Abstract
Foot ulcers are common in diabetic patients, have a cumulative lifetime incidence rate as high as 25% and frequently become infected. The spread of infection to soft tissue and bone is a major causal factor for lower-limb amputation. For this reason, early diagnosis and appropriate treatment are essential, including treatment which is both local (of the foot) and systemic (metabolic), and this requires coordination by a multidisciplinary team. Optimal treatment also often involves extensive surgical debridement and management of the wound base, effective antibiotic therapy, consideration for revascularization and correction of metabolic abnormalities such as hyperglycemia. This article focuses on diagnosis and management of diabetic foot infections in the light of recently published data in order to help clinicians in identification, assessment and antibiotic therapy of diabetic foot infections.
Keywords: Diabetic foot ulcer, Infection, Management
INTRODUCTION
Foot ulcers are common in diabetic patients with prevalence as high as 25%[1]. Infection is a frequent (40%-80%) and costly[2] complication of these ulcers and represents a major cause of morbidity and mortality. It is estimated to be the most common cause of diabetes-related admission to hospital and remains one of the major pathways to lower-limb amputation[3-5]. A recent report estimated that the risk of hospitalization and lower-extremity amputation was ≈ 56 and 155 times greater for diabetic people who had a foot infection than for those without, respectively[6]. In the same report, 9.1% of the 1 666 consecutively enrolled patients in a program of prevention and treatment of diabetic foot complications developed a foot infection over a 2-year period. Most infections involved soft tissue but 20% of patients with foot infection had bone culture-proven osteomyelitis. In the Eurodiale study, 58% of 1 229 diabetic patients consecutively attending one of 14 foot clinics in ten European countries with a new foot ulcer had a wound which was clinically infected[7]. In our own experience, prognosis of diabetic foot infection (DFI) remains poor as nearly half of patients admitted in specialized French foot clinics for DFI had some forms of lower-limb amputation and 23 of the 291 patients included into this study died during the one year study period[8].
A prior foot wound is an almost obligatory prerequisite for DFI even though the wound may have closed over before the time of presentation in some cases[6,9]. A hematogeneous origin is very rare. The clinical diagnosis of infection may, however, be made difficult. Indeed, the presence of peripheral arterial disease, neuropathy or impaired leukocyte functions may reduce the local inflammatory response and classical signs or symptoms of local infection[10,11]. Moreover, systemic signs of toxicity such as leukocytosis or fever may be lacking or appear late, even in severe cases[12-14]. Thus, neither local nor systemic inflammatory signs or symptoms and biological markers should be regarded as reliable for diagnosing foot infection in diabetic individuals. Other aspects of DFI are still open to debate, as recently emphasized by Ulbrecht and colleagues[15]: how to differentiate infection from colonization, when and how to do bacterial cultures, when to use antibiotic treatment and for how long?
DIFFERENTIATING INFECTION FROM COLONIZATION
Clinical findings
As all chronic wounds are colonized by microorganisms, the diagnosis of DFI should not be based on the microbiological analysis of a wound culture but on clinical findings[5,9,16]. The International Working Group on the Diabetic Foot (IWGDF) and the Infectious Diseases Society of America (IDSA) have developed clinical criteria for recognizing and classifying the severity of DFI (Table 1)[3,17]. These criteria have been recently endorsed by the French-speaking Society of Infectious Diseases[9]. Briefly, DFI is defined by the presence at least two inflammatory manifestations (purulence or erythema, pain, tenderness, warmth or induration) and divided in three grades of severity from mild (grade 2) to severe (grade 4) infection, according to the extent of tissue involvement and presence of systemic toxicity or metabolic derangement. This classification system has been validated by a prospective study[18]. Nevertheless, as mentioned above, clinical signs or symptoms of foot wound infection may be much reduced in diabetic patients. This means that reliance on these signs for diagnosis may result in some infections being undetected when they first present. The resultant delay in starting treatment may lead to progression of infection from limited to severe and limb-threatening. This progression is often rapid because of associated ischemia, diabetic immunopathy and the particular anatomic characteristics of the foot[16]. On the other hand, the routine administration of antibiotic therapy to all patients with diabetic foot ulcers (DFUs), including those that are clinically uninfected, favors the emergence of antimicrobial resistance, increases financial costs and may cause adverse events[3]. Experts in the field discourage the use of antibiotics in clinically uninfected ulcers[3,5,9,17,19].
Table 1.
International consensus on the diabetic foot classification of foot wound infections (adapted from reference [17])
| Grade 1 | No symptoms, no signs of infection |
| Grade 2 | Lesion only involving the skin (no subcutaneous tissue lesion or systemic disorders) with at least two of the following signs: |
| Local warmth | |
| Erythema > 0.5 cm - 2 cm around the ulcer | |
| Local tenderness or pain | |
| Local swelling or induration | |
| Purulent discharge (thick, opaque to white or sanguineous secretion) | |
| Other causes of inflammation of the skin must be eliminated (for example: trauma, gout, acute Charcot foot, fracture, thrombosis, venous stasis) | |
| Grade 3 | Erythema > 2 cm and one of the findings described above |
| or | |
| Infection involving structures beneath the skin and subcutaneous tissue, such as deep abscess, lymphangitis, osteomyelitis, septic arthritis or fasciitis | |
| There must not be any systemic inflammatory response (see Grade 4) | |
| Grade 4 | Regardless of the local infection, in the presence of systemic signs corresponding to at least two of the following characteristics: |
| Temperature > 39°C or < 36°C | |
| Pulse > 90 bpm | |
| Respiratory rate > 20/min | |
| PaCO2 < 32 mmHg | |
| Leukocytes > 12 000 or < 4 000/mm3 | |
| 10% of immature leukocytes |
Various factors have been proposed as being suggestive of the presence of DFI when classical signs are not obvious. These include the identification of friable granulation tissue, delay in healing despite otherwise adequate ulcer management and the occurrence of an unexplained hyperglycemia[20-22]. From a practical point of view, however, such signs are either too subtle or too non-specific for general use. Recently Lipsky et al[23] developed a 10 item DFI wound score incorporating semi-quantitative grading of both wound measurements and various infection parameters. This score could be a reliable and useful tool for predicting clinical outcomes.
Biochemical parameters
The measurement of circulating markers of inflammation is usually regarded as lacking sensitivity and specificity to reliably identify infection: the sedimentation rate and leukocyte count are normal in approximately 50% of cases, even in severe deep infections. In contrast, measurement of some other inflammatory markers might be of value in discriminating between infected and non-infected DFUs. They could help in the more rationale use of antibiotic agents[24]. In diabetic patients with clinically uninfected ulcers, circulating levels of orosomucoid, haptoglobin, serum albumin, C-reactive protein (CRP) and procalcitonin (PCT) did not significantly differ from those found in non-ulcerated diabetic controls. They were statistically lower than those in diabetic patients with infected ulcers. However, the differences were unrelated to the severity of the infection. The combination of CRP and PCT measurement appeared to differentiate uninfected and infected ulcers: using a computer-derived formula combining CRP and PCT circulating levels, a cut-off value could discriminate grade 1 (uninfected) from grade 2 (mildly infected) ulcers with a sensitivity and a specificity of 0.91 and 0.83 respectively[24].
Assessment of bacterial load
As there may be a continuum from colonization to infection, some experts have advocated quantification of the bacterial burden as a means of assessing the clinical significance of bacteria which are known to be present on the wound surface. It has been suggested that a bacterial concentration greater than 105 colony-forming units (CFU) per gram or mm3 of tissue[25,26] indicates the presence of “critical colonization”- a degree of colonization which host defenses are no longer able to contain[21]. Critical colonization may itself hinder healing or may be a precursor of spreading clinical infection. Nevertheless, the use of a bacterial colony count to define infection and decide antibiotic therapy is neither useful nor practical. Some authorities argue that the identity of specific pathogens is more important than the density of microorganisms[26]. It is also possible that (1) a high bacterial colony count may itself be the consequence of other factors (tissue hypoxia related to ischemia, diabetic immunopathy, etc.) and it is these which are really responsible for impaired healing; and (2) the relationship between colonization and impaired healing is one of association and not truly causal. Moreover, the variability of bacterial virulence factors must also be taken into account as well as the level of host resistance. The importance of individual virulence potential was recently illustrated by the Sidestep study: favorable clinical response to ertapenem was noticed in patients in whom Enteroccus spp. and Pseudomonas aeruginosa were isolated despite the ertapenem resistance of the latter isolates[27]. Ten years ago, Dow et al had already observed that β-hemolytic streptococci at 102 CFU per gram of tissue were able to induce tissue damage while a count greater than 105 CFU per gram of tissue of less pathogenic organisms was of little clinical significance[25]. The various organisms isolated from infected wounds do not have an identical pathogenetic importance and rather than counting bacterial load, evaluating the intrinsic virulence potential of isolated bacteria to identify their real pathogenicity seems a promising way. Moreover, quantification of the bacterial burden requires a tissue biopsy for each wound and this may be unrealistic in routine practice as well as potentially harmful to the patient. Rather than bacterial load, it is the type of identified bacteria that make their pathogenicity. Thus well-known virulence bacteria (S. aureus, β-hemolytic streptococci, Enterobacteriaceae, anaerobes) are implied in DFI. However low virulence bacteria including the well-described commensal flora such as coagulase-negative Staphylococcus, Corynebacterium sp. and Propionibacterium sp. together with Enterococcus sp., Stenotrophomonas maltophilia and Pseudomonas sp. could be pathogens. When there is a doubt, specimens must be repeated and these bacteria will be taken into consideration when they are isolated on several occasions in patients with clinical signs of DFI or when the patient’s septic state is worrisome.
When and how to undertake bacterial culture?
A multidisciplinary management of the patient is essential and requires good coordination between all health care professionals involved[28,29]. Organization of the diabetic foot clinic is essential, promoting a multidisciplinary strategy by a team made up of trained diabetologists, microbiologists and specialists in infectious diseases, radiologists, orthopedic and vascular surgeons, physiotherapists, podiatrists and dedicated nurses with regular meetings and easily available advice. In this multidisciplinary approach, the management of DFU, notably the best use of microbiological sampling, must be organized.
National and international consensus documents on DFI emphasize some essential points[3,5,17]: (1) DFI must be diagnosed on clinical signs and symptoms and must be always confirmed and classified by an expert in the field; (2) bacteriological sampling is only indicated if DFI is clinically confirmed, corresponding to grade 2-4 infections using the International Consensus grading system[3]; (3) before sampling, the wound must be mechanically debrided with a sterile curette or scalpel and cleansed using gauze soaked in sterile physiological saline (antiseptics can be used but they must be eliminated by sterile physiological saline before taking the specimen); (4) the best sampling technique remains a matter of debate[8,30,31]. Samples must be obtained by scrapping or curetting the wound base, by aspirating purulent secretion using a needle through healthy skin or by tissue biopsy as recommended in many guidelines[3,9,17,32]. Superficial swabbing of the wound is discouraged but swabbing the base of the ulcer (“deep swab technique”) is allowed if it is the only possible option. For osteomyelitis, performing a bone biopsy is promoted by surgery or percutaneously through healthy skin[9]; (5) sampling must be repeated only if the wound infection does not improve despite antibiotic treatment or if the patient remains frankly septic; (6) samples must be sent to the microbiology laboratory as rapidly as possible, requiring good collaboration between clinicians, nurses and couriers and use of adapted transport medium; and (7) only clinically infected DFU warrants microbiological sampling and antibiotic therapy[3,5,9,17].
Following these recommendations, the purpose of microbiological sampling is to identify the organisms which are likely to be responsible for infection and not to diagnose infection itself.
Since 2003, we have progressively changed our policy on managing patients with DFU in our University hospital. Regarding microbiological sampling, we changed our technique to using deep tissue sampling obtained by biopsy or curettage (scraping the debrided ulcer base after cleansing the wound) instead of superficial sampling (obtained by rolling a cotton swab across the surface of the wound). As a result, there was a striking decrease in the number of pathogens per sample from 4.1 to 1.9. In parallel, the recovery rate of Gram-negative bacilli decreased steadily, mirroring the increased rate of Gram-positive cocci[33]. Moreover, the prevalence rate of multidrug-resistant organisms (MDRO) dramatically and steadily decreased, halving from 2003 to 2007; the most important decrease was for methicillin-resistant Staphylococcus aureus (MRSA) whose prevalence was nearly cut down by three. Conversely, the prevalence rate of bacteria considered as low-virulence pathogens or commensal flora was cut down by two from 40.1% to 16.4%. From the point of view of health economics, cost-saving for our hospital was estimated to be at least €125 000 (~$170 000) due to a decrease in microbiology laboratory workload and a decreased prescription of extended-spectrum antibiotics (in line with a decrease of defined daily doses for those antibiotics) as a consequence of the reduction in MDRO prevalence. The decrease of amputation rate was also an indirect marker of the beneficial impact of the multidisciplinary approach and the efficiency of the guidelines.
Assessing bacterial virulence and resistance
Identification of the virulence power of bacteria might also be of value in the future routine diagnosis of infection. New technologies such as DNA micro-array and multiplex real-time PCR offer a unique opportunity to analyze both the virulence and resistance potential of microorganisms. This method of miniaturized genotyping can rapidly and reliably detect the presence of genes encoding for various virulence and antibiotic resistance factors[34,35]. Using this method, both virulence and resistance genes were far more often present in clinically infected diabetic foot wounds than in uninfected wounds[36]. In cultures from foot wounds from diabetic individuals who had not been exposed to recent antibiotic treatment and were positive only for S. aureus, we previously found that virulence factors were present in < 10% of isolates from patients with uninfected ulcers but in > 98% patients with ulcers that were clinically infected[36]. In addition, the presence of virulence factors at either presentation or at follow-up in patients with clinically uninfected wounds appeared to be predictive of a poor clinical outcome. In another study, we confirmed and extended our previous results using multiplex PCR assays to evaluate 31 of the most prevalent virulence-associated genes in S. aureus strains isolated from DFUs[37]. The combination of 5 genes (sea, sei, lukE, hlgv and cap8) was the most predictive for differentiating grade 1 (clinically uninfected) from grade 2-4 ulcers with a sensitivity and a specificity of 0.98 and 0.87 respectively. For discriminating infected from uninfected diabetic foot ulceration due to S. aureus, the determination of only five virulence-associated genes may be of practical value, especially as the method we used is relatively easy to perform, has low cost (~US$5 per assay) and gives rapidly available results. The observation of the coexistence of two S. aureus populations (a colonizing versus and an infecting one) in DFI whether or not they were methicillin-resistant is important for diagnosis and these populations are not linked to presence or absence of methicillin-resistance. These results confirm the importance of a good microbiological sampling method including the debridement of the wound as debridement decreases the bacterial load and contributes to remove the colonizing bacteria. Analysis of virulence potential (by genotyping methods) would be of value for the diagnosis of diabetic foot wounds in routine clinical practice.
ANTIBIOTIC TREATMENT: WHEN TO USE AND FOR HOW LONG
Systemic antibiotics must be given as early as possible in cases of clinically infected DFUs and the use of topical antibiotics and antiseptics is not recommended as the sole treatment of infection[9,17,38,39]. Nevertheless, many experts use additional topical metronidazole to control odor in patients with extensive tissue devitalization when anaerobic organisms are often involved. Moreover, a recent double blind randomized controlled trial has shown that pexiganan acetate cream, a synthetic antimicrobial cationic peptide analogue of the magainin 2 (a host defense peptide isolated from frog skin)[40], might be as effective as oral ofloxacin in promoting clinical improvement, microbial eradication and wound healing in mildly infected DFUs[41]. Silver-containing dressings have been advocated for use in wounds with superficial infection because of the antibacterial properties of silver ion but their efficacy in DFUs is far from being proved[42,43]. In conclusion, topical antimicrobial therapy, although not currently advisable for most clinically infected chronic wounds, does have a role in specific circumstances. It could be used: (1) in a properly managed wound that is failing to heal and presented subclinical infection; and (2) to help in the removal of biofilms which have been implicated in persistent infections[39].
Many systemically active antibiotic agents have demonstrable in vitro activity against strains isolated from DFI including newer preparations such as linezolid, ertapenem, doripenem, ceftobiprole, dalbavancin, daptomycin or tigecycline[44-48]. Moreover, randomized clinical trials have shown that many antibiotics are of clinical value in DFI[3] including the most recently available agents[3,49,50]. However, as emphasized by Lipsky et al and recently confirmed in a systematic review[27,32,49-51], no one particular antimicrobial agent or regimen has yet been shown to be superior to others in curing DFI.
The increasing prevalence of antibiotic-resistant bacteria in DFUs, particularly MRSA, both as colonizers or pathogens[52-54] is problematic. MRSA requires targeted antibiotic treatment and its involvement is generally considered to be associated with a poor outcome[55,56]. In a prospective study we recently conducted, S. aureus was the most common pathogen isolated, accounting for 36.5% of all isolates and 37.4% of these were MRSA. Overall, MRSA was isolated in 37 of the 188 (19.7%) patients included in the study[57]. Using multivariate analysis, however, we found that the presence of MRSA did not appear to have a significant effect on wound healing time, confirming some previously published studies[58-60]. Although diabetic foot clinics are considered as a major reservoir for MRSA, a recent study suggests that they do not play a key role in transmission and spread of MRSA[61]. Potential sources of acquisition of MRSA to patients may include prior hospitalizations and the nurses who provide care at home for dressing changes. There is, therefore, no evidence that colonization by MRSA requires specific intervention even though infection with MRSA requires early aggressive treatment, as in our study. Such treatment should be based on empiric broad-spectrum antibiotic treatment (including agents active against MRSA) and this should be later adjusted according to microbiological isolates, in vitro sensitivities and the clinical response to empiric therapy. Using this approach, it would appear that the isolation of MRSA - even from wounds, which are clinically infected - does not appear to be associated with a worse prognosis. Thus, the importance of early identification of MRSA in DFI is to ensure that clinicians engage in early aggressive appropriate therapy. Accordingly, new technologies (such as GeneXpert®, Cepheid SA) are developed to diagnose the presence of MRSA in less than 1 h.
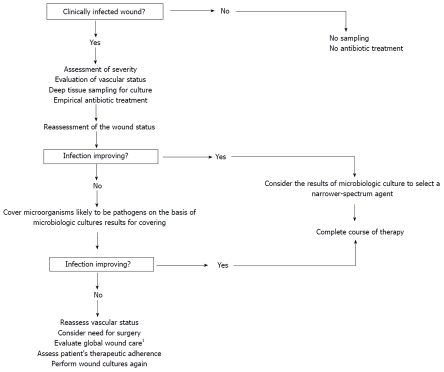
Initial antibiotic therapy is usually empirical and chosen without knowledge of initial microbiological culture results. The antibiotic regimen must always include an agent active against Gram-positive cocci, particularly S. aureus. Other factors must be taken into account in the selection of an appropriate antibiotic combination and the route of administration (Table 2): severity of infection, previous allergy or intolerance, patient compliance, renal and/or hepatic dysfunction, peripheral arterial disease and any devitalization of the tissues surrounding the wound, recent exposure to antibiotic therapy or hospital admission, chronicity of the wound, knowledge of local potential pathogens and antibiotic sensitivity patterns[62]. Once antibiotic treatment is initiated, the wound must be regularly and carefully inspected to assess if the infection is responding to treatment. If the clinical condition is improving, empirical therapy can be continued; however, if microbiological culture and sensitivity testing reveal that the initial regimen had an unnecessarily broad spectrum of activity, the most effective narrow-spectrum regimen should be selected. If the condition of the wound infection is not improving on empirical treatment, the choice of antibiotics should be adjusted on the basis of the results of microbiological culture (Figure 1).
Table 2.
Factors to be considered for antibiotic prescription in diabetic foot infection (adapted from reference [17])
| Criteria | Comments |
| Severity of infection | Broad-spectrum therapy via parenteral route for severe infection |
| Renal dysfunction | Avoid nephrotoxic agents (aminoglycosides, glycopeptides) |
| Hepatic dysfunction | Avoid hepatotoxic agents (macrolides, amoxicillin/clavulanate) |
| Ischemic limb | Use relatively high doses of oral antibiotics or prefer IV route to achieve adequate antibiotic level at the site of infection if revascularization procedure is unfeasible |
| Consider anti-anaerobic bacteria when there is ischaemia or extensive devitalized tissue | |
| Impaired gastrointestinal function (gastroparesis) | Prefer parenteral route |
| Local antibiotic resistance patterns | Cover MRSA if indicated |
| Drug allergies | Review patient's medical history carefully |
| History of recent antibiotic treatment | May need an extended coverage against gram-negative bacilli and Enterococcus |
| Chronicity of the wound | Give preference to broad-spectrum therapy initially |
| Poor therapeutic compliance | Consider IV route and/or hospitalization |
MRSA: methicillin-resistant Staphylococcus aureus.
Figure 1.
Algorithm for selecting antibiotic treatment in diabetic patients with clinically infected foot wound. 1Including off-loading, wound care treatment (debridement, dressings) and glycemic control.
Some uncertainty surrounds the need to cover less virulent bacteria like coagulase-negative staphylococci and corynebacteria (as well as organisms such as P. aeruginosa or enterococci which do not usually behave in a pathogenic way in DFUs). As a rule, antibiotic treatment of these bacteria is not generally thought to be necessary but if the clinical signs of infection do not improve (under prophylactic treatment), wound sampling should be repeated and if these organisms are isolated again or if the patient is critically ill, they should regarded as potential opportunistic pathogens and treated[9,63].
The optimal duration of antibiotic treatment is not clearly defined and depends on severity of infection and response to treatment[3,5,9,16,17,64]. Most authorities would suggest that one to 2 wk may suffice for mild infections whereas treatment must be extended for up to 1 mo for more severe infections.
Other approaches have been advocated in order to reduce the duration of antibiotic treatment while ensuring effective eradication of infection, rapid wound healing and prevention of lower-limb amputation.
Randomized clinical trials studied the possible effect of applying or injecting granulocyte-colony-stimulating factor (G-CSF) in infected diabetic ulcers[65-69] but the results are difficult to interpret due to differences in G-CSF formulations, severity of infection, route of administration, endpoints and the quality and design of the studies. According to a meta-analysis[70] and a recent Cochrane report[71], G-CSF treatment appeared to have no significant effect on duration of intravenous antibiotic treatment, resolution of infection or rate of wound healing but might reduce the risk of lower-limb amputation, other infection-related invasive interventions or the duration of hospitalization.
Systemic hyperbaric oxygen therapy (HBOT) has been also proposed as infection and ischemia are considered the two main indications for this procedure. It has been claimed that HBOT is effective in reducing amputations in diabetic patients with foot ulcers[72] and in facilitating healing of chronic DFU[73]. However, in this otherwise well-designed study, cases of infected ulcers are not specified and patients with acute infection were included, but once the acute period was resolved. So, it seems that available data are insufficient to justify use of HBOT[74,75] and the results of further large well-designed and more specific trials are needed. Without more robust evidence, the routine use of HBOT is not recommended, especially as it is both expensive and not widely available.
CONCLUSION
Infection of foot ulcers in diabetic patients is estimated to be the most common cause of diabetes-related admission to hospital and remains one of the major pathways to lower-limb amputation. For all these reasons, diabetic foot infections are a real public health problem and early diagnosis and appropriate treatment are essential. Identification and management of foot infection in diabetic patients are often problematic due to difficulty in (1) differentiating infection from colonization; (2) understanding the actual extent of the infectious process when suspected; (3) treating infection because of the increasing frequency of multidrug-resistant bacteria and altered pharmacokinetic properties of antibiotic agents due to poor quality of arterial supply to the foot; and (4) determining duration of therapy as the optimal duration of antibiotic treatment is not clearly defined and criteria for cure are poor, especially for osteomyelitis. However, development of clinical criteria for recognizing and classifying the severity of diabetic foot infection, optimization of sampling technique using deep tissue sampling, development of DNA micro-array and/or PCR technology to analyze both the virulence and resistance potential of microorganisms and development of new antibiotic agents active against S. aureus should help the clinician to resolve this challenge.
Acknowledgments
We thank all the members of Working Group on the Diabetic Foot (GP30).
Footnotes
Supported by Institut National de la Santé Et de la Recherche Médicale, the French Speaking Association for Diabetes and Metabolic Diseases (ALFEDIAM grant) and the University of Montpellier 1, the Languedoc-Roussillon Area (Chercheur d’avenir Grant) and the City of Nîmes
Peer reviewer: Rachel Mary Hudacko, MD, Department of Pathology and Laboratory Medicine, Robert Wood Johnson Medical School - UMDNJ, One Robert Wood Johnson Place, New Brunswick, NJ 08901, United States
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
Table 1 International consensus on the diabetic foot classification of foot wound infections (adapted from reference [17])
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Prompers L, Huijberts M, Schaper N, Apelqvist J, Bakker K, Edmonds M, Holstein P, Jude E, Jirkovska A, Mauricio D, et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia. 2008;51:1826–1834. doi: 10.1007/s00125-008-1089-6. [DOI] [PubMed] [Google Scholar]
- 3.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- 4.Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003;26:1435–1438. doi: 10.2337/diacare.26.5.1435. [DOI] [PubMed] [Google Scholar]
- 5.Jeffcoate WJ, Lipsky BA, Berendt AR, Cavanagh PR, Bus SA, Peters EJ, van Houtum WH, Valk GD, Bakker K. Unresolved issues in the management of ulcers of the foot in diabetes. Diabet Med. 2008;25:1380–1389. doi: 10.1111/j.1464-5491.2008.02573.x. [DOI] [PubMed] [Google Scholar]
- 6.Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29:1288–1293. doi: 10.2337/dc05-2425. [DOI] [PubMed] [Google Scholar]
- 7.Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18–25. doi: 10.1007/s00125-006-0491-1. [DOI] [PubMed] [Google Scholar]
- 8.Richard JL, Lavigne JP, Got I, Hartemann A, Malgrange D, Tsirtsikolou D, Baleydier A, Senneville E. Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study. Diabetes Metab. 2010:in press. doi: 10.1016/j.diabet.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Management of diabetic foot infections. Long text. Société de Pathologie Infectieuse de Langue Française. Med Mal Infect. 2007;37:26–50. doi: 10.1016/j.medmal.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Edmonds M. Infection in the neuroischemic foot. Int J Low Extrem Wounds. 2005;4:145–153. doi: 10.1177/1534734605280597. [DOI] [PubMed] [Google Scholar]
- 11.Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. Clin Infect Dis. 2004;39 Suppl 2:S83–S86. doi: 10.1086/383267. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong DG, Lavery LA, Sariaya M, Ashry H. Leukocytosis is a poor indicator of acute osteomyelitis of the foot in diabetes mellitus. J Foot Ankle Surg. 1996;35:280–283. doi: 10.1016/s1067-2516(96)80075-5. [DOI] [PubMed] [Google Scholar]
- 13.Eneroth M, Larsson J, Apelqvist J. Deep foot infections in patients with diabetes and foot ulcer: an entity with different characteristics, treatments, and prognosis. J Diabetes Complications. 1999;13:254–263. doi: 10.1016/s1056-8727(99)00065-3. [DOI] [PubMed] [Google Scholar]
- 14.Eneroth M, Apelqvist J, Stenström A. Clinical characteristics and outcome in 223 diabetic patients with deep foot infections. Foot Ankle Int. 1997;18:716–722. doi: 10.1177/107110079701801107. [DOI] [PubMed] [Google Scholar]
- 15.Ulbrecht JS, Cavanagh PR, Caputo GM. Foot problems in diabetes: an overview. Clin Infect Dis. 2004;39 Suppl 2:S73–S82. doi: 10.1086/383266. [DOI] [PubMed] [Google Scholar]
- 16.Lipsky BA. Infectious problems of the foot in diabetic patients. In: Levin and O’Neal’s The diabetic Foot (7th ed), editor. In: Bowker JH, Pfeifer MA, editors. Philadelphia: Mosby Elsevier; 2008. pp. 305–318. [Google Scholar]
- 17.Lipsky BA. A report from the international consensus on diagnosing and treating the infected diabetic foot. Diabetes Metab Res Rev. 2004;20 Suppl 1:S68–S77. doi: 10.1002/dmrr.453. [DOI] [PubMed] [Google Scholar]
- 18.Lavery LA, Armstrong DG, Murdoch DP, Peters EJ, Lipsky BA. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis. 2007;44:562–565. doi: 10.1086/511036. [DOI] [PubMed] [Google Scholar]
- 19.Chantelau E, Tanudjaja T, Altenhöfer F, Ersanli Z, Lacigova S, Metzger C. Antibiotic treatment for uncomplicated neuropathic forefoot ulcers in diabetes: a controlled trial. Diabet Med. 1996;13:156–159. doi: 10.1002/(SICI)1096-9136(199602)13:2<156::AID-DIA59>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Cutting KF, White RJ. Criteria for identifying wound infection--revisited. Ostomy Wound Manage. 2005;51:28–34. [PubMed] [Google Scholar]
- 21.Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. Clin Infect Dis. 2004;39 Suppl 2:S83–S86. doi: 10.1086/383267. [DOI] [PubMed] [Google Scholar]
- 22.Frykberg RG. An evidence-based approach to diabetic foot infections. Am J Surg. 2003;186:44S–54S; discussion 61S-64S. doi: 10.1016/j.amjsurg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Lipsky BA, Polis AB, Lantz KC, Norquist JM, Abramson MA. The value of a wound score for diabetic foot infections in predicting treatment outcome: a prospective analysis from the SIDESTEP trial. Wound Repair Regen. 2009;17:671–677. doi: 10.1111/j.1524-475X.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeandrot A, Richard JL, Combescure C, Jourdan N, Finge S, Rodier M, Corbeau P, Sotto A, Lavigne JP. Serum procalcitonin and C-reactive protein concentrations to distinguish mildly infected from non-infected diabetic foot ulcers: a pilot study. Diabetologia. 2008;51:347–352. doi: 10.1007/s00125-007-0840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dow G, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage. 1999;45:23–27, 29-40; quiz 41-42. [PubMed] [Google Scholar]
- 26.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, double-blinded, multicentre trial. Lancet. 2005;366:1695–1703. doi: 10.1016/S0140-6736(05)67694-5. [DOI] [PubMed] [Google Scholar]
- 28.Hartemann-Heurtier A, Ha Van G, Danan JP, Koskas F, Jacqueminet S, Golmard JL, Grimaldi A. Outcome of severe diabetic foot ulcers after standardised management in a specialised unit. Diabetes Metab. 2002;28:477–484. [PubMed] [Google Scholar]
- 29.Sumpio BE. Foot ulcers. N Engl J Med. 2000;343:787–793. doi: 10.1056/NEJM200009143431107. [DOI] [PubMed] [Google Scholar]
- 30.Citron DM, Goldstein EJ, Merriam CV, Lipsky BA, Abramson MA. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol. 2007;45:2819–2828. doi: 10.1128/JCM.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slater RA, Lazarovitch T, Boldur I, Ramot Y, Buchs A, Weiss M, Hindi A, Rapoport MJ. Swab cultures accurately identify bacterial pathogens in diabetic foot wounds not involving bone. Diabet Med. 2004;21:705–709. doi: 10.1111/j.1464-5491.2004.01221.x. [DOI] [PubMed] [Google Scholar]
- 32.Consensus Development Conference on Diabetic Foot Wound Care: 7-8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care. 1999;22:1354–1360. doi: 10.2337/diacare.22.8.1354. [DOI] [PubMed] [Google Scholar]
- 33.Sotto A, Richard JL, Combescure C, Jourdan N, Schuldiner S, Bouziges N, Lavigne JP. Beneficial effects of implementing guidelines on microbiology and costs of infected diabetic foot ulcers. Diabetologia. 2010;53:2249–2255. doi: 10.1007/s00125-010-1828-3. [DOI] [PubMed] [Google Scholar]
- 34.Monecke S, Ehricht R. Rapid genotyping of methicillin-resistant Staphylococcus aureus (MRSA) isolates using miniaturised oligonucleotide arrays. Clin Microbiol Infect. 2005;11:825–833. doi: 10.1111/j.1469-0691.2005.01243.x. [DOI] [PubMed] [Google Scholar]
- 35.Heller MJ. DNA microarray technology: devices, systems, and applications. Annu Rev Biomed Eng. 2002;4:129–153. doi: 10.1146/annurev.bioeng.4.020702.153438. [DOI] [PubMed] [Google Scholar]
- 36.Sotto A, Richard JL, Jourdan N, Combescure C, Bouziges N, Lavigne JP. Miniaturized oligonucleotide arrays: a new tool for discriminating colonization from infection due to Staphylococcus aureus in diabetic foot ulcers. Diabetes Care. 2007;30:2051–2056. doi: 10.2337/dc07-0461. [DOI] [PubMed] [Google Scholar]
- 37.Sotto A, Lina G, Richard JL, Combescure C, Bourg G, Vidal L, Jourdan N, Etienne J, Lavigne JP. Virulence potential of Staphylococcus aureus strains isolated from diabetic foot ulcers: a new paradigm. Diabetes Care. 2008;31:2318–2324. doi: 10.2337/dc08-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agence Française de Sécurité Sanitaire des Produits de Santé. Prescription des antibiotiques des antibiotiques par voie locale dans les infections cutanées bactériennes primitives et secondaires. Paris, juillet. Philadelphia: Mosby Elsevier; 2004. Available from: http://agmed.sante.gouv.fr/pdf/5/rbp/dermreco.pdf. [Google Scholar]
- 39.Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis. 2009;49:1541–1549. doi: 10.1086/644732. [DOI] [PubMed] [Google Scholar]
- 40.Lamb HM, Wiseman LR. Pexiganan acetate. Drugs. 1998;56:1047–1052; discussion 1053-1054. doi: 10.2165/00003495-199856060-00011. [DOI] [PubMed] [Google Scholar]
- 41.Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis. 2008;47:1537–1545. doi: 10.1086/593185. [DOI] [PubMed] [Google Scholar]
- 42.Bergin SM, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2006:CD005082. doi: 10.1002/14651858.CD005082.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Vermeulen H, van Hattem JM, Storm-Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev. 2007:CD005486. doi: 10.1002/14651858.CD005486.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Sotto A, Lemaire X, Jourdan N, Bouziges N, Richard JL, Lavigne JP. [In vitro activity of ertapenem against strains isolated from diabetic foot infections] Med Mal Infect. 2008;38:146–152. doi: 10.1016/j.medmal.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Sotto A, Bouziges N, Jourdan N, Richard JL, Lavigne JP. In vitro activity of tigecycline against strains isolated from diabetic foot ulcers. Pathol Biol (Paris) 2007;55:398–406. doi: 10.1016/j.patbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein EJ, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT. In vitro activity of ceftobiprole against aerobic and anaerobic strains isolated from diabetic foot infections. Antimicrob Agents Chemother. 2006;50:3959–3962. doi: 10.1128/AAC.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein EJ, Citron DM, Warren YA, Tyrrell KL, Merriam CV, Fernandez HT. In vitro activities of dalbavancin and 12 other agents against 329 aerobic and anaerobic gram-positive isolates recovered from diabetic foot infections. Antimicrob Agents Chemother. 2006;50:2875–2879. doi: 10.1128/AAC.00286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein EJ, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT. In vitro activities of doripenem and six comparator drugs against 423 aerobic and anaerobic bacterial isolates from infected diabetic foot wounds. Antimicrob Agents Chemother. 2008;52:761–766. doi: 10.1128/AAC.01128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipsky BA, Itani K, Norden C. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis. 2004;38:17–24. doi: 10.1086/380449. [DOI] [PubMed] [Google Scholar]
- 50.Lipsky BA, Stoutenburgh U. Daptomycin for treating infected diabetic foot ulcers: evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi-synthetic penicillins for complicated skin and skin-structure infections. J Antimicrob Chemother. 2005;55:240–245. doi: 10.1093/jac/dkh531. [DOI] [PubMed] [Google Scholar]
- 51.Nelson EA, O’Meara S, Golder S, Dalton J, Craig D, Iglesias C. Systematic review of antimicrobial treatments for diabetic foot ulcers. Diabet Med. 2006;23:348–359. doi: 10.1111/j.1464-5491.2006.01785.x. [DOI] [PubMed] [Google Scholar]
- 52.Tentolouris N, Petrikkos G, Vallianou N, Zachos C, Daikos GL, Tsapogas P, Markou G, Katsilambros N. Prevalence of methicillin-resistant Staphylococcus aureus in infected and uninfected diabetic foot ulcers. Clin Microbiol Infect. 2006;12:186–189. doi: 10.1111/j.1469-0691.2005.01279.x. [DOI] [PubMed] [Google Scholar]
- 53.Tentolouris N, Jude EB, Smirnof I, Knowles EA, Boulton AJ. Methicillin-resistant Staphylococcus aureus: an increasing problem in a diabetic foot clinic. Diabet Med. 1999;16:767–771. doi: 10.1046/j.1464-5491.1999.00132.x. [DOI] [PubMed] [Google Scholar]
- 54.Dang CN, Prasad YD, Boulton AJ, Jude EB. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med. 2003;20:159–161. doi: 10.1046/j.1464-5491.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- 55.Mantey I, Hill RL, Foster AV, Wilson S, Wade JJ, Edmonds ME. Infection of foot ulcers with Staphylococcus aureus associated with increased mortality in diabetic patients. Commun Dis Public Health. 2000;3:288–290. [PubMed] [Google Scholar]
- 56.Wagner A, Reike H, Angelkort B. [Highly resistant pathogens in patients with diabetic foot syndrome with special reference to methicillin-resistant Staphylococcus aureus infections] Dtsch Med Wochenschr. 2001;126:1353–1356. doi: 10.1055/s-2001-18655. [DOI] [PubMed] [Google Scholar]
- 57.Richard JL, Sotto A, Jourdan N, Combescure C, Vannereau D, Rodier M, Lavigne JP. Risk factors and healing impact of multidrug-resistant bacteria in diabetic foot ulcers. Diabetes Metab. 2008;34:363–369. doi: 10.1016/j.diabet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Game FL, Boswell T, Soar C. Outcome of diabetic foot ulcers with and without Staphylococcus aureus (MRSA) Diabet Med. 2003;20:30. [Google Scholar]
- 59.Hartemann-Heurtier A, Robert J, Jacqueminet S, Ha Van G, Golmard JL, Jarlier V, Grimaldi A. Diabetic foot ulcer and multidrug-resistant organisms: risk factors and impact. Diabet Med. 2004;21:710–715. doi: 10.1111/j.1464-5491.2004.01237.x. [DOI] [PubMed] [Google Scholar]
- 60.Trividic-Rumeau M, Bouyssou-Gauthier ML, Mounier M, Sparsa A, Blaise S, Bédane C, Bonnetblanc JM. [Methicilline-sensitive and methicilline-resistant Staphylococcus aureus related morbidity in chronic wounds: a prospective study] Ann Dermatol Venereol. 2003;130:601–605. [PubMed] [Google Scholar]
- 61.Lagacé-Wiens PR, Ormiston D, Nicolle LE, Hilderman T, Embil J. The diabetic foot clinic: not a significant source for acquisition of methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2009;37:587–589. doi: 10.1016/j.ajic.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Lipsky BA, Berendt AR. Principles and practice of antibiotic therapy of diabetic foot infections. Diabetes Metab Res Rev. 2000;16 Suppl 1:S42–S46. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr109>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 63.Cunha BA. Antibiotic selection for diabetic foot infections: a review. J Foot Ankle Surg. 2000;39:253–257. doi: 10.1016/s1067-2516(00)80009-5. [DOI] [PubMed] [Google Scholar]
- 64.Lipsky BA. Medical treatment of diabetic foot infections. Clin Infect Dis. 2004;39 Suppl 2:S104–S114. doi: 10.1086/383271. [DOI] [PubMed] [Google Scholar]
- 65.Gough A, Clapperton M, Rolando N, Foster AV, Philpott-Howard J, Edmonds ME. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet. 1997;350:855–859. doi: 10.1016/S0140-6736(97)04495-4. [DOI] [PubMed] [Google Scholar]
- 66.de Lalla F, Pellizzer G, Strazzabosco M, Martini Z, Du Jardin G, Lora L, Fabris P, Benedetti P, Erle G. Randomized prospective controlled trial of recombinant granulocyte colony-stimulating factor as adjunctive therapy for limb-threatening diabetic foot infection. Antimicrob Agents Chemother. 2001;45:1094–1098. doi: 10.1128/AAC.45.4.1094-1098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yönem A, Cakir B, Güler S, Azal O O, Corakçi A. Effects of granulocyte-colony stimulating factor in the treatment of diabetic foot infection. Diabetes Obes Metab. 2001;3:332–337. doi: 10.1046/j.1463-1326.2001.00142.x. [DOI] [PubMed] [Google Scholar]
- 68.Kästenbauer T, Hörnlein B, Sokol G, Irsigler K. Evaluation of granulocyte-colony stimulating factor (Filgrastim) in infected diabetic foot ulcers. Diabetologia. 2003;46:27–30. doi: 10.1007/s00125-002-0998-z. [DOI] [PubMed] [Google Scholar]
- 69.Viswanathan V, Mahesh U, Jayaraman M, Shina K, Ramachandram A. Beneficial role of granulocyte colony stimulating factor in foot infection in diabetic patients. J Assoc Physicians India. 2003;51:90–91. [PubMed] [Google Scholar]
- 70.Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Are granulocyte colony-stimulating factors beneficial in treating diabetic foot infections?: A meta-analysis. Diabetes Care. 2005;28:454–460. doi: 10.2337/diacare.28.2.454. [DOI] [PubMed] [Google Scholar]
- 71.Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev. 2009:CD. doi: 10.1002/14651858.CD006810.pub2. [DOI] [PubMed] [Google Scholar]
- 72.Niinikoski J, Bakker D, Cronjé F, Lind F, Mathieu D, Schmutz J, Hunt T, Mani R, Romanelli M, Téot L, et al. ECHM-ETRS joint conference on oxygen and tissue repair, Ravenna, Italy, October 27-28, 2006: recommendations by the international jury. Int J Low Extrem Wounds. 2007;6:139–142. doi: 10.1177/1534734607304625. [DOI] [PubMed] [Google Scholar]
- 73.Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33:998–1003. doi: 10.2337/dc09-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004:CD004123. doi: 10.1002/14651858.CD004123.pub2. [DOI] [PubMed] [Google Scholar]
- 75.Berendt AR. Counterpoint: hyperbaric oxygen for diabetic foot wounds is not effective. Clin Infect Dis. 2006;43:193–198. doi: 10.1086/505223. [DOI] [PubMed] [Google Scholar]