic in childhood. Worldwide, up to 10% of children and 80% of adults show laboratory evidence of H. pylori infection. Aydemir et al[65] (2005) provided the first association between chronic H. pylori infection and insulin resistance. The homeostasis model assessment of insulin resistance was significantly higher in 36 H. pylori-positive subjects than in 27 H. pylori-negative ones[65]. Epidemiological evidence also supports the association of H. pylori seropositivity with cardiovascular diseases and elevated parameters of metabolic syndrome[66,67].
C. pneumoniae
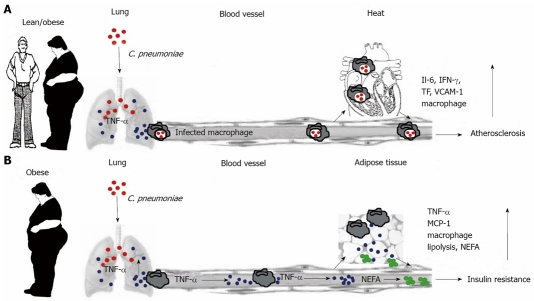
C. pneumoniae, an intracellular respiratory bacterium, causes acute or chronic bronchitis and pneumonia[68] and is responsible for 10% of the cases of community-acquired pneumonia and approximately 5% of cases of bronchitis and sinusitis in adults[68-70]. Many epidemiological surveys, experimental studies and clinical trials have provided strong evidence for the association between C. pneumoniae infection and metabolic syndrome, insulin resistance and cardiovascular disease[70-82]. The current concept of the influence of C. pneumoniae on atherosclerosis is that C. pneumoniae-infected macrophages traffic to secondary organs including arterial endothelium, induce persistent infection and lead to the local upregulation of proinflammatory molecules (Figure 1A). Subsequently, infected macrophages and smooth muscle cells transform into foam cells and result in plaque destabilization, thrombus formation and myocardial infarction in arterial endothelium[75,85]. Ased on this notion, antibiotic treatment would reduce cardiovascular events by eliminating C. pneumoniae persistent infection and preventing re-infection. However, clinical trials with anti–biotic treatment based on this concept failed. This failure to reduce cardiovascular events by antibiotic treatment requires the reformulation of the current mechanistic understanding of the association between C. pneumoniae and metabolic syndrome and cardiovascular disease[83].
Figure 1.
Schematic representation of C. pneumoniae-mediated acceleration of inflammatory diseases associated with metabolic syndrome. A: Current concept of the influence of C. pneumoniae on atherosclerosis. After respiratory infection, C. pneumoniae (red) is endocytosed by alveolar macrophages of infected lean or obese individuals. Infected macrophages re-enter circulation and traffic to secondary organs including arterial endothelium. Disseminated C. pneumoniae organisms infect other cell types, leading to up-regulation of pro-inflammatory molecules such as IL-6, IFN-γ, tissue factor and vascular-cell-adhesion molecule 1. These cytokines, in particular IFN-γ, retard chlamydial replication and induce persistent infection. In arterial endothelium, infected macrophages and smooth muscle cells transform into foam cells, affecting atheroma biology and leading to plaque destabilization, thrombus formation or myocardial infarction[85]. B: Concept of C. pneumoniae-induced exacerbation of insulin resistance developed by Wang et al[84]. C. pneumoniae infection results in lung colonization, thereby increasing TNF-α release (blue). Circulating TNF-α induces insulin resistance by inhibiting the function of insulin receptor substrate-1 in peripheral tissues and further exacerbates insulin resistance by promoting lipolysis and increased NEFA (green) production in adipose tissue. TNF-α and NEFA promote further macrophage infiltration and excess production of pro-inflammatory molecules in adipose tissue. In contrast to the atherosclerosis concept, the perpetuation of adipose tissue inflammation is driven by circulating pro-inflammatory cytokines rather than by in situ production stimulated by infected macrophages. Sustained by continuous high-fat nutrition, the inflammatory condition of adipose tissue maintains the vicious cycle of insulin resistance in the absence of C. pneumoniae organisms.
Wang et al[84] examined the influence of C. pneumoniae infection on progression of insulin resistance in dependence of host genetic background and dietary fat concentration in an obese mouse model. They concluded that murine C. pneumoniae infection enhances insulin resistance and diabetes in a genetically and nutritionally restricted manner via circulating inflammatory mediators such as TNF-α[84] and proposed a new mechanism of C. pneumoniae-induced exacerbation of insulin resistance developed in this investigation (Figure 1B). By quantifying the levels of C. pneumoniae and TNF-α transcripts in different organs, they concluded that the dispersal of a small number of C. pneumoniae organisms to secondary tissues was irrelevant to progression of insulin resistance and the early onset of type 2 diabetes. In contrast, the bulk infection of the lung caused an increase in circulating cytokines that drove the long-term exacerbation of insulin resistance (Figure 1B) and accelerated the onset of type 2 diabetes[84]. It was reported that combined pathogen burden[82] and positive serology for both H. pylori and C. pneumoniae[66] showed the strongest association with insulin resistance. These data suggest that exposure to multiple pathogens may potentiate chronic low-grade inflammation and insulin resistance and that the mechanisms whereby the pathogens affect chronic inflammatory diseases are shared.
P. gingivalis
A national survey with 9 689 subjects from 1988 to 1994 showed that periodontal disease is prevalent in the U.S. adult population[86]. Approximately 35% of the adults in the USA have periodontitis, an inflammation that involves the periodontal ligament and alveolar bone, while about 75% have gingivitis, an inflammation of the gingival tissues surrounding the teeth[8,86]. Periodontal diseases are initiated by gram-negative and anaerobic bacteria such as P. gingivalis residing in biofilms on gingival tissues and teeth[87]. Periodontal diseases had been thought of as localized conditions of concern only to dental health professionals. Emerging evidence now suggests that periodontal diseases also exacerbate systemic conditions such as metabolic syndrome and diabetes[88,89]. The oral infection causes elevated circulating IL-1β and TNF-α which lead to hyperlipidemia and development of diabetes[88,90].
HCV
HCV infection is a worldwide problem with approximately 200 million infected individuals[1,2]. Chronic HCV infection may result in liver cirrhosis and hepatocellular carcinoma and is associated with multifaceted disease such as porphyria cutanea tarda, membranoproliferative glomerulonephritis and cryoglobulinemia. While epidemiological studies suggested a linkage between HCV infections and type 2 diabetes[91-95], Shintani et al[91] confirmed the direct involvement of HCV infection in the development of insulin resistance in a mouse model using mice with the HCV core gene inserted in their genome. They showed that a high level of TNF-α was the main factor to induce insulin resistance in HCV-transgenic mice and insulin sensitivity was restored by administration of anti-TNF-α antibody.
HIV
Insulin resistance is common in HIV-infected people and the prevalence of hyperglycemia and diabetes is significantly higher in people with HIV infection treated with antiretrovirals as compared with the general population[96]. The prevalence of insulin resistance in HIV subjects is around 35% and up to 47% when they received protease inhibitor therapy, while the incidence of insulin resistance is only around 5% in the general population[97]. Presumably, HIV induces an increased inflammatory state, as evident in elevated levels of adiponectin and free fatty acids in HIV-infected individuals[96].
CONCLUSION
Ranked as one of the three major challenges to human progress along with war and famine, infectious diseases remain among the leading causes of death and disability worldwide[98]. More than 25% of annual human deaths are the direct result of infectious diseases[98,99]. Progress in diagnostic methodology now allows the consistent detection of low-number but widely-prevalent pathogens as well as sensitive and precise quantification that captures subtle elevations of inflammatory cytokines which are closely related to metabolism and the immune response. Application of these essential tools in epidemiological, pathological and experimental studies has strongly suggested an infectious influence on obesity, metabolic syndrome, diabetes and cardiovascular diseases.
Following Darwinian adaption in human evolution, thrifty genes have probably evolved to maximize food utilization during periods of mass starvation.This metabolic adaptation combines frugal use of nutrients with vigorous inflammatory responses to pathogens and thus maximizes survival[100,101]. While advantageous under selective pressure of starvation and epidemic infectious diseases, this genetic makeup of large population segments has become a liability in times of food abundance and increased hygiene that eliminates epidemic but not endemic chronic infections. The evolved frugal metabolic characteristics increase obesity under conditions of over-nutrition and intensive inflammatory responses enhance the pathological consequences of obesity under constant stimulation by previously unrecognized ubiquitous but low-level chronic infections.
In summary, the dominant influence on chronic inflammatory diseases is anchored in human genetics and the outcome is driven by food supply and consumption. In this cause-effect network with tightly integrated metabolic and immune response pathways, infections likely play a heretofore underappreciated role in modulating intensity and pathological consequences, thus potentially decisively modulating the cause-effect network in the pathogenesis of chronic inflammatory diseases. Confirmation of the infectious modulation of obesity (Table 1) and chronic inflammatory diseases (Table 2) will facilitate prevention and management of such diseases. Very likely, vaccinations against multiple infectious agents will be the sole effective and realistic approach to ameliorate pathogen-enhanced obesity and related conditions such as diabetes and atherosclerosis.
Table 1.
Pathogens associated with obesity
| Pathogen | Effect | Mechanisms | Ref |
| CDV | Increased body weight in Swiss albino mice | Altered hypothalamic integrity; increased cytokine production, hyperinsulinemia and decreased leptin and neuropeptides | [15-17,21,22] |
| RAV-7 | Stunting, anemia and increased visceral fat in white leghorn chickens | Decreased thyroxine, hyperlipidemia and hyperinsulinemia | [23,24] |
| BDV | Obesity with increased visceral fat in Lewis rats | Inflammatory lesions in hypothalamus, increased triglyceride and blood glucose | [35,36] |
| SMAM-1 | Stunting and increased visceral fat in white leghorn chickens | Impaired liver function and lipogenesis and glucagon deficiency | [49,50] |
| Scrapie agent | Increased body weight and fat accumulation in mice | Altered brain function and reduced GLUT-1 | [43,44] |
| Adenovirus | Increased body weight in chickens, mice, rats and monkeys; seropositive subjects were heavier than seronegative counterparts | Increased replication, differentiation and lipid accumulation of preadipocytes | [51-60] |
CDV: Canine distemper virus; RAV-7: Rous-associated virus-7; BDV: Borna disease virus; Ref: Reference.
Table 2.
Pathogens associated with increased chronic inflammatory diseases in humans
| Pathogen | Effect | Mechanisms | Ref |
| H. pylori | Affected subjects showed increased insulin resistance | Increased concentrations of plasma glucose and lipids | [64-67] |
| C. pneumoniae | Increased metabolic syndrome, insulin resistance and cardiovascular disease | Increased production of proinflammatory and circulating cytokines | [68-72] |
| P. gingivalis | Adults with dental infections demonstrated higher chance of insulin resistance and diabetes | Increased oxidative stress, advanced glycation end-products and altered immune function | [86-90] |
| HCV | Infected patients showed increased chance to develop insulin resistance and diabetes | Increased production of TNF and IL-6 | [92-95] |
| HIV | HIV patients showed higher insulin resistance (35%) compared to normal subjects (5%) | Impaired glucose tolerance and significant hyperinsulinaemia | [96,97] |
H. pylori: Helicobacter pylori; C. pneumoniae: Chlamydia pneumoniae; P. gingivalis: Porphyromonas gingivalis; HCV: Hepatitis C virus; HIV: human immunodeficiency virus; Ref: Reference.
Footnotes
Supported in part by NIH grant AI47202, and by grants from the Diabetes Trust Foundation, Diabetes Action Research and Education Foundation Grant #198, and by Graduate-in-Aid and Competitive Research Grant Programs at Auburn University
Peer reviewers: Moshira Abdel Hakim Rateb, MD, Assistant Professor of Human Physiology, Faculty of Medicine (Kasr Al Aini.), Cairo University, Manial El Roda, Cairo, Egypt; Edwin Mariman, PhD, Professor, Department of Human Biology, Maastricht University, 6200 MD Maastricht, The Netherlands
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ. Health (Abingdon) 2003;16:230. doi: 10.1080/1357628031000116808. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galinier A, Carrière A, Fernandez Y, Carpéné C, André M, Caspar-Bauguil S, Thouvenot JP, Périquet B, Pénicaud L, Casteilla L. Adipose tissue proadipogenic redox changes in obesity. J Biol Chem. 2006;281:12682–12687. doi: 10.1074/jbc.M506949200. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- 7.Huang J, DeGraves FJ, Lenz SD, Gao D, Feng P, Li D, Schlapp T, Kaltenboeck B. The quantity of nitric oxide released by macrophages regulates Chlamydia-induced disease. Proc Natl Acad Sci USA. 2002;99:3914–3919. doi: 10.1073/pnas.062578399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Compend Contin Educ Dent. 2008;29:402–408, 410, 412-413. [PubMed] [Google Scholar]
- 9.Begue RE, Mirza A, Compton T, Gomez R, Vargas A. Helicobacter pylori infection and insulin requirement among children with type 1 diabetes mellitus. Pediatrics. 1999;103:e83. doi: 10.1542/peds.103.6.e83. [DOI] [PubMed] [Google Scholar]
- 10.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67:1085–1093. doi: 10.1902/jop.1996.67.10s.1085. [DOI] [PubMed] [Google Scholar]
- 11.Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care. 2001;24:1044–1049. doi: 10.2337/diacare.24.6.1044. [DOI] [PubMed] [Google Scholar]
- 12.Rozenblatt S, Eizenberg O, Englund G, Bellini WJ. Cloning and characterization of DNA complementary to the canine distemper virus mRNA encoding matrix, phosphoprotein, and nucleocapsid protein. J Virol. 1985;53:691–694. doi: 10.1128/jvi.53.2.691-694.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandevelde M, Zurbriggen A. The neurobiology of canine distemper virus infection. Vet Microbiol. 1995;44:271–280. doi: 10.1016/0378-1135(95)00021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summers BA, Appel MJ. Aspects of canine distemper virus and measles virus encephalomyelitis. Neuropathol Appl Neurobiol. 1994;20:525–534. doi: 10.1111/j.1365-2990.1994.tb01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons MJ, Faust IM, Hemmes RB, Buskirk DR, Hirsch J, Zabriskie JB. A virally induced obesity syndrome in mice. Science. 1982;216:82–85. doi: 10.1126/science.7038878. [DOI] [PubMed] [Google Scholar]
- 16.Bernard A, Zwingelstein G, Meister R, Wild TF. Hyperinsulinemia induced by canine distemper virus infection of mice and its correlation with the appearance of obesity. Comp Biochem Physiol B. 1988;91:691–696. doi: 10.1016/0305-0491(88)90193-9. [DOI] [PubMed] [Google Scholar]
- 17.Bernard A, Fevre-Montange M, Giraudon P, Hardin H, Wild TF, Belin MF. [Demonstration of viral proteins and RNA in hypothalamus of mice infected by canine distemper virus] C R Acad Sci III. 1991;313:545–551. [PubMed] [Google Scholar]
- 18.Bernard A, Cohen R, Khuth ST, Vedrine B, Verlaeten O, Akaoka H, Giraudon P, Belin MF. Alteration of the leptin network in late morbid obesity induced in mice by brain infection with canine distemper virus. J Virol. 1999;73:7317–7327. doi: 10.1128/jvi.73.9.7317-7327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagashima K, Zabriskie JB, Lyons MJ. Virus-induced obesity in mice: association with a hypothalamic lesion. J Neuropathol Exp Neurol. 1992;51:101–109. doi: 10.1097/00005072-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Bencsik A, Akaoka H, Giraudon P, Belin MF, Bernard A. Inhibition of tyrosine hydroxylase expression within the substantia nigra of mice infected with canine distemper virus. J Neuropathol Exp Neurol. 1997;56:673–685. [PubMed] [Google Scholar]
- 21.Verlaeten O, Casery C, Cavagna S, Naville D, Giraudon P, Belin MF, Begeot M, Bernard A. Identification of Urop11, a novel leptin-modulated gene that is upregulated in the hypothalamus of mice with virus-induced obesity. J Mol Endocrinol. 2007;38:3–17. doi: 10.1677/jme.1.02139. [DOI] [PubMed] [Google Scholar]
- 22.Griffond B, Verlaeten O, Belin MF, Risold PY, Bernard A. Specific alteration of the expression of selected hypothalamic neuropeptides during acute and late mouse brain infection using a morbillivirus: relevance to the late-onset obesity? Brain Res. 2004;1022:173–181. doi: 10.1016/j.brainres.2003.10.077. [DOI] [PubMed] [Google Scholar]
- 23.Carter JK, Ow CL, Smith RE. Rous-associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity. Infect Immun. 1983;39:410–422. doi: 10.1128/iai.39.1.410-422.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter JK, Garlich JD, Donaldson WE, Smith RE. Influence of diet on a retrovirus-induced obesity and stunting syndrome. Avian Dis. 1983;27:317–322. [PubMed] [Google Scholar]
- 25.Carter JK, Smith RE. Specificity of avian leukosis virus-induced hyperlipidemia. J Virol. 1984;50:301–308. doi: 10.1128/jvi.50.2.301-308.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stitz L, Dietzschold B, Carbone KM. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 27.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 28.Kao M, Hamir AN, Rupprecht CE, Fu ZF, Shankar V, Koprowski H, Dietzschold B. Detection of antibodies against Borna disease virus in sera and cerebrospinal fluid of horses in the USA. Vet Rec. 1993;132:241–244. doi: 10.1136/vr.132.10.241. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- 30.Richt JA, VandeWoude S, Zink MC, Clements JE, Herzog S, Stitz L, Rott R, Narayan O. Infection with Borna disease virus: molecular and immunobiological characterization of the agent. Clin Infect Dis. 1992;14:1240–1250. doi: 10.1093/clinids/14.6.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de la Torre JC. Molecular biology of borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalmers RM, Thomas DR, Salmon RL. Borna disease virus and the evidence for human pathogenicity: a systematic review. QJM. 2005;98:255–274. doi: 10.1093/qjmed/hci039. [DOI] [PubMed] [Google Scholar]
- 33.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunopathological responses to persistent borna virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 34.Gosztonyi G. Natural and experimental Borna disease virus infections--neuropathology and pathogenetic considerations. APMIS Suppl. 2008;124:53–57. doi: 10.1111/j.1600-0463.2008.000m8.x. [DOI] [PubMed] [Google Scholar]
- 35.Gosztonyi G, Ludwig H. Borna disease--neuropathology and pathogenesis. Curr Top Microbiol Immunol. 1995;190:39–73. [PubMed] [Google Scholar]
- 36.Herden C, Herzog S, Richt JA, Nesseler A, Christ M, Failing K, Frese K. Distribution of Borna disease virus in the brain of rats infected with an obesity-inducing virus strain. Brain Pathol. 2000;10:39–48. doi: 10.1111/j.1750-3639.2000.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson RL. Viruses as an etiology of obesity. Mayo Clin Proc. 2007;82:1192–1198. doi: 10.4065/82.10.1192. [DOI] [PubMed] [Google Scholar]
- 38.Pasarica M, Dhurandhar NV. Infectobesity: obesity of infectious origin. Adv Food Nutr Res. 2007;52:61–102. doi: 10.1016/S1043-4526(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 39.Prusiner SB, Scott M, Foster D, Pan KM, Groth D, Mirenda C, Torchia M, Yang SL, Serban D, Carlson GA. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990;63:673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 40.Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YS, Carp RI, Callahan SM, Wisniewski HM. Scrapie-induced obesity in mice. J Infect Dis. 1987;156:402–405. doi: 10.1093/infdis/156.2.402. [DOI] [PubMed] [Google Scholar]
- 42.Kim YS, Carp RI, Callahan SM, Wisniewski HM. Adrenal involvement in scrapie-induced obesity. Proc Soc Exp Biol Med. 1988;189:21–27. doi: 10.3181/00379727-189-42774. [DOI] [PubMed] [Google Scholar]
- 43.Carp RI, Kim YS, Callahan SM. Pancreatic lesions and hypoglycemia-hyperinsulinemia in scrapie-injected hamsters. J Infect Dis. 1990;161:462–466. doi: 10.1093/infdis/161.3.462. [DOI] [PubMed] [Google Scholar]
- 44.Vorbrodt AW, Dobrogowska DH, Tarnawski M, Meeker HC, Carp RI. Quantitative immunogold study of glucose transporter (GLUT-1) in five brain regions of scrapie-infected mice showing obesity and reduced glucose tolerance. Acta Neuropathol. 2001;102:278–284. doi: 10.1007/s004010100382. [DOI] [PubMed] [Google Scholar]
- 45.Pereira HG, Huebner RJ, Ginsberg HS, van der Veen J. A short description of the adenovirus group. Virology. 1963;20:613–620. doi: 10.1016/0042-6822(63)90286-1. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y, Shenk TE. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 47.Rubin BA. Clinical picture and epidemiology of adenovirus infections (a review) Acta Microbiol Hung. 1993;40:303–323. [PubMed] [Google Scholar]
- 48.van Ginneken V, Sitnyakowsky L, Jeffery JE. “Infectobesity: viral infections (especially with human adenovirus-36: Ad-36) may be a cause of obesity. Med Hypotheses. 2009;72:383–388. doi: 10.1016/j.mehy.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 49.Dhurandhar NV, Kulkarni P, Ajinkya SM, Sherikar A. Effect of adenovirus infection on adiposity in chicken. Vet Microbiol. 1992;31:101–107. doi: 10.1016/0378-1135(92)90068-5. [DOI] [PubMed] [Google Scholar]
- 50.Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obes Res. 1997;5:464–469. doi: 10.1002/j.1550-8528.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 51.Wigand R, Gelderblom H, Wadell G. New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol. 1980;64:225–233. doi: 10.1007/BF01322702. [DOI] [PubMed] [Google Scholar]
- 52.Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, Augustus AS. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (Lond) 2005;29:281–286. doi: 10.1038/sj.ijo.0802830. [DOI] [PubMed] [Google Scholar]
- 53.Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord. 2000;24:989–996. doi: 10.1038/sj.ijo.0801319. [DOI] [PubMed] [Google Scholar]
- 54.Pasarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL, MohanKumar S, MohanKumar PS, Markward N, Dhurandhar NV. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity (Silver Spring) 2006;14:1905–1913. doi: 10.1038/oby.2006.222. [DOI] [PubMed] [Google Scholar]
- 55.Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, Israel BA, Bradley SM, Kemnitz JW, Allison DB, Atkinson RL. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002;132:3155–3160. doi: 10.1093/jn/131.10.3155. [DOI] [PubMed] [Google Scholar]
- 56.Vangipuram SD, Sheele J, Atkinson RL, Holland TC, Dhurandhar NV. A human adenovirus enhances preadipocyte differentiation. Obes Res. 2004;12:770–777. doi: 10.1038/oby.2004.93. [DOI] [PubMed] [Google Scholar]
- 57.Na HN, Hong YM, Kim J, Kim HK, Jo I, Nam JH. Association between human adenovirus-36 and lipid disorders in Korean schoolchildren. Int J Obes (Lond) 2010;34:89–93. doi: 10.1038/ijo.2009.207. [DOI] [PubMed] [Google Scholar]
- 58.Atkinson RL, Lee I, Shin HJ, He J. Human adenovirus-36 antibody status is associated with obesity in children. Int J Pediatr Obes. 2009:1–4. doi: 10.3109/17477160903111789. [DOI] [PubMed] [Google Scholar]
- 59.Broderick MP, Hansen CJ, Irvine M, Metzgar D, Campbell K, Baker C, Russell KL. Adenovirus 36 seropositivity is strongly associated with race and gender, but not obesity, among US military personnel. Int J Obes (Lond) 2010;34:302–308. doi: 10.1038/ijo.2009.224. [DOI] [PubMed] [Google Scholar]
- 60.Goossens VJ, Dejager SA, Grauls GE, Gielen M, Vlietinck RF, Derom CA, Loos RJ, Rensen SS, Buurman WA, Greve JW, et al. Lack of Evidence for the Role of Human Adenovirus-36 in Obesity in a European Cohort. Obesity (Silver Spring) 2010:In press. doi: 10.1038/oby.2009.452. [DOI] [PubMed] [Google Scholar]
- 61.Kanerva A, Hemminki A. Adenoviruses for treatment of cancer. Ann Med. 2005;37:33–43. doi: 10.1080/07853890410018934. [DOI] [PubMed] [Google Scholar]
- 62.So PW, Herlihy AH, Bell JD. Adiposity induced by adenovirus 5 inoculation. Int J Obes (Lond) 2005;29:603–606. doi: 10.1038/sj.ijo.0802917. [DOI] [PubMed] [Google Scholar]
- 63.de Jong JC, Wigand R, Wadell G, Keller D, Muzerie CJ, Wermenbol AG, Schaap GJ. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J Med Virol. 1981;7:105–118. doi: 10.1002/jmv.1890070204. [DOI] [PubMed] [Google Scholar]
- 64.Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J Med. 1996;100:12S–17S; discussion 17S-18S. doi: 10.1016/s0002-9343(96)80224-5. [DOI] [PubMed] [Google Scholar]
- 65.Aydemir S, Bayraktaroglu T, Sert M, Sokmen C, Atmaca H, Mungan G, Gun BD, Borazan A, Ustundag Y. The effect of Helicobacter pylori on insulin resistance. Dig Dis Sci. 2005;50:2090–2093. doi: 10.1007/s10620-005-3012-z. [DOI] [PubMed] [Google Scholar]
- 66.Ekesbo R, Nilsson PM, Lindholm LH, Persson K, Wadström T. Combined seropositivity for H. pylori and C. pneumoniae is associated with age, obesity and social factors. J Cardiovasc Risk. 2000;7:191–195. doi: 10.1177/204748730000700305. [DOI] [PubMed] [Google Scholar]
- 67.Longo-Mbenza B, Nkondi Nsenga J, Vangu Ngoma D. Prevention of the metabolic syndrome insulin resistance and the atherosclerotic diseases in Africans infected by Helicobacter pylori infection and treated by antibiotics. Int J Cardiol. 2007;121:229–238. doi: 10.1016/j.ijcard.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Grayston JT, Kuo CC, Wang SP, Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986;315:161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- 69.Kleemola M, Saikku P, Visakorpi R, Wang SP, Grayston JT. Epidemics of pneumonia caused by TWAR, a new Chlamydia organism, in military trainees in Finland. J Infect Dis. 1988;157:230–236. doi: 10.1093/infdis/157.2.230. [DOI] [PubMed] [Google Scholar]
- 70.Grayston JT. Chlamydia pneumoniae, strain TWAR pneumonia. Annu Rev Med. 1992;43:317–323. doi: 10.1146/annurev.me.43.020192.001533. [DOI] [PubMed] [Google Scholar]
- 71.Kuo CC, Jackson LA, Campbell LA, Grayston JT. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Mäkelä PH, Huttunen JK, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 73.Boman J, Söderberg S, Forsberg J, Birgander LS, Allard A, Persson K, Jidell E, Kumlin U, Juto P, Waldenström A, et al. High prevalence of Chlamydia pneumoniae DNA in peripheral blood mononuclear cells in patients with cardiovascular disease and in middle-aged blood donors. J Infect Dis. 1998;178:274–277. doi: 10.1086/517452. [DOI] [PubMed] [Google Scholar]
- 74.Gupta S, Leatham EW, Carrington D, Mendall MA, Kaski JC, Camm AJ. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation. 1997;96:404–407. doi: 10.1161/01.cir.96.2.404. [DOI] [PubMed] [Google Scholar]
- 75.Campbell LA, Moazed TC, Kuo CC, Grayston JT. Preclinical models for Chlamydia pneumoniae and cardiovascular disease: hypercholesterolemic mice. Clin Microbiol Infect. 1998;4 Suppl 4:S23–S32. [PubMed] [Google Scholar]
- 76.Ekesbo R, Nilsson PM, Lindholm LH, Persson K, Wadström T. Combined seropositivity for H. pylori and C. pneumoniae is associated with age, obesity and social factors. J Cardiovasc Risk. 2000;7:191–195. doi: 10.1177/204748730000700305. [DOI] [PubMed] [Google Scholar]
- 77.Smieja M, Mahony J, Petrich A, Boman J, Chernesky M. Association of circulating Chlamydia pneumoniae DNA with cardiovascular disease: a systematic review. BMC Infect Dis. 2002;2:21. doi: 10.1186/1471-2334-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dart AM, Martin JL, Kay S. Association between past infection with Chlamydia pneumoniae and body mass index, low-density lipoprotein particle size and fasting insulin. Int J Obes Relat Metab Disord. 2002;26:464–468. doi: 10.1038/sj.ijo.0801890. [DOI] [PubMed] [Google Scholar]
- 79.Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, Cairns R, Skene AM. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–1654. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- 80.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, et al. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–1645. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- 81.Nabipour I, Vahdat K, Jafari SM, Pazoki R, Sanjdideh Z. The association of metabolic syndrome and Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, and herpes simplex virus type 1: the Persian Gulf Healthy Heart Study. Cardiovasc Diabetol. 2006;5:25. doi: 10.1186/1475-2840-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernández-Real JM, López-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- 83.Anderson JL. Infection, antibiotics, and atherothrombosis--end of the road or new beginnings? N Engl J Med. 2005;352:1706–1709. doi: 10.1056/NEJMe058019. [DOI] [PubMed] [Google Scholar]
- 84.Wang C, Gao D, Kaltenboeck B. Acute Chlamydia pneumoniae reinfection accelerates the development of insulin resistance and diabetes in obese C57BL/6 mice. J Infect Dis. 2009;200:279–287. doi: 10.1086/599796. [DOI] [PubMed] [Google Scholar]
- 85.Campbell LA, Kuo CC. Chlamydia pneumoniae--an infectious risk factor for atherosclerosis? Nat Rev Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- 86.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 87.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol. 2001;6:125–137. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 89.Pontes Andersen CC, Flyvbjerg A, Buschard K, Holmstrup P. Relationship between periodontitis and diabetes: lessons from rodent studies. J Periodontol. 2007;78:1264–1275. doi: 10.1902/jop.2007.060491. [DOI] [PubMed] [Google Scholar]
- 90.Ritchie CS. Mechanistic links between type 2 diabetes and periodontitis. J Dent. 2009;37:S578–S579. doi: 10.1016/j.jdent.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 92.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 93.Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O’Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 94.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 95.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 96.Aboud M, Elgalib A, Kulasegaram R, Peters B. Insulin resistance and HIV infection: a review. Int J Clin Pract. 2007;61:463–472. doi: 10.1111/j.1742-1241.2006.01267.x. [DOI] [PubMed] [Google Scholar]
- 97.Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: a randomized, controlled trial. Ann Intern Med. 2004;140:786–794. doi: 10.7326/0003-4819-140-10-200405180-00008. [DOI] [PubMed] [Google Scholar]
- 98.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.World Health Organization. The World Health Report 2004. World Health Organization: Genève; 2004. [Google Scholar]
- 100.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 101.Roth J. Evolutionary speculation about tuberculosis and the metabolic and inflammatory processes of obesity. JAMA. 2009;301:2586–2588. doi: 10.1001/jama.2009.930. [DOI] [PubMed] [Google Scholar]