Abstract
Free fatty acids are known to play a key role in promoting loss of insulin sensitivity in type 2 diabetes mellitus but the underlying mechanism is still unclear. It has been postulated that an increase in the intracellular concentration of fatty acid metabolites activates a serine kinase cascade, which leads to defects in insulin signaling downstream to the insulin receptor. In addition, the complex network of adipokines released from adipose tissue modulates the response of tissues to insulin. Among the many molecules involved in the intracellular processing of the signal provided by insulin, the insulin receptor substrate-2, the protein kinase B and the forkhead transcription factor Foxo 1a are of particular interest, as recent data has provided strong evidence that dysfunction of these proteins results in insulin resistance in vivo. Recently, studies have revealed that phosphoinositidedependent kinase 1-independent phosphorylation of protein kinase Cε causes a reduction in insulin receptor gene expression. Additionally, it has been suggested that mitochondrial dysfunction triggers activation of several serine kinases, and weakens insulin signal transduction. Thus, in this review, the current developments in understanding the pathophysiological processes of insulin resistance in type 2 diabetes have been summarized. In addition, this study provides potential new targets for the treatment and prevention of type 2 diabetes.
Keywords: Adipokines, Forkhead box protein O, Insulin receptor, Insulin resistance, Insulin signaling, Insulin receptor substrate proteins, Type 2 diabetes mellitus, Phosphatidylinositol 3-kinase, Protein kinase B
INTRODUCTION
Diabetes mellitus (DM) is the most common endocrine disorder in man, currently affecting over 170 million people world-wide and, potentially, over 365 million in the year 2030[1]. Type 2 DM is rapidly emerging as one of the greatest global health challenges of the 21st century. This looming epidemic is also expected to trigger a steep rise in the complications associated with diabetes, such as ischemic heart disease, stroke, neuropathy, retinopathy, and nephropathy. Besides β cell failure, the major pathophysiological event contributing to the development of type 2 DM is the resistance of target tissues to insulin, which is usually associated with abnormal insulin secretion. Clinically, the term “insulin resistance” implies that higher-than-normal concentrations of insulin are required to maintain normoglycemia. On a cellular level, it defines the inadequate strength of insulin signaling from the insulin receptor downstream to the final substrates of insulin action involved in multiple metabolic and mitogenic aspects of cellular function[2].
The pathogenesis of type 2 diabetes involves abnormalities in both insulin action and secretion[3]. Although the precise pathophysiological sequence which leads to insulin resistance is still largely unknown, recent studies have contributed to a deeper understanding of the underlying molecular mechanisms. This review deals with the mechanisms related to type 2 diabetes. A detailed understanding of these basic pathophysiological mechanisms is critical for the development of novel therapeutic strategies to treat diabetes.
NORMAL INSULIN SIGNALING
The insulin receptor (IR) is a heterotetramer consisting of two α subunits and two β subunits that are linked by disulphide bonds. Insulin binds to the α subunit of the insulin receptor and activates the tyrosine kinase in the β subunit. Once the tyrosine kinase of insulin receptor is activated, it promotes autophosphorylation of the β subunit, where phosphorylation of three tyrosine residues (Tyr-1158, Tyr-1162, and Tyr-1163) is required for amplification of the kinase activity[4]. Most of the metabolic and antiapoptotic effects of insulin are mediated by the signaling pathway involving the phosphorylation of the insulin receptor substrate (IRS) proteins, and the activation of the phosphatidylinositol (PI) 3-kinase, Akt (also known as protein kinase B), the molecular target of rapamycin (mTOR), and p70 S6 kinase[5,6]. The insulin receptor tyrosine kinase phosphorylates the IRS proteins, and phosphotyrosine residues on IRS proteins become good targets for the p85 regulatory subunit of PI3-kinase. The activated PI3-kinase generates 3′-phosphoinositides [phosphatidyl-inositol-3,4-bisphosphate (PIP2) and phosphatidyl-inositol-3,4,5-trisphosphate (PIP3)][7], which bind to the phosphoinositidedependent kinase 1 (PDK1). Known substrates of the PDKs are the protein kinase B (PKB) and also atypical forms of the protein kinase C (PKC)[8].
PKB
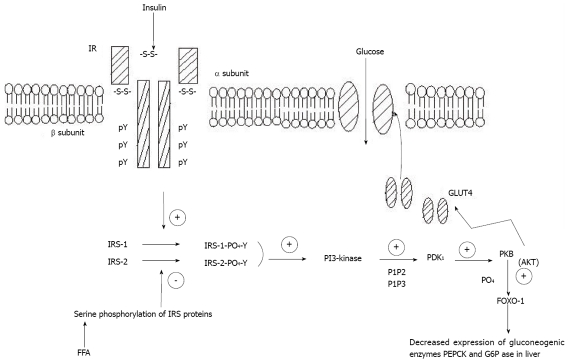
Downstream from PI3-kinase, the serine/threonine kinase Akt (also called PKB), triggers insulin effects on the liver, such as glycogen synthesis and the suppression of hepatic glucose production. Akt plays an important role by linking glucose transporter (GLUT4), the insulin-dependent glucose transporter protein, to the insulin signaling pathway. It activates GLUT4 which moves to the cell surface to transport glucose into the cell[9-11]. Recent data from PKB knockout animal models offer a clearer answer to the question of whether PKB is required for normal glucose homeostasis. While disruption of PKB/Akt1 isoform in mice did not cause any significant perturbations in metabolism, mice with a knock-out of the PKB (Akt2) isoform show insulin resistance, ending up with a phenotype closely resembling type 2 diabetes in humans[12-13]. Consistently, recent studies on inherited insulin post-receptor mutations in humans detected a missense mutation in the kinase domain of PKB (Akt2) in a family of severely insulin resistant patients. The mutant kinase was unable to phosphorylate downstream targets and to mediate inhibition of phosphoenolpyruvate carboxykinase (PEPCK), a gluconeogenic key enzyme[14]. This suggests that the impairment of insulin activity leading to insulin resistance is linked to insulin signaling defects. These insulin signaling pathways are shown in Figure 1.
Figure 1.
Insulin signaling pathway showing binding of insulin with the insulin receptor leading to the activation of glucose transporter 4 which imports glucose into the cell. Binding of insulin to the IR activates PI3-k which produces PI4, 5P2 and PI3, 4, 5P3. These serve as docking sites for PDK1 which then mediates activation of PKB. Activated PKB can regulate transcription of target genes-PEPCK and G6Pase via Foxo-1. Increased free fatty acids may cause serine phosphorylation of IRS proteins, which in turn decreases IRS-tyrosine phosphorylation, impairing downstream effectors. pY: phosphorylated tyrosine; IR: insulin receptor; IRS: insulin receptor protein; PI3-k: phosphatidylinositol 3-kinase; PDK1: phosphoinositidedependent kinase 1; PKB: protein kinase B; Foxo-1: forkhead box protein O; PEPCK: phosphoenolpyruvate carboxykinase; G6Pase: glucose-6-phosphatase; FFA: free fatty acids; PIP2: phosphatidyl-inositol-3,4-bisphosphate; PIP3: phosphatidyl-inositol-3,4,5-tris-phosp-hate; GLUT4: glucose transporter 4.
MUTATIONS IN IRS PROTEINS
In humans, rare mutations of the IRS-1 protein are associated with insulin resistance[15]. Disruption of the IRS-1 gene in mice results in insulin resistance, mainly of muscle and fat[16]. Interesting results are obtained by studying IRs in knockout mice. Heterozygous knockout mice lacking a single allele of IRS-1 gene lack any significant phenotype, whereas homozygous disruption of the IRS-1 gene results in a mild form of insulin resistance[17]. IRS-1 homozygous null mice (IRS-1-/-) do not show a clear diabetic phenotypic expression, presumably because of pancreatic β cell compensation. IRS-2-/- mice, on the other hand, developed diabetes as a result of severe insulin resistance paired with β cell failure[18,19].
INCREASE IN SERINE PHOSPORYLATION OF IRS PROTEINS
Recent studies have made it apparent that serine phosphorylation of IRS proteins can reduce the ability of IRS proteins to attract PI3-kinase, thereby minimizing its activation[20-25], and can also lead to an accelerated degradation of the IRS-1 protein[26]. This serine phosphorylation in turn decreases IRS-1 tyrosine phosphorylation, impairing downstream effectors[27]. Serine phosphorylation of IRS proteins can occur in response to a number of intracellular serine kinases[28]. The causes of serine phosphorylation of IRS-1 proteins are shown in Table 1.
Table 1.
Causes of insulin receptor substrate-1 serine phosphorylation
| mTOR |
| p70S6 kinase |
| Amino acids |
| Hyperinsulinemia |
| JNK |
| Stress |
| Hyperlipidemia |
| Inflammation |
| IKK |
| Inflammation |
| TNF-α |
| Obesity |
| Inflammation |
| Mitochondrial dysfunction |
| PKCθ |
| Hyperglycemia |
| Diacylglycerol |
| Inflammation |
mTOR: molecular target of rapamycin; JNK: c-Jun N-terminal kinase; IKK: IκB kinase; TNF: tumour necrosis factor; PKC: protein kinase C.
Recent studies have demonstrated hyper-serine phosphorylation of IRS-1 on Ser302, Ser307, Ser612, and Ser632 in several insulin-resistant rodent models[23,29-31] as well as in young lean insulin-resistant offspring of type 2 diabetic parents[32]. Further evidence for this hypothesis stems from recent studies in a muscle-specific triple serine to alanine mutant mouse (IRS-1 Ser → Ala302, Ser → Ala307, and Ser → Ala612), which has been shown to be protected from high-fat diet-induced insulin resistance in vivo[33]. Based on in vitro studies, serine phosphorylation may lead to dissociation between the insulin receptor/IRS-1 and/or IRS-1/PI3-kinase, preventing PI3-kinase activation[34,35] or increased degradation of IRS-1[36]. Furthermore, there are data linking IRS dysfunction in skeletal muscle to adipocyte biology and lipotoxicity. For example, circulating free fatty acids (FFA) and the adipokine tumour necrosis factor (TNF) may increase serine phosphorylation of IRS proteins, thereby causing impaired insulin signal transduction[37].
FORKHEAD BOX PROTEIN O-1
The fasting hyperglycemia in patients with type 2 diabetes is the clinical correlate of the increased glucose production by the liver because of insulin resistance. This is the result of the lack of inhibition of the two key gluconeogenic enzymes, PEPCK and the glucose-6-phosphatase (G6Pase) catalytic subunit. There is increasing evidence that Foxo-proteins are critically involved in the insulin dependent regulation of gluconeogenic gene expression and insulin-resistance in vivo[38,39]. Studies in hepatoma cells[40,41] suggest that transcription of reporter genes containing insulin response elements from the PEPCK and G6Pase promoters are regulated by forkhead box protein o (Foxo)-1 and 3. Furthermore, Foxo1 is phosphorylated in an insulin-responsive manner by Akt. Reduced activity of Akt2 results in decreased phosphorylation of Foxo protein, allowing it to enter the nucleus and activate the transcription of these rate-controlling enzymes of gluconeogenesis[40,42].
PI3 KINASE
A molecular mechanism that may potentially lead to insulin resistance is a disruption in the balance between the amounts of the PI3-kinase subunits[43]. The PI3-kinase family is divided into three different classes, of which class 1a[44] exists as heterodimers, consisting of a regulatory subunit p85, which is tightly associated with a catalytic subunit, p110. Normally, the regulatory subunit exists in stoichiometric excess to the catalytic one, resulting in a pool of free p85 monomers not associated with the p110 catalytic subunit. However, there exists a balance between the free p85 monomer and the p85-p110 heterodimer, with the latter being responsible for the PI3-kinase activity[45,46]. Because the p85 monomer and the p85-p110 heterodimer compete for the same binding sites on the tyrosine-phosphorylated IRS proteins, an imbalance could cause either increased or decreased PI3-kinase activity[47]. This is recently suggested by studies which showed that human placental growth hormone causes severe insulin resistance by specifically increasing the expression of the p85α subunit and subsequently affecting the ability of insulin to stimulate the association of the p85-p110 heterodimer with IRS-1, thus reducing the PI3-kinase insulin signaling[48]. Other studies in insulin-resistant states induced by obesity, type 2 diabetes[49] and short-term overfeeding of lean nondiabetic women[50] have also supported these findings. Additionally, Barbour and colleagues[51] have demonstrated that pregnancy-induced insulin resistance is probably due to increased expression of skeletal muscle p85 in response to increased concentrations of the human placental growth hormone. Furthermore, women who remain insulin resistant postpartum have been found to display higher levels of p85 in their muscle tissue[52].
PKC
The underlying mechanism of FFA-induced impairment of insulin signals is still unclear. The molecular mechanism underlying defective insulin-stimulated glucose transport activity can be attributed to increases in intramyocellular lipid metabolites such as fatty acyl CoAs and diacylglycerol, which in turn activate a serine/threonine kinase cascade, thus leading to defects in insulin signaling through the Ser/Thr phosphorylation of the insulin receptor substrate-1[53]. Diacylglycerol (DAG) has been shown to increase in muscle during both lipid infusions and fat feeding and it is also a known activator of novel PKC isoforms[53]. Some of the PKC isoforms represent such signaling molecules. PKC isoforms are classified as classical (cPKCα, βI, βII, γ), novel (nPKC δ, ε, θ, η) or atypical (aPKC ζ, λ). cPKCs are activated by Ca2+ and DAG, nPKCs are activated only by DAG and aPKCs respond to neither Ca2+ nor DAG[54]. Among all these PKC isoforms, nPKCs are said to have a modulatory role in insulin signaling. Recent reports also demonstrate a link between nPKCs and FFA induced insulin resistance; lipid infusion in rats and humans impaired insulin-stimulated glucose disposal into the muscle and concomitantly activated PKCθ and PKCδ[55,56]. The latter has been shown to be a possible candidate for phosphorylation of the IR on serine residues[57], resulting in defects in the insulin signaling pathway and imposing insulin resistance.
Clearly, the IR is one of the major targets in FFA-induced impairment of insulin activity. Studies performed in vivo have suggested that glucose uptake rather than intracellular glucose metabolism is the rate-limiting step for fatty acid-induced insulin resistance in humans[58]. This indicates a mechanism in which accumulation of intracellular fatty acids or their metabolites results in an impairment of signaling through the IRS/PI3-kinase and a decrease in the recruitment of GLUT4 transporters to the cell membrane.
PDK1 can directly phosphorylate all PKCs including nPKCs[59]. The PKCε isotype has been shown to be related to insulin resistance. PKCε has also shown PDK1-independent phosphorylation due to FFA[60,61]. This may be due to constitutive phosphorylation of PKCε by FFA in a PDK1-independent manner. It was shown that myristic acid incubation of HEPG2 cells causes myristoylation of PKCε which results in constitutive phosphorylation of PKCε at thr566/ser729 in the kinase domain required for PKCε activity. This phosphorylation was totally independent of PDK1, which was demonstrated by the researchers by using PDK1 knockout cells. In the same way, addition of palmitate to skeletal muscle cells or adipocytes may affect palmitoylation of PKCε, resulting in constitutive phosphorylation of PKCε[60,61]. Taken together, it is clear that FFA causes PDK1-independent phosphorylation of PKCε, which in turn translocates to the nucleus, and its time of entry into the nucleus coincides with the inhibition of IR gene transcription.
MOLECULAR MECHANISM OF INHIBITION OF IR GENE TRANSCRIPTION
In order to understand the molecular basis of the regulation of IR gene expression, the promoter region of the human IR gene has been identified and studied by several groups[62]. Two unique AT-rich sequences, C2 and E3, within the IR gene promoter have been identified, and both these sequences are positively regulated by transcription factor HMGA1 (earlier known as HMG1-Y)[63]. HMGA1 interacts with the AT rich regions and regulates transcriptional activation of many genes by modifying DNA conformation, which permits recruitment of transcriptional factor to the transcription start site[64,65]. HMGA1 induces transcriptional activation of the human IR gene by permitting the recruitment of SP1 and cEBPβ, the ubiquitously expressed transcription factors, to the promoter region. A recent report demonstrates that a genetic flaw which reduces the intracellular expression of HMGA1 protein can adversely affect IR expression in cells and tissues from subjects with insulin resistance and type 2 diabetes[66]. There is also a possibility that activated PKCε phosphorylates HMGA1, which inhibits its mobilization to the promoter region IR gene. It has been shown that phosphorylation of the HMGA1 protein reduces its DNA-binding ability[67]. Without the mobilization of HMGA1 to the IR promoter there is no recruitment of additional transcription factors to the promoter region of the IR gene and therefore no expression of the IR gene.
PGC-1
The PPARγ co-activator-1 (PGC-1) has been recognized as playing a major role in glucose homeostasis of the organism. Work mainly by Spiegelman’s group demonstrated the crucial role of PGC-1 in the regulation of GLUT4 gene expression in muscle cells. They showed that PGC-1 powerfully induces the expression of the endogenous GLUT4 gene in cultured myotubes, resulting in expression comparable to that seen in muscle in vivo[68]. In addition, PGC-1, a factor integrating the effects of glucocorticoids and cAMP on gluconeogenic gene expression in the liver[69,70] is also regulated by Akt and Foxo-1[71]. PGC-1 may also play a role in the regulation of genes involved in the process of oxidative phosphorylation which commonly show reduced expression in the muscles of diabetic patients[72].
OTHER CAUSES OF INSULIN RESISTANCE
Mitochondrial dysfunction
It has been known for many years that severe mitochondrial dysfunction can result in diabetes[73]. In a study using 13C/31P MRS, it was found that in healthy lean elderly volunteers with severe muscle insulin resistance, there is ~40% reduction in the rates of oxidative phosphorylation activity associated with increased intramyocellular and intrahepatic lipid content[74]. This study suggests that an acquired loss of mitochondrial function associated with aging predisposes elderly subjects to intramyocellular lipid accumulation, which results in insulin resistance[53]. Further, it was found that mitochondrial density was reduced by 38%, intramyocellular lipid content was increased by 60% and serine phosphorylation of IRS-1 was increased by 50% in the young insulin-resistant offspring of type 2 diabetes parents[32].
Adipokines
Insulin has three major target tissues-skeletal muscle, adipose tissue and the liver. Not only is IR overexpressed in the cells of these tissues, but these are also the three places where glucose is deposited and stored; no other tissue can store glucose. About 75% of insulin-dependent postprandial glucose disposal occurs into the skeletal muscle[75]; it is therefore the major target organ. Patients suffering from insulin resistance and type 2 diabetes frequently display signs of abnormal lipid metabolism, increased circulatory concentration and elevated deposition of lipids in the skeletal muscle[76]. Increase in plasma FFA reduces insulin-stimulated glucose uptake, whereas a decrease in plasma lipid content improves insulin activity in the skeletal muscle cells, adipocytes and liver[77]. Studies have shown that raising plasma fatty acids in both rodents[78] and humans[79] abolishes insulin activation of IRS-1-associated PI3-kinase activity in skeletal muscle where IRS-1 is most prevalent. Lipid-associated insulin resistance has also been shown to be linked to GLUT4 translocation defects[9].
Adipose tissue also acts as an endocrine organ producing adipokines which modulate glucose homeostasis[80]. Currently, those most intensely discussed are TNF-α, leptin, adiponectin and resistin. At a molecular level, TNF-α increases serine phosphorylation of IRS-1 and down-regulates GLUT4 expression, thereby contributing to insulin resistance[81]. Furthermore, mice lacking functional TNF-α were protected from obesity-induced insulin resistance[82]. The role of leptin in regulating food intake and energy expenditure is well established. Humans with leptin deficiency or leptin receptor mutations are severely obese[83,84]. In addition, it has direct effects on insulin sensitivity and may also reverse insulin resistance in mice with congenital lipodystrophy[85]. Adiponectin has insulin-sensitizing effects, as it enhances inhibition of hepatic glucose output as well as glucose uptake and utilization in fat and muscle. The expression of adiponectin is decreased in obese humans and mice[86]. Thus, in humans, adiponectin levels correlate with insulin sensitivity. Because of its insulin-antagonistic effects, the adipokine resistin has attracted a lot of primarily preclinical research interest. Resistin decreases insulin-dependent glucose transport in vitro and increases fasting blood glucose concentrations and hepatic glucose production in vivo[87,88].
CONCLUSION
In this review, we have summarized current developments contributing to our understanding of insulin resistance, and to the pathogenesis of type 2 diabetes. Among the many molecules involved in the intracellular processing of the signal provided by insulin, IRS-2, PKB, the Foxo protein and p85 regulatory subunit of PI-3 kinase have attracted particular interest, because their dysfunction results in insulin resistance in vivo. The identification of signaling defects and an understanding of the complex relationship of the different factors modulating insulin sensitivity is an important prerequisite for the development of novel and more specific anti-diabetic compounds. By elucidating the cellular and molecular mechanisms responsible for insulin resistance, these studies provide potential new targets for the treatment and prevention of type 2 diabetes.
Footnotes
Peer reviewer: Filip K Knop, MD, PhD, Department of Internal Medicine F, Gentofte Hospital, University of Copenhagen, Hellerup, DK 2900, Denmark
S- Editor Zhang HN L- Editor Herholdt A E- Editor Liu N
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saltiel RA. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 4.White MF, Shoelson SE, Keutmann H, Kahn CR. A cascade of tyrosine autophosphorylation in the beta-subunit activates the phosphotransferase of the insulin receptor. J Biol Chem. 1988;263:2969–2980. [PubMed] [Google Scholar]
- 5.Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepherd PR, Navé BT, Siddle K. Insulin stimulation of glycogen synthesis and glycogen synthase activity is blocked by wortmannin and rapamycin in 3T3-L1 adipocytes: evidence for the involvement of phosphoinositide 3-kinase and p70 ribosomal protein-S6 kinase. Biochem J. 1995;305(Pt 1):25–28. doi: 10.1042/bj3050025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 8.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, et al. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 10.Kupriyanova TA, Kandror KV. Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. J Biol Chem. 1999;274:1458–1464. doi: 10.1074/jbc.274.3.1458. [DOI] [PubMed] [Google Scholar]
- 11.Martin S, Millar CA, Lyttle CT, Meerloo T, Marsh BJ, Gould GW, James DE. Effects of insulin on intracellular GLUT4 vesicles in adipocytes: evidence for a secretory mode of regulation. J Cell Sci. 2000;113 Pt 19:3427–3438. doi: 10.1242/jcs.113.19.3427. [DOI] [PubMed] [Google Scholar]
- 12.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 13.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 14.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitehead JP, Humphreys P, Krook A, Jackson R, Hayward A, Lewis H, Siddle K, O'Rahilly S. Molecular scanning of the insulin receptor substrate 1 gene in subjects with severe insulin resistance: detection and functional analysis of a naturally occurring mutation in a YMXM motif. Diabetes. 1998;47:837–839. doi: 10.2337/diabetes.47.5.837. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, Takahashi Y, Yoshizawa F, Aizawa S, Akanuma Y, et al. Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol. 1996;16:3074–3084. doi: 10.1128/mcb.16.6.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araki E, Lipes MA, Patti ME, Brüning JC, Haag B 3rd, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 18.Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- 19.Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105:199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao LY, Goldberg JL, Russell JC, Sun XJ. Identification of enhanced serine kinase activity in insulin resistance. J Biol Chem. 1999;274:10625–10632. doi: 10.1074/jbc.274.15.10625. [DOI] [PubMed] [Google Scholar]
- 21.White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 22.Birnbaum MJ. Turning down insulin signaling. J Clin Invest. 2001;108:655–659. doi: 10.1172/JCI13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 24.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 25.Qiao LY, Zhande R, Jetton TL, Zhou G, Sun XJ. In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin-resistant rodents. J Biol Chem. 2002;277:26530–26539. doi: 10.1074/jbc.M201494200. [DOI] [PubMed] [Google Scholar]
- 26.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 28.Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes. 2006;55:2392–2397. doi: 10.2337/db06-0391. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morino K, Neschen S, Bilz S, Sono S, Tsirigotis D, Reznick RM, Samuel V, Philbrick WM, Shulman GI. IRS-1 serine phosphorylation is a key molecular event in the pathogenesis of fat-induced insulin resistance in skeletal muscle in vivo (Abstract) Diabetes. 2005;54 Suppl 1:A339. [Google Scholar]
- 34.Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Häring HU, Lehmann R. Protein kinase C-zeta-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem. 2004;279:25157–25163. doi: 10.1074/jbc.M402477200. [DOI] [PubMed] [Google Scholar]
- 35.Li J, DeFea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. J Biol Chem. 1999;274:9351–9356. doi: 10.1074/jbc.274.14.9351. [DOI] [PubMed] [Google Scholar]
- 36.Egawa K, Nakashima N, Sharma PM, Maegawa H, Nagai Y, Kashiwagi A, Kikkawa R, Olefsky JM. Persistent activation of phosphatidylinositol 3-kinase causes insulin resistance due to accelerated insulin-induced insulin receptor substrate-1 degradation in 3T3-L1 adipocytes. Endocrinology. 2000;141:1930–1935. doi: 10.1210/endo.141.6.7516. [DOI] [PubMed] [Google Scholar]
- 37.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 38.Nakae J, Biggs WH 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J. 2004;378:839–849. doi: 10.1042/BJ20031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall RK, Yamasaki T, Kucera T, Waltner-Law M, O'Brien R, Granner DK. Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin. The role of winged helix/forkhead proteins. J Biol Chem. 2000;275:30169–30175. doi: 10.1074/jbc.M004898200. [DOI] [PubMed] [Google Scholar]
- 41.Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 42.Wolfrum C, Besser D, Luca E, Stoffel M. Insulin regulates the activity of forkhead transcription factor Hnf-3beta/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. Proc Natl Acad Sci USA. 2003;100:11624–11629. doi: 10.1073/pnas.1931483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueki K, Fruman DA, Brachmann SM, Tseng YH, Cantley LC, Kahn CR. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol Cell Biol. 2002;22:965–977. doi: 10.1128/MCB.22.3.965-977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shepherd PR, Withers DJ, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333(Pt 3):471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mauvais-Jarvis F, Ueki K, Fruman DA, Hirshman MF, Sakamoto K, Goodyear LJ, Iannacone M, Accili D, Cantley LC, Kahn CR. Reduced expression of the murine p85α subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. J Clin Invest. 2000;109:141–149. doi: 10.1172/JCI13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueki K, Fruman DA, Yballe CM, Fasshauer M, Klein J, Asano T, Cantley LC, Kahn CR. Positive and negative roles of p85 alpha and p85 beta regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J Biol Chem. 2003;278:48453–48466. doi: 10.1074/jbc.M305602200. [DOI] [PubMed] [Google Scholar]
- 47.Giorgino F, Pedrini MT, Matera L, Smith RJ. Specific increase in p85alpha expression in response to dexamethasone is associated with inhibition of insulin-like growth factor-I stimulated phosphatidylinositol 3-kinase activity in cultured muscle cells. J Biol Chem. 1997;272:7455–7463. doi: 10.1074/jbc.272.11.7455. [DOI] [PubMed] [Google Scholar]
- 48.Barbour LA, Shao J, Qiao L, Leitner W, Anderson M, Friedman JE, Draznin B. Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology. 2004;145:1144–1150. doi: 10.1210/en.2003-1297. [DOI] [PubMed] [Google Scholar]
- 49.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes. 2005;54:2351–2359. doi: 10.2337/diabetes.54.8.2351. [DOI] [PubMed] [Google Scholar]
- 50.Cornier MA, Bessesen DH, Gurevich I, Leitner JW, Draznin B. Nutritional upregulation of p85alpha expression is an early molecular manifestation of insulin resistance. Diabetologia. 2006;49:748–754. doi: 10.1007/s00125-006-0148-0. [DOI] [PubMed] [Google Scholar]
- 51.Barbour LA, Mizanoor Rahman S, Gurevich I, Leitner JW, Fischer SJ, Roper MD, Knotts TA, Vo Y, McCurdy CE, Yakar S, et al. Increased P85alpha is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J Biol Chem. 2005;280:37489–37494. doi: 10.1074/jbc.M506967200. [DOI] [PubMed] [Google Scholar]
- 52.Kirwan J, Varastehpour A, Jing M, Presley L, Shao J, Friedman JE, Catalano PM. Reversal of insulin resistance post-partum is linked to enhanced skeletal muscle insulin signaling. J Clin Endocrinol Metab. 2004;89:4678–4684. doi: 10.1210/jc.2004-0749. [DOI] [PubMed] [Google Scholar]
- 53.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 Suppl 2:S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32 Suppl 3:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 56.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 57.Strack V, Stoyanov B, Bossenmaier B, Mosthaf L, Kellerer M, Häring HU. Impact of mutations at different serine residues on the tyrosine kinase activity of the insulin receptor. Biochem Biophys Res Commun. 1997;239:235–239. doi: 10.1006/bbrc.1997.7457. [DOI] [PubMed] [Google Scholar]
- 58.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toker A, Newton A C. Cellular signaling: pivoting around PDK-1. Cell. 2000;103:185–188. doi: 10.1016/s0092-8674(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 60.Dey D, Basu D, Roy SS, Bandyopadhyay A, Bhattacharya S. Involvement of novel PKC isoforms in FFA induced defects in insulin signaling. Mol Cell Endocrinol. 2006;246:60–64. doi: 10.1016/j.mce.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Dey D, Mukherjee M, Basu D, Datta M, Roy SS, Bandyopadhyay A, Bhattacharya S. Inhibition of insulin receptor gene expression and insulin signaling by fatty acid: interplay of PKC isoforms therein. Cell Physiol Biochem. 2005;16:217–228. doi: 10.1159/000089847. [DOI] [PubMed] [Google Scholar]
- 62.Lee JK, Tam JW, Tsai MJ, Tsai SY. Identification of cis- and trans-acting factors regulating the expression of the human insulin receptor gene. J Biol Chem. 1992;267:4638–4645. [PubMed] [Google Scholar]
- 63.Brunetti A, Manfioletti G, Chiefari E, Goldfine ID, Foti D. Transcriptional regulation of human insulin receptor gene by the high-mobility group protein HMGI(Y) FASEB J. 2001;15:492–500. doi: 10.1096/fj.00-0190com. [DOI] [PubMed] [Google Scholar]
- 64.Bustin M, Reeves R. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog. Nucleic Acids Res Mol Biol. 1996;54:35–100. doi: 10.1016/s0079-6603(08)60360-8. [DOI] [PubMed] [Google Scholar]
- 65.Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- 66.Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, Manfioletti G, Barbetti F, Brunetti A, Croce CM, et al. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat Med. 2005;11:765–773. doi: 10.1038/nm1254. [DOI] [PubMed] [Google Scholar]
- 67.Reeves R, Beckerbaur L. HMG 1/Y proteins: flexible regulators of transcription and chromatin structure. Biochem Biophys Acta Mol Cell Res. 2001;1519:13–29. doi: 10.1016/s0167-4781(01)00215-9. [DOI] [PubMed] [Google Scholar]
- 68.Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA. 2001;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 70.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 71.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 72.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 74.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klip A, Pâquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990;13:228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- 76.McGarry , Banting lecture. Dysregulation of fatty acid me–tabolism in the etiology of type 2 diabetes. Diabetes. 2001;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 77.Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 78.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 81.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 83.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 84.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 85.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 86.Stumvoll M, Häring H. Resistin and adiponectin--of mice and men. Obes Res. 2002;10:1197–1199. doi: 10.1038/oby.2002.162. [DOI] [PubMed] [Google Scholar]
- 87.Moon B, Kwan JJ, Duddy N, Sweeney G, Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am J Physiol Endocrinol Metab. 2003;285:E106–E115. doi: 10.1152/ajpendo.00457.2002. [DOI] [PubMed] [Google Scholar]
- 88.Pravenec M, Kazdová L, Landa V, Zidek V, Mlejnek P, Jansa P, Wang J, Qi N, Kurtz TW. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J Biol Chem. 2003;278:45209–45215. doi: 10.1074/jbc.M304869200. [DOI] [PubMed] [Google Scholar]