Use of Soller slits to remove back-fluorescence from the reference foil helps provide more accurate transmission X-ray absorption spectra of weak samples.
Keywords: Soller slits, transmission, X-ray absorption spectroscopy
Abstract
Measurement of X-ray absorption spectroscopy (XAS) in transmission is the method of choice for strong or concentrated samples. In a typical XAS experiment above 5 keV the sample is placed between the first (I 0) and second (I 1) ion chambers and a standard foil is placed between the second (I 1) and third (I 2) ion chambers for simultaneous calibration of energy during sample analysis. However, some fluorescence from the foil may be registered in I 1, causing anomalies in the transmission signal of the sample, especially when the sample edge jump is relatively small. To remedy this, Soller slits were constructed and placed between the foil and I 1 to minimize back-fluorescence from the foil. A comparison of blank and standard samples, measured with or without Soller slits or under a worst-case scenario, demonstrates the advantages of Soller slits when analyzing weak signal samples via transmission XAS.
1. Introduction
Since their first application in 1924 (Soller, 1924 ▶), Soller slits have been used in a variety of experimental configurations to select for beams of interest, whether parallel, converging or diverging. Generally Soller slits comprise of closely spaced blades composed of a material that strongly absorbs stray beams and does not fluoresce in the energy range of interest. In fluorescence X-ray absorption spectroscopy (XAS), with a non-energy-dispersive detector, Soller slits are used in combination with X-ray filters to preferentially eliminate scattered radiation from the sample (Lytle, 1975 ▶; Samant et al., 1987 ▶). With energy-dispersive detectors the combination of filter and Soller slits provides relief from electronic dead-time detector non-linearities caused by concentrated samples (Cramer et al., 1988 ▶).
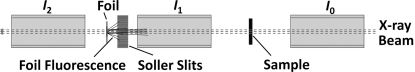
A typical transmission XAS measurement (Fig. 1 ▶) involves a sample between ion chamber detectors I 0 and I 1 with a standard foil downstream between I 1 and I 2, for internal energy calibration. Back-fluorescence from the foil into I 1 (Fig. 1 ▶) can appear as an inverted edge superimposed on the transmission data of the sample [T = log(I 0/I 1)] (Fig. 2 ▶). This effect becomes more pronounced when the foil is closer to I 1 or the signal from the sample is weak (not illustrated). In the present example the elemental Se foil has a lower excitation energy than the selenate sample, leading to a trough in the spectrum, but in other cases the foil fluorescence may occur underneath the absorption spectrum of the sample. In either case this will result in anomalies in the spectrum of the sample. To minimize this effect, Soller slits were designed and placed between the foil and I 1 to minimize the amount of back-fluorescence from the foil.
Figure 1.
Plan view of a typical transmission X-ray absorption spectroscopy set-up.
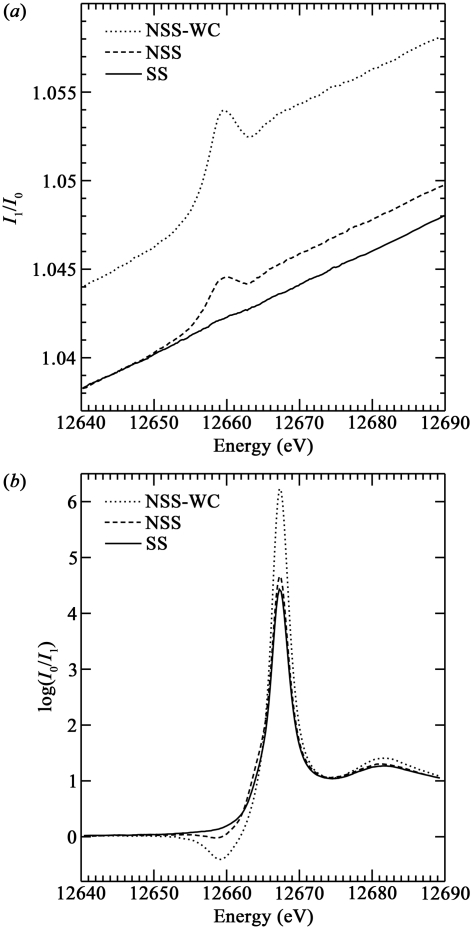
Figure 2.
(a) Spectra from the blank under the three conditions, plotted as I 1/I 0 to emphasize the back-fluorescence signal. (b) Background-subtracted and normalized transmission spectra from 4.3 mM Na2SeO4 run under the three conditions. The 12663 eV shoulder is a small fraction of selenite produced by selenate photoreduction in the X-ray beam even at 10 K.
2. Materials and methods
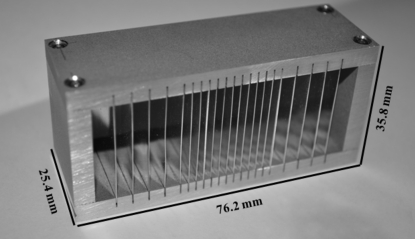
The Soller slit assembly consisted of an Al body with slots accommodating up to 21 vertical blades (Fig. 3 ▶). The assembly, fabricated by Vantec Design & Manufacturing Ltd, was the same width as the ion chamber for easy alignment. The transmitted beam travelled through 13 ‘inner blades’ spaced 2.0 mm apart in the middle of the Soller slit, while 8 ‘outer blades’ were spaced 4.0 mm apart; the closer-spaced blades removed more back-fluorescence. Silver (Ag) was chosen for the blade material owing to its high Z, long-term stability, machinability and availability. Sheets of 0.25 mm-thick Ag (Surepure Chemetals) were cut with an X-Acto No. 2 knife into blades measuring 30 mm (height) × 25 mm (length along the beam). All blade edges were sanded with 3M diamond lapping film to remove any burrs acquired during cutting. A thickness of 0.25 mm allowed for 0% transmission at energies below 15 keV and up to 2.2% transmission at 21 keV (McMaster et al., 1969 ▶).
Figure 3.
Image of the Soller slit assembly.
To demonstrate the effectiveness of the Soller slit assembly, a Se-free blank and 4.3 mM Na2SeO4 standard were measured under three conditions: SS, with Soller slits between the standard foil and I 1 with ∼3 mm on either side; NSS, without the Soller slits; NSS-WC, worst-case scenario with no Soller slits and I 1 moved downstream flush with the foil to maximize back-fluorescence entering I 1. The blank was a standard biochemical buffer solution, 100 mM MOPS (pH 7), with 30% v/v glycerol added to reduce ice crystal formation upon freezing. The 4.3 mM Na2SeO4 solution comprised a 1:5 dilution of 21.4 mM Na2SeO4 in 100 mM MOPS (pH 7) and 30% glycerol. Samples were measured on beamline 7-3 at the Stanford Synchrotron Radiation Lightsource (SSRL, Menlo Park, CA, USA) in 2 mm path length Vero White (Objet FullCure830) polymer cuvettes printed on a rapid prototyper (Objet Eden500V), placed at 90° to the incident beam and maintained at 10 K (Oxford Instruments CF1208 liquid-helium-flow cryostat). The energy was selected using a Si(220) double-crystal monochromator, with a 15 keV cut-off achieved by adjusting the angle of the upstream Rh-coated vertically collimating mirror. Focusing optics were not present. A 1.0 mm × 8.0 mm (height × width) beam was defined using slits upstream of I 0.
3. Results and discussion
The effects of foil fluorescence entering I 1 are shown in Fig. 2 ▶. In NSS-WC and even NSS the blank spectra (Fig. 2a ▶) showed a significant negative feature, which also appeared as a trough in the pre-edge region of the 4.3 mM Na2SeO4 spectrum (Fig. 2b ▶). Substantial reduction of back-fluorescence from the foil into I 1 was observed (Fig. 2b ▶) in the presence of the Soller slits. Though residual back-fluorescence is observed, the contribution is negligible compared with NSS and NSS-WC. Estimated from the height of the foil white line above background, the ratio of back-fluorescence intensities in the three cases NSS-WC:NSS:SS is 2.45:1:0.09.
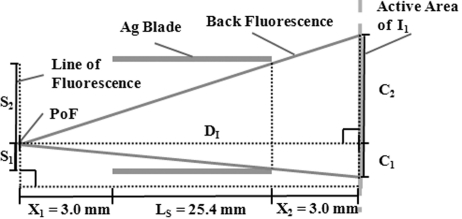
The effectiveness of the Soller slit assembly in the horizontal dimension was predicted from a general single point of fluorescence (PoF) between two blades (Fig. 4 ▶). The current configuration predicted a projected horizontal length (P H) of 2.21 mm (see equation in Fig. 4 ▶), equivalent to an angle of 71 mrad. Regardless of the PoF position on a line normal to the blades, P H remained the same. In the absence of Soller slits the angle calculated from the width of the ion chamber opening (approximately 64 mm) at a distance D I (Fig. 4 ▶) was 1.60 rad. Thus, the calculated intensity ratio NSS:SS is about 1:0.04, slightly better than the observed ratio of 1:0.09, possibly due to uncertainties in measuring the very small SS back-fluorescence (Fig. 2a ▶). Quantifying foil fluorescence into I 1 for NSS-WC was difficult owing to the acute angles occurring with the foil so close to I 1; however, more back-fluorescence is expected for NSS-WC compared with SS and NSS owing to the proximity of the foil.
Figure 4.
Schematic demonstrating the amount of foil fluorescence entering I 1 in the horizontal plane from any point of fluorescence (PoF) along the line of fluorescence. L s = length of Soller slit blades. D s = distance between Soller slit blades (S1 + S2). D I = distance from foil to I 1 (L s + X 1 + X 2). X 1 = distance from foil to Soller slit. X 2 = distance from Soller slit to I 1. P H = horizontal projection onto I 1. Since P H = C 1 + C 2, and C 1/S 1 = C 2/S 2 = D I/(D I − X 1), then P H = D I D S/(D I − X 2).
In summary, the use of Soller slits to remove foil fluorescence is advantageous when collecting transmission XAS of weaker samples. Foil back-fluorescence anomalies are essentially removed by the Soller slits, allowing for more accurate spectra. The simplicity of the Soller slit design and the relatively low cost of materials allow easy creation of custom Soller slits for any XAS beamline. We recommend their routine use in the collection of transmittance XAS data.
Acknowledgments
We thank George and Pickering research group members and staff at the SSRL for help with data acquisition. JJT is a CIHR-THRUST Fellow and is supported by a NSERC-Cameco CRD Grant (D. Janz). Research at the University of Saskatchewan is also funded by the CIHR, SHRF, NSERC and the Canada Research Chairs program (GNG, IJP). SSRL is supported by DOE OBES. The SSRL Structural Molecular Biology Program is supported by DOE OBER, and by NIH NCRR BTP.
References
- Cramer, S. P., Tench, O., Yocum, M. & George, G. N. (1988). Nucl. Instrum. Methods Phys. Res. A, 266, 586–591.
- Lytle, F. W. (1975). Phys. Rev. B, 11, 4825–4835.
- McMaster, W. H., Kerr Del Grande, N., Mallet, J. H. & Hubbell, J. H. (1969). Compilation of X-ray Cross Sections. Lawrence Livermore National Laboratory Report. Lawrence Livermore National Laboratory, Livermore, CA, USA.
- Samant, M. G., Borges, G. L., Gordon, J. G. II, Melroy, O. R. & Blum, L. (1987). J. Am. Chem. Soc. 109, 5970–5974.
- Soller, W. (1924). Phys. Rev. 24, 158–167.