Abstract
Proinflammatory stimuli, after amyloid β (Aβ) deposition, have been hypothesized to create a self-reinforcing positive feedback loop that increases amyloidogenic processing of the Aβ precursor protein (APP), promoting further Aβ accumulation and neuroinflammation in Alzheimer’s disease (AD). Interleukin-6 (IL-6), a proinflammatory cytokine, has been shown to be increased in AD patients implying a pathological interaction. To assess the effects of IL-6 on Aβ deposition and APP processing in vivo, we overexpressed murine IL-6 (mIL-6) in the brains of APP transgenic TgCRND8 and TG2576 mice. mIL-6 expression resulted in extensive gliosis and concurrently attenuated Aβ deposition in TgCRND8 mouse brains. This was accompanied by up-regulation of glial phagocytic markers in vivo and resulted in enhanced microglia-mediated phagocytosis of Aβ aggregates in vitro. Further, mIL-6-induced neuroinflammation had no effect on APP processing in TgCRND8 and had no effect on APP processing or steady-state levels of Aβ in young Tg2576 mice. These results indicate that mIL-6-mediated reactive gliosis may be beneficial early in the disease process by potentially enhancing Aβ plaque clearance rather than mediating a neurotoxic feedback loop that exacerbates amyloid pathology. This is the first study that methodically dissects the contribution of mIL-6 with regard to its potential role in modulating Aβ deposition in vivo.—Chakrabarty, P., Jansen-West, K., Beccard, A., Ceballos-Diaz, C., Levites, Y., Verbeeck, C., Zubair, A. C., Dickson, D., Golde, T. E., Das, P. Massive gliosis induced by interleukin-6 suppresses Aβ deposition in vivo: evidence against inflammation as a driving force for amyloid deposition.
Keywords: Alzheimer’s disease, neuroinflammation, APP, recombinant adeno-associated virus
A pathological hallmark of Alzheimer’s disease (AD) is reactive gliosis, which is especially prominent in the vicinity of extracellular Aβ plaques (1, 2). It has been proposed that plaque-associated chronic glial activation results in a self-reinforcing positive feedback loop that promotes further Aβ deposition by increasing amyloidogenic processing of Aβ precursor protein (APP). Such a positive feedback loop may originate from a proinflammatory stimuli driving increased Aβ production and aggregation via several pathways including increased expression of APP, increased β-secretase activity, and increased expression of acute-phase proteins that codeposit with Aβ plaques (3,4,5,6,7,8).
Activated microglia may promote neurodegeneration but could also play a neuroprotective role. For example, local chronic up-regulation of microgliosis in the APP transgenic mouse brain leads to focal amelioration of plaque pathology (9). Similarly, up-regulation of brain microgliosis by peripheral administration of colony stimulating factor leads to attenuated Aβ deposition and improved behavior in APP transgenic mice (10). Similarly, astroglial overexpression of TGF-β decreases Aβ plaque load by promoting microglial activation (11). Conversely, reducing microglial activation by knocking out the chemokine receptor CCR2 is reported to exacerbate Aβ pathology in APP-transgenic mice (12). In any case, the precise role of glial activation in AD-type neurodegenerative diseases is still contentious and may very well be dependent on the context, timing, and exact mediator of the inflammatory response.
IL-6, a pleiotropic proinflammatory cytokine, has been reported to be elevated in the plasma, cerebrospinal fluid, and brain parenchyma of patients with AD (13,14,15,16,17,18). An IL-6 promoter polymorphism has been associated with increased AD risk in selected ethnic populations (19, 20). Whether IL-6 is simply a marker for increased activation of the innate immune system in the proximity of Aβ plaques or it is mechanistically linked to the disease process remains an unanswered question. To characterize the role of IL-6-induced neuroinflammation in AD and to specifically test whether IL-6 induces a proamyloidogenic positive feedback loop in vivo, we used recombinant adeno-associated virus serotype 1 (AAV1) to express mIL-6 in the brains of neonatal and adult APP TgCRND8 mice. mIL-6 overexpression led to widespread gliosis and significantly decreased Aβ plaque burden in APP TgCRND8 mice. Our studies provide compelling evidence that mIL-6 does not drive a positive feedback loop resulting in enhanced Aβ deposition in vivo. Instead, mIL-6-mediated inflammatory responses appear to limit Aβ deposition early in the disease by enhancing glia-mediated amyloid plaque clearance from the brain.
MATERIALS AND METHODS
Mice
All animal husbandry procedures performed were approved by the Mayo Clinic Institutional Animal Care and Use Committee. TgCRND8 and Tg2576 mice were generated and maintained as described previously (21).
AAV1 preparation and injection
AAV1 viruses expressing mIL-6 (Image clone 2645608) or EGFP, under the control of the cytomegalovirus enhancer/chicken β-actin promoter, were generated as described previously (21). The injection procedures were performed as described previously (21). Briefly, cryoanesthetized mouse pups [0–12 h old (P0) or 36–48 h old (P2)] were bilaterally injected with 2 μl of AAV1 construct (1012 particles/ml) in the cerebral ventricles. Negative control groups (total n=10) were noninjection (n=5) and AAV1-EGFP-injected (n=5) groups. Experimental groups consisted of mice injected with AAV1-mIL-6 on P0 or P2 (n=9–12 transgenics/group). For Tg2576 mice, AAV1-mIL-6 and AAV1-EGFP (P0 injection) injected or uninjected mice were euthanized after 3 mo (n=5 and 6, respectively). For stereotactic injections, mice (n=5–6/group) were anesthetized with 1.5% isoflurane in 1% oxygen and secured into a Kopf apparatus (Model 900 Small Animal Stereotactic Instrument, David Kopf Instruments, Tujunga, CA, USA). The coordinates for injection were −1.7 caudal, −1.6 lateral, and −1.2 ventral from the bregma. A UMP2 Microsyringe Injector and Micro4 Controller (World Precision Instruments, Sarasota, FL, USA) was used to inject 2 μl of virus at a constant rate over a 10 min period. After an additional 10 min were allowed, the needle was raised slowly and the scalp incision was closed aseptically.
Quantitative real-time PCR
Total RNA from mouse forebrains was isolated using the RNaqueous kit (Ambion, Austin, TX, USA) and reverse transcribed using Superscript III (Invitrogen, Carlsbad, CA, USA). Since a precedence exists for using whole mouse forebrains rather than purified glial preparations, we used pericardially transfused mouse forebrains for transcriptional profiling (9, 22). The quantitative PCR (initial denaturation cycle of 95°C/10 min, followed by 40 amplification cycles of 95°C/15 s and 60°C/1 min) was performed with ABI Prism 7900 Real Time PCR System (Applied Biosystems, Foster City, CA, USA) using SYBR Green to detect the amplification products. Relative quantification of mRNA expression was calculated by the ΔCT method described by the manufacturer (ABI Prism 7700 Sequence Detection System, User Bulletin 2) after the levels were adjusted to the corresponding internal actin control for each sample. Primers were designed following Roche Universal Probe Library sequences (Roche Diagnostics Corp., Indianapolis, IN, USA).
Preparation of brain homogenate for immunoblotting, IL-6 ELISA, and Aβ ELISA assay
Snap-frozen hemi-forebrains from the P0 and P2 groups were sequentially extracted in RIPA buffer, 2% SDS buffer, and 70% formic acid (FA) as described previously (21). For adult injected mice, the brain was coronally dissected 1 mm anterior and posterior to the point of injection and used for subsequent analysis. Membranes containing protein samples separated on Bis-Tris 12% XT gels (Bio-Rad, Hercules, CA, USA) were probed with the antibody CT20 (anti-APP C-terminal 20 amino acid; 1:1000; T.E.G.), 82E1 (anti-Aβ 1–16; 1:1000), glial fibrillary acidic protein (GFAP; 1:1000; Cell Signaling, Danvers, MA, USA), cd11b (1:500; Novus Biological, Littleton, CO, USA), BACE1 (1:500; Chemicon, Temecula, CA, USA), apolipoprotein E (ApoE; Cell Signaling; 1:500), and anti-β-actin (1:1000; Sigma, St. Louis, MO, USA). Relative band intensity was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Sandwich capture IL-6 ELISA assays using RIPA soluble lysates were done with mouse specific reagents (BD Biosciences) or human specific reagents (Raybiotech, Norcross, GA, USA).
Aβ levels from brain lysates of APP transgenic animals were determined biochemically using human Aβ end-specific sandwich ELISA as described previously (21). Endogenous mouse Aβ40 was detected from diethylamine extracted brains of 5-mo-old nontransgenic mice injected on P0 using capture antibody 32.4.1 (mouse Aβx-40 specific). All ELISA results were analyzed using SoftMax Pro software (Molecular Devices, Sunnyvale, CA, USA).
Immunohistochemical imaging and image processing
For P0-injected mice, sagittal sections initiating from the midline of paraffin embedded brains were used for immunostaining, whereas for adult mice injected in hippocampus, the brain was coronally dissected at the point of injection for analysis. Immunohistochemical staining was done using pan-Aβ antibody 33.1.1 (1:1500; T.E.G.), GFAP (1:1000; Sigma), Iba-1 (1:1000; Wako, Richmond, VA, USA), EGFP (1:1000; Invitrogen), and von Willebrand factor (1:500, Chemicon). For free floating sections, paraformaldehyde-fixed brains were stained with cd11b (1:200; Serotec, Raleigh, NC, USA), GFAP-Cy5 (1:500; Sigma), and Iba-1 (1:250; Wako) and developed using fluorescent labeled secondary antibodies.
Immunohistochemically stained sections were captured using the Scanscope XT image scanner (Aperio, Vista, CA, USA) and analyzed using the ImageScope program. Fluorescently labeled free floating sections were captured using a Zeiss microscope and analyzed using the Metamorph program (Carl Zeiss, Oberkochen, Germany). Brightness and contrast alterations were applied identically on captured images using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA, USA).
Quantification of Aβ deposition
Paraformaldehyde-fixed paraffin-embedded brain tissue sections were immunostained with 33.1.1 antibody. The Aβ plaque burden was calculated using the Positive Pixel Count program (Aperio). At least 3 sections/sample, 30 μm apart, were averaged by a blinded observer to calculate plaque burden.
Quantitation of circumscription score per plaque
The density of Iba-1-positive microglia (immunostained using DAB stain) around thioflavin-S-positive fluorescent plaques in paraffin-embedded brain sections was quantified by assigning a score (1 through 4) for the percentage of microglia directly associated per plaque (0–25%=1, 25–50%=2, 50–75%=3, and 75–100%=4, respectively). The higher the score, the higher is the plaque area circumscribed by Iba-1-positive microglia. This was done by 2 independent observers, blinded to the experiment. There were 25–28 plaques from 4–5 separate animals from each experimental group analyzed. The circumscription score was calculated by averaging the percentage of plaque circumscription by Iba-1 positivity per field per sample.
Microglia phagocytosis assay
Primary microglia cells were obtained from the cerebral cortices of neonate mice (1–2 d old) as described previously (23). All studies were conducted on cultures in which >95% of cells were positive for cd11b. Hilyte 488 labeled Aβ42 (AnaSpec, Freemont, CA, USA) was aggregated in PBS buffer at 37°C for 6 h and then sonicated (3×10 s burst) to generate smaller fibrillar structures. Microglia, pretreated with mIL-6 (R&D Systems, Minneapolis, MN, USA) for 4 h, were treated with Hilyte 488-Aβ42 for 10 min at 37°C. Cells were washed, fixed, and mounted for visualization. For FACS analysis, after washes, glia were collected by trypsinization and resuspended in FACS buffer containing BSA. The scan was collected using Becton-Dickinson FacsVantage SE and analyzed with DIVA software (BD Biosciences).
Statistical analysis
Two-way ANOVA with post hoc Holm-Sidak multiple-comparison test or 2-tailed Student’s t test was used for statistical comparison (SigmaStat 3.0; Systat, San Jose, CA, USA) unless otherwise mentioned. Graphical analyses were done using Prism 4 (GraphPad Software), and final images were created using Photoshop CS2 (Adobe).
RESULTS
AAV1-mediated expression of mIL-6 leads to widespread gliosis in the brains of TgCRND8 mice
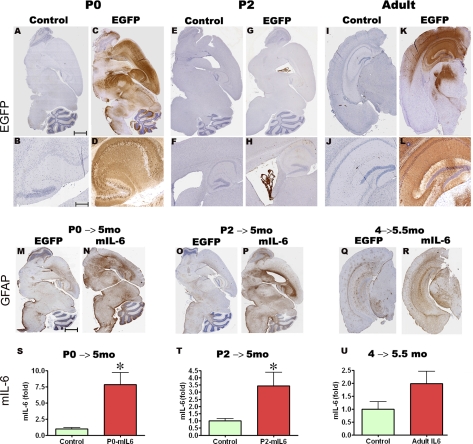
Three paradigms were used to evaluate the effects of AAV1-mediated expression of mIL-6 on gliosis and Aβ pathology in TgCRND8 mice. The first 2 paradigms investigated the effects of mIL-6 expression initiated before the onset of plaque deposition. Mouse pups, P0 and P2, were transduced by bilateral injection of AAV1-EGFP or AAV1-mIL-6 into the cerebral ventricles and were then aged to 5 mo for analysis (P0→5 mo and P2→5 mo, respectively). Whereas P0 delivery of AAV1-EGFP leads to transgene expression in neurons throughout the cortex, hippocampus, and midbrain (Fig. 1A–D), P2 delivery results in expression largely localized in the choroid plexus and ependymal cells lining the ventricles, along with a few neurons in the hippocampus overlying the lateral ventricle (Fig. 1E–H). The third paradigm evaluated the effects of AAV1-mIL-6 or AAV1-EGFP delivery to the hippocampus of adult 4-mo-old TgCRND8 mice using stereotactic injection. Immunohistochemical analysis with anti-EGFP antibody shows that the viral transgene is predominantly expressed in the hippocampal CA neurons, parts of the dentate gyrus, neuronal projections in the cortex, and some overlying cortical neurons after 6 wk expression of AAV1-EGFP (Fig. 1I–L, 4→5.5 mo). In previous studies, since AAV1-EGFP expression had no effect on amyloid pathology or gliosis when compared wtih uninjected mice (21), AAV1-EGFP-injected animals were used as the control cohort in this study.
Figure 1.
AAV1-mediated transduction of mIL-6 in mouse brain results in widespread expression and robust gliosis. A–L) AAVI-EGFP (2×109 genome particles/ventricle) was injected into the cerebral ventricles of TgCRND8 pups on day P0 (A–D, P0) or P2 (E–H, P2) or injected stereotaxically into the hippocampus of 4-mo-old mice (I–L, Adult). Mice were then sacrificed after 4–6 wk (n=5/group). Age-matched controls were injected with saline in all cases. Representative images of whole-brain sections (top panels) and hippocampus (bottom panels) show widespread EGFP expression in both forebrain and hindbrain areas in P0-injected mice (A–D), whereas P2 injection results in localized transduction of the choroid plexus (E–H). AAVI-EGFP injection into the hippocampus of 4-mo-old mice results in transduction of the hippocampal pyramidal layer as well as neuronal projections in the cortex and thalamus (I–L) at least 1 mm anterior and posterior to the point of injection. M–R) AAV1-mIL-6 or AAV1-EGFP (2×109 genome particles/ventricle) was injected into the cerebral ventricles of TgCRND8 mice on either P0 (M, N; P0→5 mo) or P2 (O, P; P2→5 mo) and sacrificed after 5 mo (n=9–12/group). GFAP immunostaining shows increased astrogliosis in both P0 → 5 mo and P2 → 5 mo mIL-6-injected mice compared with EGFP-injected control mice. Stereotactic injection of AAV1 mIL-6 into the hippocampus of 4-mo TgCRND8 mice and analyzed after 6 wk (Q, R; 4→5.5 mo) shows increased astrogliosis (n=5–6/group). S–U) Levels of mIL-6 in injected mouse brains were analyzed using sandwich ELISA technique using RIPA-soluble brain lysates. Results are expressed as fold over control (n=5/group). *P < 0.05. Scale bars = 600 μm (A, C, E, G, M–P); 500 μm (I, K, Q, R); 150 μm (B, D); 250 μm (F, H); 125 μm (J, L).
To assess the effects of AAV1-directed mIL-6 expression in the brain, we evaluated the extent of astrogliosis by immunohistochemistry using anti-GFAP antibody and also direct biochemical determination of mIL-6 protein levels. AAV1-mIL-6 expression in P0 → 5 mo and P2 → 5 mo resulted in widespread up-regulation of GFAP reactive astrocytes compared wtih AAV1-EGFP-expressing age-matched control cohorts (Fig. 1M–P) as well as up-regulation of mIL-6 protein levels (Fig. 1S, T). The average levels of mIL-6 were significantly increased by 7.8-fold over controls in P0 → 5 mo-injected group and 3.4-fold over controls in P2 → 5 mo-injected group. Although the P0 and P2 paradigms show markedly different spatial patterns of expression of the viral transgene, the extent of gliosis was quite similar, suggesting widespread distribution of secreted mIL-6 throughout the brain in the P2 paradigm. Similarly, robust astrogliosis was readily observed in the coronal brain sections at least 1 mm anterior and 1 mm posterior to the injection site in adult mice stereotaxically injected in the hippocampus with AAV1-mIL-6 at 4 mo and analyzed after 6 wk (Fig. 1Q, R). When AAV1-mIL-6 was injected into the hippocampus of adult TgCRND8 animals, analysis of the injected region revealed a 2.0-fold increase in mIL-6 levels in the 4 → 5.5 mo group (Fig. 1U).
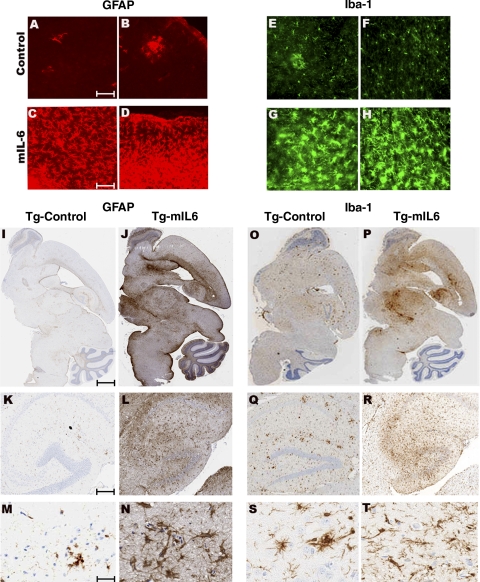
Detailed immunohistochemical analysis showed significant numbers of GFAP immunoreactive astrocytes as well as Iba-1 reactive microglia displaying hypertrophic processes in the cortex, hippocampus, midbrain, and cerebellum in 5-mo-old TgCRND8 mice injected with mIL-6 on P0 compared with controls (Fig. 2I–T). While Iba-1-positive microglia or GFAP-positive astrocytes are predominantly clustered around plaques in control TgCRND8 mice (Fig. 2A, B, E, F), mIL-6 injection resulted in robust widespread gliosis in APP mouse brain, independent of plaque association (Fig. 2C, D, G, H). Immunoblot analysis showed that there was a 3.4- and 2.8-fold increase in GFAP reactivity in P0 → 5 mo- and P2 → 5 mo-injected TgCRND8 mice, respectively, compared with age-matched controls (Supplemental Fig. 1A, B). Similar levels of activated astrocytes and microglia were also noted in P0-injected CRND8 nontransgenic littermates overexpressing mIL-6 (Supplemental Fig. 1C–N). In addition, focal expression of AAV1-mIL-6 in the hippocampus of adult TgCRND8 mice also resulted in robust reactive astrogliosis and microgliosis in and around the hippocampus compared with AAV1-EGFP-expressing control mice (Supplemental Fig. 2). In addition to reactive gliosis, animals injected on P0 and P2 display prominent neovascularization, as evident by immunohistochemistry with von Willebrand factor, an endothelial cell-specific marker (Supplemental Fig. 3). We found no evidence of increased cerebral amyloid angiopathy in mIL-6-expressing TgCRND8 mice compared with controls in all paradigms tested (data not shown). Moreover, despite such widespread neuroinflammation, we did not detect any appreciable demyelination in the brains of these mice or gross neuronal atrophy in all paradigms tested (data not shown).
Figure 2.
AAV1-mIL-6 expression in transgenic CRND8 mice results in extensive induction of astrogliosis and microgliosis. A–H) Up-regulation of activated astrocytes (C, D; GFAP) and microglia (G, H; Iba-1) in the cortex of P0 → mIL-6-injected TgCRND8 mice compared with control TgCRND8 mice (A, B; GFAP; E, F; Iba-1) detected by immunofluorescent staining of free-floating fixed sections (GFAP, red; Iba-1, green). I–N) Reactive astrocytes (GFAP immunoreactivity) in paraffin-embedded sections of P0 → 5 mo TgCRND8 mice injected with either mIL-6 (Tg-mIL6) or EGFP (Tg-Control). Whole-brain sections (I, J) along with higher magnification pictures (K–N, bottom panels) show detailed morphology of the activated astrocytes in and around the corresponding hippocampus. O–T) Iba-1 immunoreactivity in whole brain sections (O–P) and higher magnifications of the hippocampus (Q–T, bottom panels) in P0 → 5 mo TgCRND8 mice. Abundant activated microglia displaying hypertrophic processes are present in mIL-6-injected mice (Tg-mIL6) compared with EGFP-expressing control mice (Tg-Control). Scale bars = 50 μm (A–H); 600 μm (I, J, O, P), 150 μm (K, L, Q, R), and 25 μm (M, N, S, T).
mIL-6 overexpression attenuates Aβ deposition in TgCRND8 mice
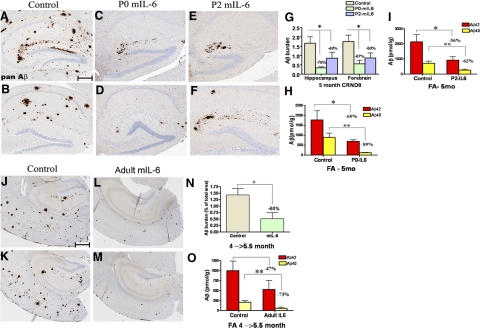
We next analyzed the effects of mIL-6 expression on Aβ deposition in all expression paradigms. There was a statistically large significant decrease in Aβ plaque burden in 5-mo-old mIL-6-expressing animals injected on either P0 or P2, as shown by Aβ immunostaining (Fig. 3A–F). In the forebrain and hippocampus of the P0 → 5 mo mIL-6-injected TgCRND8 mice, there was a 67 and 79% reduction in plaque burden, respectively (Fig. 3G). Similarly, in the forebrain and hippocampus of the P2 → 5 mo mIL-6-expressing mice, there was a 50 and 48% reduction in Aβ plaque burden, respectively (Fig. 3G). Notably, in a few mIL-6-treated animals, we could detect almost no Aβ plaques in the vicinity of the ventricle and hippocampus indicating a dramatic decrease in deposited Aβ plaques (Supplemental Fig. 4). Biochemical analysis of Aβ levels, as measured by end-specific Aβ sandwich ELISA using sequentially extracted RIPA, SDS, and FA solubilized mouse brain lysates, showed a similar magnitude of decrease in Aβ levels after mIL-6 expression. In P0 → 5 mo mIL-6-injected TgCRND8 mice, Aβ42 and Aβ40 levels decreased by 56 and 79%, respectively, in the SDS fractions compared with control mice (Supplemental Fig. 5A). Similarly, Aβ42 and Aβ40 levels were decreased by 60 and 89%, respectively, in the FA fractions compared with control mice (Fig. 3H). In the P2 → 5 mo group, Aβ42 and Aβ40 levels decreased by 12 and 44% in the SDS fractions in mIL-6-injected animals (Supplemental Fig. 5B), whereas the corresponding decrease in the FA fraction was 56 and 62%, respectively, in mIL-6-injected mice (Fig. 3L). RIPA soluble Aβ levels also decreased in both P0 and P2 mIL-6-injected cohorts compared with controls (Supplemental Fig. 5D, E).
Figure 3.
Attenuation of Aβ deposition in AAV1-mIL-6-expressing TgCRND8 mice. A–F). Representative brain sections stained with pan-Aβ1–16 antibody (mAb 33.1.1) show Aβ plaque immunoreactivity in the hippocampus of P0 → 5 mo mIL-6-expressing (C, D; P0-mIL-6), P2 → 5 mo mIL-6-expressing (E, F; P2-mIL-6), and age-matched P0 → 5 mo EGFP-expressing TgCRND8 mice (A, B; Control). G) There was a significant decrease in total forebrain Aβ as well as hippocampal Aβ plaque burdens in both P0 → 5 mo and P2 → 5 mo injection groups compared with control mice. H–I) Biochemical analyses of FA extractable Aβ42 and Aβ40 levels in P0 → 5 mo mIL-6-expressing TgCRND8 mice (H) and P2 → 5 mo mIL-6-expressing CRND8 mice (I) compared with EGFP-expressing age matched controls. J–M) 4-mo-old TgCRND8 mice were stereotaxically injected in the hippocampus with either AAV1-mIL-6 or AAV1-EGFP and sacrificed after 6 wk (n=5–6/group). Representative brain sections stained with 33.1.1 antibody (pan-Aβ 1–16) depict attenuation of Aβ deposition in mIL-6-expressing mice (L, M; Adult mIL-6) compared with controls (J, K; Control) in the immediate vicinity of the injection site. N) Aβ plaque burden analysis shows a significant decrease in amyloid deposition in mIL-6-injected mice compared with control EGFP-injected mice O) Biochemical analyses of Aβ42 and Aβ40 levels by ELISA show significant reductions in FA fraction in mIL-6-injected mice compared with controls. *P < 0.05; **P < 0.05. Scale bars = 150 μm.
Figure 4.
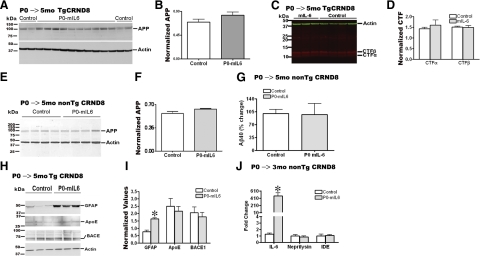
APP-processing, Aβ-production, or Aβ-degradation enzymes are not significantly altered in AAV1-mIL-6-expressing mice. A) Representative anti-CT20 immunoblot showed no significant changes in APP levels in AAV1-mIL-6-expressing P0 → 5 mo TgCRND8 compared with age-matched controls. B) Intensity analysis of anti-CT20 immunoreactive APP levels was normalized to β-actin in P0 → 5 mo TgCRND8 mouse cohort. C) Representative anti-CT20 immunoblot analysis of CTFα and CTFβ levels showed no significant changes in P0 → 5 mo TgCRND8 mice injected with AAV1-mIL-6 compared with age-matched controls. D). Intensity analysis of anti-CT20 immunoreactive CTF bands was normalized to β-actin in P0 → 5 mo TgCRND8 mouse cohort. E) Representative immunoblot showed no significant changes in APP levels in P0 → 5 mo mIL-6-expressing nontransgenic CRND8 littermates compared with age-matched controls. F). Intensity analysis of anti-CT20 immunoreactive APP levels was normalized to β-actin in P0 → 5 mo nontransgenic CRND8 mouse cohort. G) No change in diethylamine-soluble endogenous Aβ40 levels was seen in mIL-6-expressing P0 → 5 mo nontransgenic CRND8 littermates compared with age-matched controls. H) Representative immunoblot analysis of GFAP, ApoE, and BACE1 using RIPA-soluble lysates of P0 → 5 mo TgCRND8 mice showed minimal changes in ApoE or BACE1 levels (n=3/group), whereas GFAP reactivity was significantly increased in mIL-6-expressing mice. I) Intensity analysis of GFAP, ApoE, and BACE1 levels was quantified after normalization to β-actin levels in P0 → 5 mo TgCRND8 mouse cohort. *P < 0.05. J) Quantitative RT-PCR analysis of mRNA levels of Aβ degrading enzymes Neprilysin and IDE in mIL-6-expressing mouse forebrain. Data (fold change over control) represent average values obtained by quantitative PCR on 3-mo-old non-Tg CRND8 mice injected with AAV1-EGFP (control) or AAV1-mIL-6 on P0. Data reflect 2 independent experiments; n = 4 mice/group. *P < 0.001.
Adult TgCRND8 mice stereotaxically injected with AAV1 constructs at 4 mo (after Aβ plaque deposition has started) were analyzed at 5.5 mo. After immunohistochemical staining for Aβ, a 60% decrease in Aβ plaque burden was seen within the dissected coronal section in the mIL-6-expressing mice compared with EGFP-injected mice (Fig. 3J–N). There was a 50 and 68% decrease in SDS extractable Aβ42 and Aβ40 levels, respectively, in mIL-6-expressing mice compared with controls (Supplemental Fig. 5C). The FA fractions showed a 47% reduction in Aβ42 and a 73% reduction in Aβ40 levels in AAV1-mIL-6-expressing mice compared with controls (Fig. 3O). RIPA extractable Aβ levels also decreased in mIL-6-expressing animals compared with control animals (Supplemental Fig. 5F).
mIL-6 expression does not alter APP processing and Aβ production in vivo
As previous studies have shown that proinflammatory cytokines can increase APP expression in vitro, we first measured APP levels in AAV1-mIL-6-expressing mouse brains. We did not detect any significant changes in APP or C-terminal fragment (CTF) levels between control and mIL-6-overexpressing animals in P0 → 5 mo cohorts (Fig. 4A–D) or in 4 → 5.5 mo-injected adult TgCRND8 animals (data not shown). Further, we did not detect any increase in either the endogenous APP levels (Fig. 4E, F) or steady-state levels of endogenous Aβx-40 levels in the 5-mo-old mIL-6-injected nontransgenic mice compared with control cohorts (Fig. 4G) suggesting that mIL-6 does not influence the endogenous levels of APP or Aβ through cellular interaction with transcriptional or post-transcriptional mechanisms.
To investigate whether mIL-6 expression could alter the steady-state Aβ levels in vivo, P0 Tg2576 pups were injected with AAV1-mIL-6 or AAV1-EGFP, and Aβ levels and APP processing were analyzed at 3 mo of age. In contrast to TgCRND8 mice, which deposit Aβ as early as 6 wk of age, 3-mo-old Tg2576 mice have no Aβ deposition, enabling an accurate assessment of steady-state Aβ levels. Despite a 5.5-fold increase in mIL-6 protein levels and increased gliosis compared with the control cohort (Supplemental Fig. 6A, B), there are no statistically significant changes in either SDS or RIPA extractable Aβ40 levels and APP levels in AAV1-mIL-6-expressing mice compared with the EGFP-injected mice (Supplemetnal Fig. 6C–E).
Brain Aβ levels are regulated by a balance between Aβ-generating enzymes, such as β-secretase and the γ-secretase complex; Aβ-degrading enzymes, such as insulin degrading enzyme (IDE) and neprilysin; and other associated factors, such as ApoE. No significant changes in BACE1 or ApoE protein levels as well as Neprilysin or IDE mRNA levels were seen in mIL-6-expressing CRND8 mice (Fig. 4H–J).
Enhanced microglial activation and Aβ phagocytosis underlie Aβ attenuation in mIL-6-overexpressing APP mice
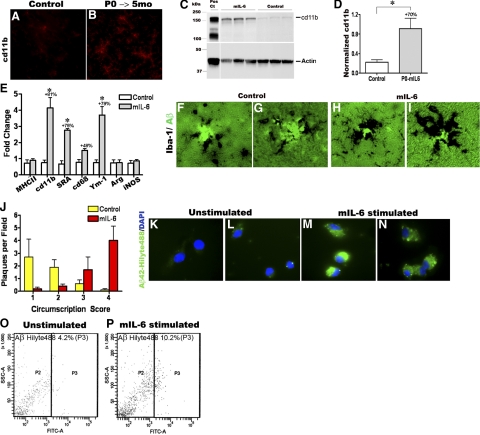
In the absence of detectable changes in steady-state Aβ levels, APP expression, and APP processing, we explored whether the amelioration of Aβ deposition in young TgCRND8 mice could be due to enhanced microglial phagocytotic activity induced by mIL-6. We first evaluated levels of cd11b/Mac, a microglial pattern recognition receptor and one of the primary phagocytic receptors on professional phagocytes (24). Immunofluorescence and immunoblotting analysis confirmed that cd11b levels were increased in mIL-6-expressing TgCRND8 mice (Fig. 5A–D). Next, we wanted to quantitatively evaluate the levels of multiple microglial surface activation markers in vivo. An increase in cd11b transcript levels (81%) was noted in mIL-6-expressing mice (Fig. 5E), confirming our earlier data. However, there was no change in MHC Class II expression in the same cohort (Fig. 5E). mRNA transcripts of two members of the scavenger receptor family, Class A Scavenger Receptor (SRA) and CD68/macrosialin, were found to be upregulated in mIL6-expressing mice by 75 and 48%, respectively (Fig. 5E). Since microglia in close apposition to the plaques could display different phenotypes, we decided to further investigate whether mIL-6 overexpression led to the establishment of a neuroprotective alternative M2 glial phenotype, marked by expression of Ym-1 and arginase or whether there was a prevalence of the classical M1 glial phenotype, marked by expression of inducible nitric oxide synthase (22, 25). Up-regulation of alternative microglial marker Ym-1 (79%) but no change in arginase or inducible nitric oxide synthase levels was noted in mIL-6-expressing animals (Fig. 5E). This is reminiscent of the establishment of an alternative macrophage M2 phenotype, albeit incomplete, described in PS1/APP mice where such plaque-associated microglia were shown to have beneficial phagocytic capabilities (22). Taken together, our data suggest that mIL-6-induced activated glia adopt a predominantly beneficial alternative phenotype that may help clear Aβ via enhanced phagocytosis.
Figure 5.
mIL6-induced persistent microglial up-regulation results in efficacious plaque clearance. A, B) Analysis of cd11b/Mac in mIL-6-expressing P0 → 5 mo TgCRND8 mice (n=3–4/group). Representative image showing up-regulation of cd11b/Mac in mIL-6-expressing TgCRND8 mice (B) compared with controls (A) detected by immunofluorescent staining on free-floating fixed sections. View ×200. C) Representative immunoblot analysis of cd11b in RIPA soluble brain extracts from mIL-6-injected P0 → 5 mo TgCRND8 mice compared with age-matched controls. D) Quantitative analysis of cd11b immunoblotting after normalizing to β-actin levels. *P < 0.05. E) Quantitative RT-PCR analysis of levels of microglial markers in mIL-6-expressing mouse forebrain. Data (fold change over control) represent average values obtained by QPCR on 3-mo-old mice injected with AAV1-EGFP (control) or AAV1-mIL-6 on P0; 2 independent experiments; n = 4 mice/group. *P < 0.001; 2-way ANOVA with Bonferroni’s posttests. F–I) Representative images of thioflavin-S-stained Aβ plaques (fluorescent green labeling) decorated with Iba-1 immunoreactive microglia (black immunostain) in mIL-6-expressing P0 → 5 mo TgCRND8 mice (H, I) and controls (F, G). View ×400. J) Quantitative analysis of the extent of Iba-1 immunodeposits circumscribing individual plaques shows increased association of activated microglia with plaques in mIL-6-expressing P0 → 5 mo TgCRND8 mice compared with controls (n = 4–5 mice/group). K–N) mIL-6-treated primary microglia appear to be more efficient in the uptake of fAβ42-Hilyte488 (green fluorescence, M, N) compared with unstimulated glia (K, L). Blue fluorescence indicates DAPI-stained glial nuclei. Data from 2 independent experiments; view ×600. O–P) Microglial cells with internalized Aβ42-Hilyte 488 (FITC channel on x-axis) were quantified by FACS. Percentage of positive cells in mIL-6 stimulated microglial cells was 10.2% (P, P3) compared with 4.2% in control unstimulated cells (O, P3).
To investigate whether the physical association of microglia and Aβ plaques may underlie efficient degradation of plaques in vivo (26, 27), we analyzed glial activation in conjunction with Aβ plaque immunostaining. Immunohistologic analysis showed that Iba-1-positive microglia are more hypertrophic and are intimately associated with thioflavin-S-stained Aβ plaques in mIL-6-injected animals compared with controls (Fig. 5F–I). A quantitative assessment of the actual number of microglia around each plaque was difficult, given the intimate association of the two. Instead, we analyzed the extent of plaques being circumscribed by Iba-1 immunoreactivity. After quantitative analysis based on a score of 1 to 4 of increasing plaque circumscription by microglia, we found that on an average there is a significant increase in microglial immunoreactivity in direct contact with Aβ plaques in mIL-6-injected animals compared with control cohorts (Fig. 5J).
Finally, we tested whether microglia have an enhanced ability to phagocytose Aβ aggregates in the presence of recombinant mIL-6 in vitro. Primary wild-type mouse microglia treated with recombinant mIL-6 show increased internalization of fluorescent tagged fibrillar Hilyte 488-Aβ42 compared with untreated glia (Fig. 5K–N). We then used FACS analysis to quantify the percentage of glial population positive for phagocytosed Hilyte 488 fAβ42. After fAβ42 uptake, cells were trypsinized to degrade any cell-surface-associated Hilyte488 fAβ42. FACS analysis showed that 10.2% of mIL-6-primed glial cells were positive for fAβ42-Hilyte488 compared with only 4.2% in untreated microglial cells (Fig. 5O, P), indicating increased uptake of Aβ42 in the presence of mIL-6.
DISCUSSION
The proinflammatory cytokine IL-6 has been proposed to play a potentiating role in AD based on increased IL-6 levels in the brains, cerebrospinal fluid, and plasma of AD patients and the proximity of IL-6 reactivity around Aβ plaques (2, 15, 28, 29). Here we show that chronic overexpression of mIL-6 using AAV1 induces a massive reactive gliosis in both APP transgenic mice and nontransgenic littermates; markedly suppresses Aβ deposition when initiated in either predepositing neonatal CRND8 mice or in adult TgCRND8 mice (Supplemental Table 1); does not significantly alter APP levels, APP processing, or steady-state Aβ generation in vivo, in contrast to earlier observations reported from in vitro studies; and results in establishment of a beneficial microglial phagocytic phenotype in vivo. These data suggest that mIL-6-induced neuroinflammation does not exacerbate Aβ plaque pathology and that the observed attenuation in Aβ levels is most likely due to enhanced Aβ phagocytosis. Such data largely refute the hypothesis that a strong proinflammatory stimulus like IL-6 creates a self-reinforcing neurotoxic feedback loop that promotes Aβ deposition.
Multiple studies (1, 3, 4) indicate that the expression of inflammatory mediators including receptor for advanced glycation end products (RAGE), monocyte chemoattractant protein 1 (MCP-1), TNF-α, IL-1β, IL-6, and α1-anti-chymotrypsin enhance amyloid processing in vitro and therefore possibly influence Aβ deposition in vivo. Proinflammatory stimuli driven APP expression and concomitant Aβ production may result in an amyloidogenic feedback loop leading to the development of AD type neurodegeneration (30,31,32). However, after a detailed analysis of APP processing under different conditions in this study, we observed no such correlation in vivo. Neither steady-state nor long-term APP expression is affected by chronic mIL-6 expression and neuroinflammation. In addition, we did not observe any changes in endogenous APP or Aβ levels in nontransgenic cohorts, strongly indicating that neuroinflammation does not transcriptionally alter the amyloidogenic pathway in mice, at least in the early phases of plaque deposition. This refutes the possible occurrence of a “cytokine cycle” whereby proinflammatory stimuli may trigger or drive Aβ production in the initial stages of AD pathology (4). Activated microglia appear to phagocytose Aβ efficiently as demonstrated in several studies (33,34,35,36), and this may delay pathology progression in transgenic mouse models (9, 11, 37,38,39). Other studies (11, 12, 34, 40,41,42) also suggest that glial activation may be beneficial in removing plaques in APP transgenic models after exposure to various inflammatory mediators or anti-Aβ immunotherapy. Our data support these studies by demonstrating that the reduction in Aβ deposition is likely mediated by enhanced microglial clearance of the Aβ deposits. Indeed, we provide several lines of evidence that mIL-6 activated microglial cells have enhanced phagocytic capacity, based on our observation that mIL-6-induced microglia display increased phenotypic activation markers as well as phagocytic markers (CD68) and scavenger receptors and are more intimately associated with plaques in vivo.
Although there is focal plaque associated glial activation in both humans with AD and mouse models of Aβ deposition, massive buildup of plaques occurs over time (43, 44). Given our current data and data from others (9, 26, 27, 45, 46), it appears that although activated glia can clear Aβ deposits, in the natural disease course glial clearance of Aβ is ineffective. One simple explanation is that ongoing Aβ accumulation overwhelms the phagocytic capacity of the glial cells. Another explanation proposed by others (22, 47, 48) is that the glial activation in AD and in mouse models may favor a neurotoxic classical activation phenotype rather than a more neuroprotective phagocytic phenotype, especially with age. Although such studies are quite intriguing and could play a role in late onset AD, they do presuppose that aging and not simply time (age) is a key determinant of AD associated pathological alterations. At least in certain mouse models such as TgCRND8 that rapidly develop Aβ deposition and in genetically driven forms of AD with extremely young ages of onset, it is clear that rapid changes in Aβ production can overwhelm the inherent clearance capacity even in the absence of age-associated pathological alterations. In contrast, our data suggest that, in the timeline studied, the mIL-6-induced neuroinflammatory phenotype in the central nervous system results in up-regulation of glial phagocytosis leading to attenuation in Aβ deposition. Nevertheless, we are presently investigating whether sustained long-term expression of mIL-6 can result in decreased Aβ clearance capacity of activated glia in older TgCRND8 mice. Another final scenario for successful Aβ clearance is via enhanced recruitment of bone-marrow-derived macrophages into the brain (38, 39, 49). We have not examined this issue in the current study; however, it remains an intriguing hypothesis.
A concern in the P0 and parenchymal injections using AAV1 brain transgenesis is that the transgene expression is mostly neuronal. Although it is classically thought that microglia and astrocytes produce the majority of the cytokines present in the brain, there is increasing evidence that neurons may also express many cytokines including IL-6, especially under pathological conditions (2, 50). Nevertheless, we compared the effect of widespread neuronal mIL-6 expression that is observed when the AAV1 virus is delivered to P0 pups to the much more restricted expression that is observed when the AAV1 is delivered to P2 pups. Despite the difference in the patterns of transgene expression, the P0 and P2 delivery of AAV1-mIL-6 resulted in qualitatively similar phenotypes with respect to glial activation and suppression of Aβ deposition. Although the P0 studies did result in slightly higher levels of mIL-6, a more uniform gliosis, and a larger degree of suppression of Aβ deposition compared with the P2-injected group, all of these effects were readily apparent in the P2 cohorts as well. Consistent with the known biology of mIL-6, these results strongly support the idea that the effects of mIL-6 in the central nervous system are noncell autonomous. More generally, this study establishes a novel paradigm to evaluate the effects of secreted factors like IL-6 when expressed largely in neurons (intracerebroventricular delivery on P0 or direct injection into the parenchyma of adult mice) or when expressed primarily from non-neuronal cells within and lining the cerebral ventricles (intracerebroventricular delivery on P2). This methodology can be utilized to address the long-term consequences of many inflammatory mediators not only on Aβ deposition but also on neuronal function and viability in vivo.
The physiological and pathophysiological role of neuroinflammatory and immune responses in AD remain largely unresolved. These responses could trigger, potentiate, limit, or have no effect on the pathogenic cascade that leads to neuronal dysfunction and clinical dementia. Our current data support the idea that inflammatory mediators, which enhance the phenotypic and immunological activation of glia, do not promote Aβ accumulation but limit Aβ deposition at least in the early preclinical phases of the disease. It remains an open question as to whether such glial activation plays a role in driving downstream pathological cascades that may contribute to the neurodegenerative process.
Supplementary Material
Acknowledgments
The authors thank Monica Castanedes-Casey, V. A. Phillips, and Linda Rousseau for assistance with tissue processing and immunohistochemical analyses; Sunita Malik for help with FACS recording; and Faith Conkle, Deborah Maloy, and the Mayo Clinic Veterinary Medicine staff for animal maintenance. This work was supported by the Mayo Clinic (to T.G.), the National Institutes of Health/National Institute on Aging (grants RO1AG18454, RO1AG29886, and P01AG25531 to T.G. and P01AG71216 to D. D.), the American Health Assistance Foundation (grant AHAF3J to P.D.), and the Robert H. and Clarice Smith Foundation postdoctoral fellowship (to P.C.).
References
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole G M, Cooper N R, Eikelenboom P, Emmerling M, Fiebich B L, Finch C E, Frautschy S, Griffin W S, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie I R, McGeer P L, O'Banion M K, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel F L, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringheim G E, Szczepanik A M, Petko W, Burgher K L, Zhu S Z, Chao C C. Enhancement of beta-amyloid precursor protein transcription and expression by the soluble interleukin-6 receptor/interleukin-6 complex. Brain Res Mol Brain Res. 1998;55:35–44. doi: 10.1016/s0169-328x(97)00356-2. [DOI] [PubMed] [Google Scholar]
- Griffin W S, Sheng J G, Royston M C, Gentleman S M, McKenzie J E, Graham D I, Roberts G W, Mrak R E. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a “cytokine cycle” in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H M, Jiang J, Wilson B, Zhang W, Hong J S, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Heneka M T, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Klockgether T, Heneka M T. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Cho H J, Kim S K, Jin S M, Hwang E M, Kim Y S, Huh K, Mook-Jung I. IFN-gamma-induced BACE1 expression is mediated by activation of JAK2 and ERK1/2 signaling pathways and direct binding of STAT1 to BACE1 promoter in astrocytes. Glia. 2007;55:253–262. doi: 10.1002/glia.20451. [DOI] [PubMed] [Google Scholar]
- Shaftel S S, Kyrkanides S, Olschowka J A, Miller J N, Johnson R E, O'Banion M K. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu G Q, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman S E, Means T K, Terada K, Geula C, Luster A D. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Shibata N, Ohnuma T, Takahashi T, Baba H, Ishizuka T, Ohtsuka M, Ueki A, Nagao M, Arai H. Effect of IL-6 polymorphism on risk of Alzheimer disease: genotype-phenotype association study in Japanese cases. Am J Med Genet. 2002;114:436–439. doi: 10.1002/ajmg.10417. [DOI] [PubMed] [Google Scholar]
- Ershler W B, Keller E T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis L J, Ferri C, Casadei V, Grimaldi L M. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Venturelli E, Fenoglio C, Guidi I, Villa C, Bergamaschini L, Cortini F, Scalabrini D, Baron P, Vergani C, Bresolin N, Scarpini E. Intrathecal levels of IL-6, IL-11 and LIF in Alzheimer’s disease and frontotemporal lobar degeneration. J Neurol. 2008;255:539–544. doi: 10.1007/s00415-008-0737-6. [DOI] [PubMed] [Google Scholar]
- Baranowska-Bik A, Bik W, Wolinska-Witort E, Martynska L, Chmielowska M, Barcikowska M, Baranowska B. Plasma beta amyloid and cytokine profile in women with Alzheimer’s disease. Neuro Endocrinol Lett. 2008;29:75–79. [PubMed] [Google Scholar]
- Sokolova A, Hill M D, Rahimi F, Warden L A, Halliday G M, Shepherd C E. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol. 2008;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F, Grimaldi L M, Bonafe M, Martina C, Olivieri F, Cavallone L, Giovanietti S, Masliah E, Franceschi C. Interleukin-6 gene alleles affect the risk of Alzheimer’s disease and levels of the cytokine in blood and brain. Neurobiol Aging. 2003;24:921–926. doi: 10.1016/s0197-4580(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Paola F, Maioli F, Martelli M, Montesi F, Bastagli L, Bianchin M, Chiappelli M, Tumini E, Bolondi L, Licastro F. Interleukin-1beta and interleukin-6 gene polymorphisms as risk factors for AD: a prospective study. Exp Gerontol. 2006;41:85–92. doi: 10.1016/j.exger.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kim J, Miller V M, Levites Y, West K J, Zwizinski C W, Moore B D, Troendle F J, Bann M, Verbeeck C, Price R W, Smithson L, Sonoda L, Wagg K, Rangachari V, Zou F, Younkin S G, Graff-Radford N, Dickson D, Rosenberry T, Golde T E. BRI2 (ITM2b) inhibits Abeta deposition in vivo. J Neurosci. 2008;28:6030–6036. doi: 10.1523/JNEUROSCI.0891-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, Ruano D, Vizuete M, Gutierrez A, Vitorica J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008;28:11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke R L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Ehlers M R. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2:289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones T L, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman D M, Bacskai B J, Hyman B T. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S, Bauer J, Ganter U, Jonas U, Berger M, Volk B. Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab Invest. 1992;66:223–230. [PubMed] [Google Scholar]
- Huell M, Strauss S, Volk B, Berger M, Bauer J. Interleukin-6 is present in early stages of plaque formation and is restricted to the brains of Alzheimer’s disease patients. Acta Neuropathol. 1995;89:544–551. doi: 10.1007/BF00571510. [DOI] [PubMed] [Google Scholar]
- Ge Y W, Lahiri D K. Regulation of promoter activity of the APP gene by cytokines and growth factors: implications in Alzheimer’s disease. Ann N Y Acad Sci. 2002;973:463–467. doi: 10.1111/j.1749-6632.2002.tb04684.x. [DOI] [PubMed] [Google Scholar]
- Giri R K, Selvaraj S K, Kalra V K. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J Immunol. 2003;170:5281–5294. doi: 10.4049/jimmunol.170.10.5281. [DOI] [PubMed] [Google Scholar]
- Lahiri D K, Chen D, Vivien D, Ge Y W, Greig N H, Rogers J T. Role of cytokines in the gene expression of amyloid beta-protein precursor: identification of a 5′-UTR-binding nuclear factor and its implications in Alzheimer’s disease. J Alzheimers Dis. 2003;5:81–90. doi: 10.3233/jad-2003-5203. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Rogers J, Strohmeyer R, Kovelowski C J, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis C A, Klunk W E, Kohsaka S, Jucker M, Calhoun M E. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee C Y, Koenigsknecht-Talboo J, Holtzman D M, Landreth G E. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease–a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Simard A R, Soulet D, Gowing G, Julien J P, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely C A, Tan J, Duman R S, Flavell R A. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin A H, Lambris J D, Alexander J J, Quigg R J, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci U S A. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier M, Peng Y, Jiang L, Seabrook T J, Carroll M C, Lemere C A. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard K L, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter L S, Barron E, Chui H C. Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett. 1990;119:32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- Dickson D W. Microglia in Alzheimer’s disease and transgenic models. How close the fit? Am J Pathol. 1999;154:1627–1631. doi: 10.1016/S0002-9440(10)65416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike J D, Brionne T C, Lu E, Anankov R, Yan F, Silverstein S C, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Hickman S E, Allison E K, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky A S, Graves M C, Gustavson A, Sayre J, Sofroni E, Suarez T, Chiappelli F, Bernard G. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2005;7:221–232. doi: 10.3233/jad-2005-7304. discussion 255–262. [DOI] [PubMed] [Google Scholar]
- Malm T M, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang S M, Iwata N, Saido T C, Maeda J, Suhara T, Trojanowski J Q, Lee V M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.