Abstract
Human T-cell leukemia virus type 1 (HTLV-1), the first human retrovirus discovered, is the etiological agent of adult-T-cell leukemia/lymphoma. The HTLV-1 encoded Tax protein is a potent oncoprotein that deregulates gene expression by constitutively activating nuclear factor-κB (NF-κB). Tax activation of NF-κB is critical for the immortalization and survival of HTLV-1-infected T cells. In this review, we summarize the present knowledge on mechanisms underlying Tax-mediated NF-κB activation, with an emphasis on post-translational modifications of Tax.
Keywords: Adult-T-cell leukemia/lymphoma, Human T-cell leukemia virus type 1, Nuclear factor-κB, Human T-cell leukemia virus type 1-associated myelopathy/tropical spastic paraparesis, IKK
INTRODUCTION
The human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult-T-cell leukemia/lymphoma (ATLL) and a neuroinflammatory disease termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP)[1-3]. Ten million to 20 million people worldwide are infected with HTLV-1, predominantly in endemic areas in Southern Japan, the Caribbean, Western Africa and South America. ATLL develops in approximately 5% to 10% of HTLV-1-infected individuals, after a long period of latency, suggesting a multistep process of T-lymphocyte transformation[4]. In ATLL patients, the malignant cells typically consist of oligoclonal or monoclonal outgrowths of CD4+ and CD25+ T lymphocytes carrying a complete or defective provirus of HTLV-1[5]. ATLL consists of four clinical subtypes including acute, lymphoma, chronic and smoldering. The current therapies for acute ATLL, which is the most aggressive form, are largely ineffective since the average time of survival after diagnosis with acute ATLL is only 6 mo[6]. HTLV-1 can be transmitted through sexual contact, blood transfusions, and from mother to child via breast-feeding[7-9]. The route of transmission appears to be one of the factors that determines the type of disease that occurs, with blood transmissions increasing the risk for HAM/TSP and mucosal transmissions (breast-feeding) increasing the risk for ATLL[4]. HTLV-1 predominantly infects CD4+ T cells in vivo, although recent studies indicate that other cell types such as CD8+ T-cells and dendritic cells (DCs) may also serve as reservoirs for HTLV-1[10].
HTLV-1 infects cells by transmission of virions between cells (infectious transmission) or by transmission of a provirus to the two daughter cells of a dividing infected cell (mitotic transmission). At least two receptors for HTLV-1 have been identified, including glucose transporter type 1 and neuropilin-1 (NRP1)[11,12]. Heparin sulfate proteoglycans also play an important role in facilitating the entry of HTLV-1[13,14]. There is also evidence for cell-type specific receptors since a recent study has reported that HTLV-1 enters DCs by binding to the receptor DC-SIGN[15]. Infected cells that express viral antigens are rapidly targeted by cytotoxic T cells, therefore the viral load is maintained predominantly by cells harboring silent provirus spread by mitotic transmission[12]. HTLV-1 transmission by free virions is very inefficient, at least in T cells, however, recent studies indicate that cell-free HTLV-1 virions are highly infectious for DCs[16]. When an HTLV-1 infected cell contacts an uninfected cell, a microtubule-organizing center (MTOC) is polarized at the cell-cell junction, and then viral proteins, such as Gag and viral genome RNA, accumulate at this junction allowing viral products, such as Gag and viral genome RNA, to accumulate at the junction with subsequent transfer of the viral complex into the target cell[17]. In HTLV-1-infected cells, expression of intercellular adhesion molecule 1 (ICAM-1) is upregulated, which increases the polarization of the MTOC at the point of contact in HTLV-1-infected cells, suggesting that increased expression of ICAM-1 facilitates cell-to-cell transmission of HTLV-1[18]. The frequencies of HTLV-1 provirus integration into transcription units (from the first exon to the last exon) are 26.8% (15/56) in carriers and 33.9% (20/59) in ATL, equivalent to the frequency calculated based on random integration (33.2%)[19]. However, there is evidence that HTLV-1 provirus is prone to integration near the transcriptional start sites in leukemic cells[19].
The HTLV-1 genome is 9032 nucleotides in length and encodes the structural proteins necessary to form the viral core particle (Gag, Env, and Pol) and the enzymatic retroviral proteins (reverse transcriptase, integrase and protease) and is flanked on both ends by long terminal repeats (LTRs) that contain cis-elements that regulate viral gene expression[20]. In addition, the HTLV-1 genome contains a cluster of at least five open reading frames (ORFs) within the pX region that are generated by alternative splicing[1]. The tax gene is the most extensively studied and encodes a protein of 40 kDa. The other pX genes encode p12I, p27I, p13II, and p30II and all function as HTLV-1 accessory proteins[21]. The HTLV-1 accessory proteins encoded in the pX region have diverse functions, many of which involve modulation of host signaling pathways[22]. For example, p12 triggers early interleukin-2 (IL-2) expression by increasing the level of intracellular calcium and selectively activating nuclear factor of activated T cells (NFAT)[23]. p13 protein accumulates in mitochondria and may function as a negative regulator of cell growth[24]. The p30 protein modulates cell cycle and apoptosis regulatory genes[25]. Very little is known regarding p27 function. Recently, a novel ORF has been identified in the complementary strand of the pX region and encodes the HTLV-1 basic leucine zipper gene (HBZ)[26]. There are two transcripts of HBZ representing spliced and unspliced forms. The spliced form of HBZ is expressed in ATLL and has been proposed to regulate cell proliferation[27,28]. HBZ also functions as a repressor of HTLV-1 transcription by forming heterodimers with CREB, CREB-2, CREM, and ATF-1 and forming inactive complexes impaired in binding to Tax-responsive elements[26,29]. During the late stages of ATLL, HBZ, which is probably the only viral product expressed at this time[30,31], may support proliferation and growth of ATLL cells.
THE HTLV-1 ONCOPROTEIN TAX
Tax is a 40 kDa phosphoprotein that contains both nuclear localization (NLS) and nuclear export sequences that enable it to shuttle between the nucleus and cytoplasm[32-35]. Tax is a trans-activating protein that regulates both viral and cellular gene expression[36,37]. With regard to viral gene expression, Tax recruits the transcription factor CREB, and the co-activators CBP/p300 and PCAF, to the HTLV-1 LTR viral promoters[38-40]. The expression of Tax is required for HTLV-1 viral gene expression. In addition to regulating viral gene expression, Tax also regulates cellular proliferation, apoptosis, genetic instability, telomerase activation, and inactivation of tumor suppressors[41-43]. Tax modulates the activation of host transcription factors to deregulate gene expression, which favors cell growth and survival[44]. Nuclear factor-κB (NF-κB) is a key target of Tax since Tax mutants, unable to activate NF-κB, are defective for cell immortalization[45]. Furthermore, NF-κB is required for the survival of HTLV-1 transformed cells[46].
Tax plays an essential role in HTLV-1-mediated leukemogenesis, in part, by driving cellular proliferation and enhancing cell survival[47]. Consistent with these functions, Tax was shown to be necessary and sufficient for the immortalization of CD4+ T-cells, a hallmark of ATLL[45,48]. Transgenic mice, expressing Tax under the control of the HTLV-1 LTR promoter, develop neurofibromas and mesenchymal tumors[49]. When Tax expression is regulated by the granzyme B promoter, mice developed large granular lymphocytic leukemias comprising CD8+ T cells and natural killer cells[50]. Two recent studies with novel Tax transgenic mice have yielded phenotypes that more closely resemble ATLL[51,52]. Transgenic mice expressing Tax under the Lck proximal promoter were shown to develop thymus-derived immature T-cell leukemia with clinical, pathological, and immunologic features characteristic of acute ATLL[51]. In an independent study, Tax expressed in lymphocytes in a conditional manner resulted in progressive alopecia, hyperkeratosis and skin lesions commonly observed in the preleukemic phase of ATLL[52]. Importantly, mice expressing the Tax M22 point mutant, defective for NF-κB activation, did not develop this phenotype[52]. Collectively, these studies provide in vivo evidence that Tax is both necessary and sufficient for tumor formation.
The expression of Tax promotes the dysregulation of hundreds of cellular genes including proto-oncogenes, cytokines, growth factor receptors, cyclin-dependent kinases, inhibitors of cyclin-dependent kinases, and genes involved in DNA repair and cell adhesion[47,53]. Tax also upregulates the expression of the T-cell growth factor IL-2 as well as its high affinity receptor IL-2R (also known as CD25)[54]. In the early phases of infection, HTLV-1-infected cells are dependent upon the presence of IL-2, possibly contributing to the early clonal expansion of infected T cells through an IL-2/IL-2R autocrine/paracrine loop. Disease progression, however, occurs in the absence of IL-2 secretion or expression. HTLV-1-infected cells are not dependent on IL-2, which is concomitant with constitutively activated Janus kinases and signal transducers and activators of transcription, leading to the induction of Shc/Ras/Raf/mitogen-activated protein kinase and PI3K/AKT pathways[55]. Tax mainly exerts its pleiotropic functions through direct interaction with numerous cellular proteins, many of which regulate signal transduction pathways[56-59]. In this review, we will focus on recent studies illustrating the importance of Tax post-translational modifications as well as Tax targeting of NF-κB negative regulatory proteins.
REGULATION OF NF-κB BY TAX
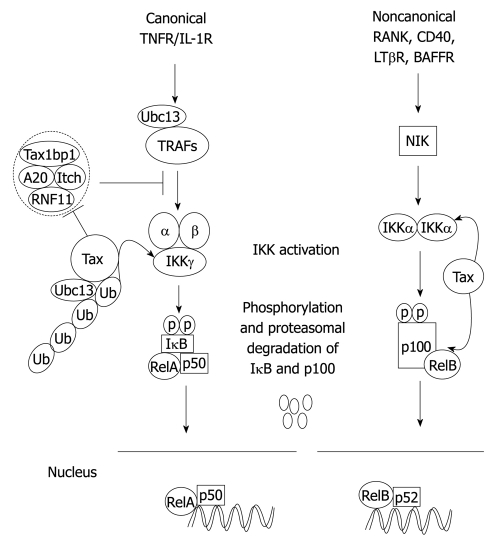
In mammalian cells, NF-κB is composed of five structurally related proteins, RelA (p65), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2), organized in different homo- and hetero-dimer NF-κB complexes. NF-κB1 and NF-κB2 are translated as precursor proteins, p105 and p100, for which proteasome-mediated processing generates the mature NF-κB subunits, p50 and p52, respectively. All NF-κB proteins share a common Rel-homology domain mediating their dimerization, DNA binding and NLS. NF-κB is normally sequestered as an inactive form through physical interaction with inhibitory κB (IκB) regulatory proteins in the cytoplasm. There are two distinct NF-κB signaling pathways: the canonical and noncanonical or alternative pathways. Generally, the canonical pathway regulates inflammation and cell survival, whereas the noncanonical pathway regulates lymphoid organogenesis and B-cell survival (Figure 1)[60]. The canonical NF-κB pathway is induced in response to diverse stimuli, including the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and IL-1, engagement of the T-cell receptor or exposure to viral and bacterial products. Following induction by various stimuli, the IκBs are phosphorylated by the IKK complex, which is composed of the catalytic subunits IKKα and IKKβ and a non-catalytic scaffolding subunit IKKγ/NEMO, leading to their ubiquitination and degradation, thus freeing NF-κB dimers to translocate to the nucleus[61]. The noncanonical pathway regulates the processing of p100 to p52 and is induced by TNF superfamily members, including CD40 Ligand, CD70, B-cell activating factor and RANK Ligand. In response to these specific TNF superfamily ligands, the MAP3K, NF-κB inducing kinase (NIK) phosphorylates IKKα, which in turn, phosphorylates p100 triggering proteasome-dependent processing to p52[62,63]. NIK stability, and hence p100 processing, is regulated by an E3 ubiquitin ligase complex consisting of TRAF2, TRAF3, cIAP1 and cIAP2[64,65].
Figure 1.
Canonical and noncanonical nuclear factor-κB (NF-κB) activation pathways. The binding of a specific ligand to a receptor (i.e. tumor necrosis factor-α (TNF-α) binding to TNFR1) leads to the recruitment and activation of an IKK complex comprising IKKα, IKKβ catalytic subunits and the regulatory subunit IKKγ/NEMO. The IKK complex then phosphorylates IκBα leading to degradation by the proteasome and concomitant translocation of NF-κB to the nucleus where it activates target genes. The NF-κB negative regulators, A20, TAX1BP1, Itch and RNF11, form a complex and inhibit activation of NF-κB upstream of IKK in the canonical pathway. In the noncanonical pathway, NIK is activated downstream of select TNFR superfamily members, and phosphorylates IKKα that in turn phosphorylates p100 resulting in its ubiquitination, limited degradation by the proteasome and nuclear mobilization of RelB/p52 dimers. Ubc13: Ubiquitin-conjugating enzyme 13.
Most human cancers exhibit constitutively activated NF-κB[66], in stark contrast to the transient NF-κB activation observed upon stimulation of cells with proinflammatory cytokines TNF-α or IL-1. NF-κB is constitutively activated in both HTLV-1-transformed T-cell lines and freshly isolated ATL cells[67]. Tax stimulates both canonical and non-canonical pathways, and constitutively activates NF-κB in HTLV-1 infected cells, by interacting with several NF-κB members, including RelA, p50, and p52[66,68], and also members of the IκB family such as IκBα and the precursor proteins p105 and p100. Tax interaction with NF-κB transcription factors does not fully explain Tax-mediated NF-κB activation since the completion of this process also requires cytoplasmic events. A key event in Tax-mediated NF-κB activation is binding with IKKγ/NEMO[69-72]. Tax interacts with IKKγ/NEMO in transfected cells as well as HTLV-1 transformed cell lines[73]. Notably, Tax activation of NF-κB is defective in T-cells genetically deficient for IKKγ[74]. Thus, it is likely that Tax binds to IKKγ/NEMO as a mechanism to be assembled into IKK complexes[75]. Tax interactions with IKKγ/NEMO are also essential for activation of the noncanonical pathway as well; however, Tax does not require NIK to trigger p100 processing[75]. Tax likely triggers the activation of the IKK catalytic subunits by recruiting upstream kinases, such as TGF-β activating kinase 1, to IKK[72]. Tax therefore promotes IκB degradation at multiple levels, thereby allowing nuclear translocation of NF-κB independently of external stimuli (Figure 2). In HTLV-1 transformed cell lines, Tax has been shown to promote the relocalization of IKK subunits to a perinuclear compartment co-localizing to the Golgi apparatus[73,76]. Consistent with these finding, another study has indicated that Tax hijacks IKK to lipid raft microdomains in the Golgi where it is activated[77]. Therefore, the Golgi appears to be a cellular compartment where Tax triggers the activation of IKKs.
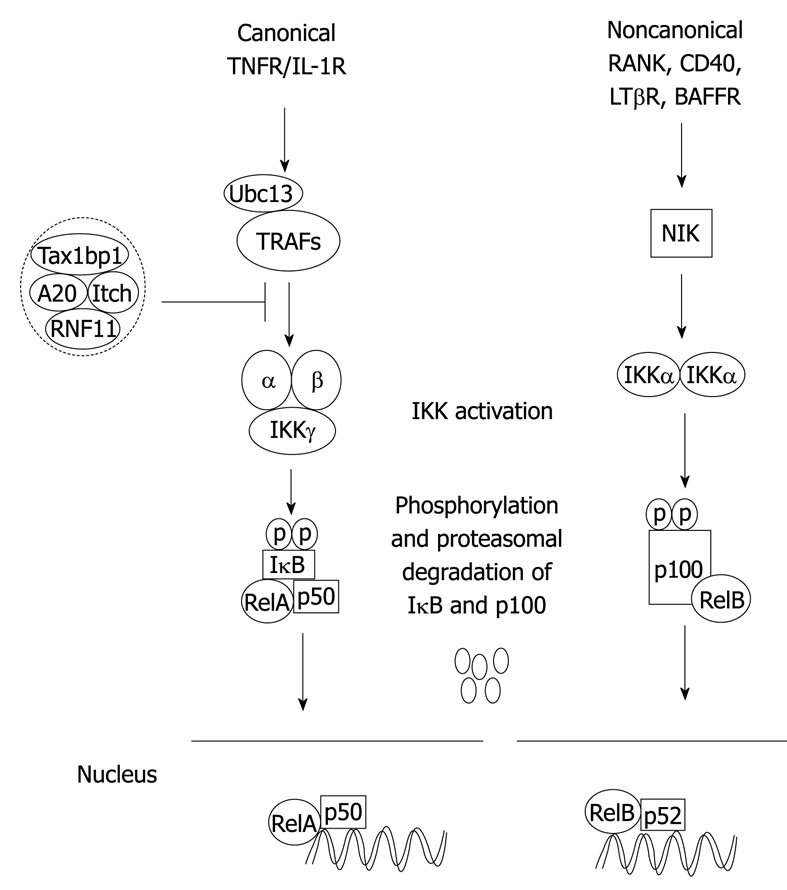
Figure 2.
Mechanisms of Tax activation of the canonical and noncanonical NF-κB pathways. In the canonical pathway, Tax interacts with TAX1BP1 to disrupt the formation and function of the A20 ubiquitin-editing complex. Ubiquitinated Tax interacts with IKKγ and activates the canonical NF-κB pathway. Tax triggers activation of the noncanonical pathway downstream of NIK by recruiting IKKα to p100 thus stimulating phosphorylation, ubiquitination, and processing to p52.
In order to promote a persistent NF-κB response, it can be predicted that Tax would impair the function of NF-κB inhibitory proteins. Indeed, recent studies from our laboratory demonstrated that Tax targets the NF-κB negative regulatory protein A20 for inactivation. A20 (also known as TNFAIP3) is a zinc finger protein that is essential for the termination of NF-κB signaling. A20-deficient mice die prematurely because of uncontrolled multi-organ inflammation and cachexia[78]. A20 functions as an ubiquitin-editing enzyme that targets ubiquitinated signaling proteins, such as RIP1 and TRAF6, for inactivation[79-81]. A20 contains a deubiquitination domain of the ovarian tumor family and seven C-terminal zinc finger domains[82]. A20 first removes lysine K63 (K63)-linked polyubiquitin chains from RIP1 and then polyubiquitinates RIP1 with lysine 48 (K48)-linked chains leading to its degradation[82]. A20 is an essential component of a ubiquitin-editing complex together with the regulatory proteins TAX1BP1, Itch and RNF11[79]. TAX1BP1 was originally cloned as an interacting protein of Tax in a yeast two-hybrid screen[83,84]. Mice lacking TAX1BP1 have been generated by two groups, and TAX1BP1 has been shown to be an essential negative regulator of NF-κB by serving as an adaptor molecule for A20[80,85]. Shembade and coworkers have shown that ectopic expression of Tax leads to the disruption of the A20 ubiquitin-editing complex[79-81]. The mechanism by which Tax disrupts the A20 ubiquitin-editing enzyme complex is unclear, although Tax may potentially impair protein-protein interactions by steric hindrance or by modifying post-translational modifications.
POST-TRANSLATIONAL MODIFICATION OF TAX
Post-translational modifications of Tax are important in the constitutive activation of NF-κB pathways, inhibition of DNA repair, activation of the p53 tumor suppressor and cell cycle control. Tax undergoes numerous post-translational modifications including phosphorylation, ubiquitination and sumoylation[86-89]. Phosphorylation of Tax at multiple sites on serine residues is important for Tax localization to nuclear bodies and for Tax-mediated activation of gene expression via both the ATF/CREB and NF-κB pathways[90,91]. However, the kinases for Tax phosphorylation and activation of NF-κB have not yet been identified.
Polyubiquitination of Tax leads to its cytoplasmic retention and is critical for the activation of IKK and NF-κB[86-88,92]. Shembade and others have shown Tax polyubiquitination is predominantly composed of K63-linked polyubiquitin chains[86,93,94]. Tax polyubiquitination can occur on multiple lysine residues, although lysine 263, 280, and/or 284 are the most critical sites[87]. Physical interaction of Tax with ubiquitin-conjugating enzyme 13, an E2 enzyme for K63 linked polyubiquitination, is essential for Tax ubiquitination and interaction with NEMO[86]. However, the Tax E3 ligase is currently unknown, although it is likely to be an E3 ligase capable of K63-linked polyubiquitination. Recently, the regulatory molecules TAX1BP1 and NRP/Optineurin were shown to be required for Tax polyubiquitination and activation of NF-κB[76]. However, how these molecules promote Tax polyubiquitination is not completely understood.
In addition to phosphorylation and polyubiquitination, Tax also undergoes sumoylation[87]. Tax sumoylation leads to its nuclear retention and the formation of nuclear bodies that include NF-κB, p300 and CBP as well as components of the transcription and splicing machineries[87]. The sites of Tax sumoylation overlap with polyubiquitination[87] thus the localization of Tax may determine whether it becomes polyubiquitinated or sumoylated. Nevertheless, it is clear from published studies that ubiquitination and sumoylation act in concert for Tax-mediated activation of gene expression via the NF-κB pathway.
Yet another post-translational modification of Tax is acetylation, which modulates transcription factor functions such as DNA binding affinity, stability and ability to interact with coactivators and corepressors[95,96]. Tax acetylation occurs at a lysine residue at amino acid position 346 in the carboxy-terminal domain of Tax by the transcriptional coactivator p300[95]. When Tax is acetylated, it favors activation of gene expression via the NF-κB pathway, suggesting that Tax oncogenic potential depends on Tax acetylation[95]. This modification may also compete with ubiquitination or sumoylation for overlapping targeted lysine residues.
CONCLUSION
HTLV-1 Tax interacts with several host proteins to activate IKK and NF-κB for proliferation and transformation of HTLV-1 infected cells. Tax activates both the canonical and noncanonical NF-κB pathways. It is clear that Tax ubiquitination is critical for interaction with IKKγ/NEMO for NF-κB activation; however it is unknown whether Tax ubiquitination is important for activation of the noncanonical NF-κB pathway. Future studies will be necessary to identify host factors such as adaptor molecules and E3 ligases that Tax requires to activate NF-κB.
Footnotes
Supported by Grants from the United States Public Health Service/National Institutes of Health, No. RO1CA135362, RO1GM083143 and PO1CA128115
Peer reviewer: Vishnu Suppiramaniam, PhD, Department of Pharmacal Sciences, 401 Walker Building, Auburn University, Auburn, AL 36849, United States
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
References
- 1.Grant C, Barmak K, Alefantis T, Yao J, Jacobson S, Wigdahl B. Human T cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J Cell Physiol. 2002;190:133–159. doi: 10.1002/jcp.10053. [DOI] [PubMed] [Google Scholar]
- 2.Osame M, Izumo S, Igata A, Matsumoto M, Matsumoto T, Sonoda S, Tara M, Shibata Y. Blood transfusion and HTLV-1 associated myelopathy. Lancet. 1986;2:104–105. doi: 10.1016/s0140-6736(86)91636-3. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barmak K, Harhaj E, Grant C, Alefantis T, Wigdahl B. Human T cell leukemia virus type I-induced disease: pathways to cancer and neurodegeneration. Virology. 2003;308:1–12. doi: 10.1016/s0042-6822(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura M, Maeda M, Yasunaga J, Kawakami H, Kaji R, Adachi A, Uchiyama T, Matsuoka M. Influence of cytokine and mannose binding protein gene polymorphisms on human T-cell leukemia virus type I (hTLV-I) provirus load in HTLV-1 asymptomatic carriers. Hum Immunol. 2003;64:453–457. doi: 10.1016/s0198-8859(02)00829-7. [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y, Tomonaga M. The current status of therapy for adult T-cell leukaemia-lymphoma in Japan. Leuk Lymphoma. 2003;44:611–618. doi: 10.1080/1042819021000055039. [DOI] [PubMed] [Google Scholar]
- 7.Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-1) J Emerg Med. 2000;18:109–119. doi: 10.1016/s0736-4679(99)00173-0. [DOI] [PubMed] [Google Scholar]
- 8.Ohsugi T, Koito A. Human T cell leukemia virus type I is resistant to the antiviral effects of APOBEC3. J Virol Methods. 2007;139:93–96. doi: 10.1016/j.jviromet.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka M. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene. 2003;22:5131–5140. doi: 10.1038/sj.onc.1206551. [DOI] [PubMed] [Google Scholar]
- 10.Nagai M, Brennan MB, Sakai JA, Mora CA, Jacobson S. CD8(+) T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood. 2001;98:1858–1861. doi: 10.1182/blood.v98.6.1858. [DOI] [PubMed] [Google Scholar]
- 11.Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115:449–459. doi: 10.1016/s0092-8674(03)00881-x. [DOI] [PubMed] [Google Scholar]
- 12.Bangham CR. The immune response to HTLV-1. Curr Opin Immunol. 2000;12:397–402. doi: 10.1016/s0952-7915(00)00107-2. [DOI] [PubMed] [Google Scholar]
- 13.Piñon JD, Klasse PJ, Jassal SR, Welson S, Weber J, Brighty DW, Sattentau QJ. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J Virol. 2003;77:9922–9930. doi: 10.1128/JVI.77.18.9922-9930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones KS, Fugo K, Petrow-Sadowski C, Huang Y, Bertolette DC, Lisinski I, Cushman SW, Jacobson S, Ruscetti FW. Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complexes to enter T cells. J Virol. 2006;80:8291–8302. doi: 10.1128/JVI.00389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceccaldi PE, Delebecque F, Prevost MC, Moris A, Abastado JP, Gessain A, Schwartz O, Ozden S. DC-SIGN facilitates fusion of dendritic cells with human T-cell leukemia virus type 1-infected cells. J Virol. 2006;80:4771–4780. doi: 10.1128/JVI.80.10.4771-4780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 17.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-1 between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 18.Barnard AL, Igakura T, Tanaka Y, Taylor GP, Bangham CR. Engagement of specific T-cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood. 2005;106:988–995. doi: 10.1182/blood-2004-07-2850. [DOI] [PubMed] [Google Scholar]
- 19.Doi K, Wu X, Taniguchi Y, Yasunaga J, Satou Y, Okayama A, Nosaka K, Matsuoka M. Preferential selection of human T-cell leukemia virus type I provirus integration sites in leukemic versus carrier states. Blood. 2005;106:1048–1053. doi: 10.1182/blood-2004-11-4350. [DOI] [PubMed] [Google Scholar]
- 20.Azran I, Schavinsky-Khrapunsky Y, Aboud M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology. 2004;1:20. doi: 10.1186/1742-4690-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cereseto A, Berneman Z, Koralnik I, Vaughn J, Franchini G, Klotman ME. Differential expression of alternatively spliced pX mRNAs in HTLV-1-infected cell lines. Leukemia. 1997;11:866–870. doi: 10.1038/sj.leu.2400665. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19:475–496. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Ding W, Albrecht B, Green PL, Lairmore MD. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J Biol Chem. 2003;278:15550–15557. doi: 10.1074/jbc.M210210200. [DOI] [PubMed] [Google Scholar]
- 24.Silic-Benussi M, Cavallari I, Zorzan T, Rossi E, Hiraragi H, Rosato A, Horie K, Saggioro D, Lairmore MD, Willems L, et al. Suppression of tumor growth and cell proliferation by p13II, a mitochondrial protein of human T cell leukemia virus type 1. Proc Natl Acad Sci USA. 2004;101:6629–6634. doi: 10.1073/pnas.0305502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael B, Nair AM, Hiraragi H, Shen L, Feuer G, Boris-Lawrie K, Lairmore MD. Human T lymphotropic virus type-1 p30II alters cellular gene expression to selectively enhance signaling pathways that activate T lymphocytes. Retrovirology. 2004;1:39. doi: 10.1186/1742-4690-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol. 2002;76:12813–12822. doi: 10.1128/JVI.76.24.12813-12822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavanagh MH, Landry S, Audet B, Arpin-André C, Hivin P, Paré ME, Thête J, Wattel E, Marriott SJ, Mesnard JM, et al. HTLV-1 antisense transcripts initiating in the 3'LTR are alternatively spliced and polyadenylated. Retrovirology. 2006;3:15. doi: 10.1186/1742-4690-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata K, Hayashibara T, Sugahara K, Uemura A, Yamaguchi T, Harasawa H, Hasegawa H, Tsuruda K, Okazaki T, Koji T, et al. A novel alternative splicing isoform of human T-cell leukemia virus type 1 bZIP factor (HBZ-SI) targets distinct subnuclear localization. J Virol. 2006;80:2495–2505. doi: 10.1128/JVI.80.5.2495-2505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemasson I, Lewis MR, Polakowski N, Hivin P, Cavanagh MH, Thébault S, Barbeau B, Nyborg JK, Mesnard JM. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J Virol. 2007;81:1543–1553. doi: 10.1128/JVI.00480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-1 basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA. 2006;103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satou Y, Matsuoka M. Implication of the HTLV-1 bZIP factor gene in the leukemogenesis of adult T-cell leukemia. Int J Hematol. 2007;86:107–112. doi: 10.1532/IJH97.07103. [DOI] [PubMed] [Google Scholar]
- 32.Gatza ML, Marriott SJ. Genotoxic stress and cellular stress alter the subcellular distribution of human T-cell leukemia virus type 1 tax through a CRM1-dependent mechanism. J Virol. 2006;80:6657–6668. doi: 10.1128/JVI.02270-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alefantis T, Barmak K, Harhaj EW, Grant C, Wigdahl B. Characterization of a nuclear export signal within the human T cell leukemia virus type I transactivator protein Tax. J Biol Chem. 2003;278:21814–21822. doi: 10.1074/jbc.M211576200. [DOI] [PubMed] [Google Scholar]
- 34.Smith MR, Greene WC. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 35.Meertens L, Chevalier S, Weil R, Gessain A, Mahieux R. A 10-amino acid domain within human T-cell leukemia virus type 1 and type 2 tax protein sequences is responsible for their divergent subcellular distribution. J Biol Chem. 2004;279:43307–43320. doi: 10.1074/jbc.M400497200. [DOI] [PubMed] [Google Scholar]
- 36.Franklin AA, Nyborg JK. Mechanisms of Tax Regulation of Human T Cell Leukemia Virus Type I Gene Expression. J Biomed Sci. 1995;2:17–29. doi: 10.1007/BF02257921. [DOI] [PubMed] [Google Scholar]
- 37.Yao J, Wigdahl B. Human T cell lymphotropic virus type I genomic expression and impact on intracellular signaling pathways during neurodegenerative disease and leukemia. Front Biosci. 2000;5:D138–D168. doi: 10.2741/yao. [DOI] [PubMed] [Google Scholar]
- 38.Koga H, Ohshima T, Shimotohno K. Enhanced activation of tax-dependent transcription of human T-cell leukemia virus type I (HTLV-1) long terminal repeat by TORC3. J Biol Chem. 2004;279:52978–52983. doi: 10.1074/jbc.M409021200. [DOI] [PubMed] [Google Scholar]
- 39.Giebler HA, Loring JE, van Orden K, Colgin MA, Garrus JE, Escudero KW, Brauweiler A, Nyborg JK. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong SJ, Lu H, Cho WK, Park HU, Pise-Masison C, Brady JN. Coactivator-associated arginine methyltransferase 1 enhances transcriptional activity of the human T-cell lymphotropic virus type 1 long terminal repeat through direct interaction with Tax. J Virol. 2006;80:10036–10044. doi: 10.1128/JVI.00186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marriott SJ, Semmes OJ. Impact of HTLV-1 Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene. 2005;24:5986–5995. doi: 10.1038/sj.onc.1208976. [DOI] [PubMed] [Google Scholar]
- 42.Taylor JM, Nicot C. HTLV-1 and apoptosis: role in cellular transformation and recent advances in therapeutic approaches. Apoptosis. 2008;13:733–747. doi: 10.1007/s10495-008-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatza ML, Watt JC, Marriott SJ. Cellular transformation by the HTLV-1 Tax protein, a jack-of-all-trades. Oncogene. 2003;22:5141–5149. doi: 10.1038/sj.onc.1206549. [DOI] [PubMed] [Google Scholar]
- 44.Harhaj EW, Harhaj NS. Mechanisms of persistent NF-kappaB activation by HTLV-1 tax. IUBMB Life. 2005;57:83–91. doi: 10.1080/15216540500078715. [DOI] [PubMed] [Google Scholar]
- 45.Robek MD, Ratner L. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol. 1999;73:4856–4865. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitajima I, Shinohara T, Bilakovics J, Brown DA, Xu X, Nerenberg M. Ablation of transplanted HTLV-1 tax-transformed tumors in mice by antisense inhibition of NF-kappa B. Science. 1993;259:1523. doi: 10.1126/science.8456277. [DOI] [PubMed] [Google Scholar]
- 47.Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24:5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- 48.Grassmann R, Dengler C, Müller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar MC, Sodroski JG, Haseltine WA. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grossman WJ, Ratner L. Transgenic mouse models for HTLV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13 Suppl 1:S162–S169. doi: 10.1097/00042560-199600001-00025. [DOI] [PubMed] [Google Scholar]
- 50.Grossman WJ, Kimata JT, Wong FH, Zutter M, Ley TJ, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasegawa H, Sawa H, Lewis MJ, Orba Y, Sheehy N, Yamamoto Y, Ichinohe T, Tsunetsugu-Yokota Y, Katano H, Takahashi H, et al. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat Med. 2006;12:466–472. doi: 10.1038/nm1389. [DOI] [PubMed] [Google Scholar]
- 52.Kwon H, Ogle L, Benitez B, Bohuslav J, Montano M, Felsher DW, Greene WC. Lethal cutaneous disease in transgenic mice conditionally expressing type I human T cell leukemia virus Tax. J Biol Chem. 2005;280:35713–35722. doi: 10.1074/jbc.M504848200. [DOI] [PubMed] [Google Scholar]
- 53.Ng PW, Iha H, Iwanaga Y, Bittner M, Chen Y, Jiang Y, Gooden G, Trent JM, Meltzer P, Jeang KT, et al. Genome-wide expression changes induced by HTLV-1 Tax: evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-kappaB activation. Oncogene. 2001;20:4484–4496. doi: 10.1038/sj.onc.1204513. [DOI] [PubMed] [Google Scholar]
- 54.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris JC, Waldmann TA. Advances in interleukin 2 receptor targeted treatment. Ann Rheum Dis. 2000;59 Suppl 1:i109–i114. doi: 10.1136/ard.59.suppl_1.i109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 57.Neuveut C, Low KG, Maldarelli F, Schmitt I, Majone F, Grassmann R, Jeang KT. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol Cell Biol. 1998;18:3620–3632. doi: 10.1128/mcb.18.6.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haller K, Wu Y, Derow E, Schmitt I, Jeang KT, Grassmann R. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol Cell Biol. 2002;22:3327–3338. doi: 10.1128/MCB.22.10.3327-3338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu K, Bottazzi ME, de la Fuente C, Deng L, Gitlin SD, Maddukuri A, Dadgar S, Li H, Vertes A, Pumfery A, et al. Protein profile of tax-associated complexes. J Biol Chem. 2004;279:495–508. doi: 10.1074/jbc.M310069200. [DOI] [PubMed] [Google Scholar]
- 60.Lo JC, Basak S, James ES, Quiambo RS, Kinsella MC, Alegre ML, Weih F, Franzoso G, Hoffmann A, Fu YX. Coordination between NF-kappaB family members p50 and p52 is essential for mediating LTbetaR signals in the development and organization of secondary lymphoid tissues. Blood. 2006;107:1048–1055. doi: 10.1182/blood-2005-06-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 63.Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 64.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun SC, Yamaoka S. Activation of NF-kappaB by HTLV-1 and implications for cell transformation. Oncogene. 2005;24:5952–5964. doi: 10.1038/sj.onc.1208969. [DOI] [PubMed] [Google Scholar]
- 67.Ballard DW, Böhnlein E, Lowenthal JW, Wano Y, Franza BR, Greene WC. HTLV-1 tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 68.Higuchi M, Tsubata C, Kondo R, Yoshida S, Takahashi M, Oie M, Tanaka Y, Mahieux R, Matsuoka M, Fujii M. Cooperation of NF-kappaB2/p100 activation and the PDZ domain binding motif signal in human T-cell leukemia virus type 1 (HTLV-1) Tax1 but not HTLV-2 Tax2 is crucial for interleukin-2-independent growth transformation of a T-cell line. J Virol. 2007;81:11900–11907. doi: 10.1128/JVI.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chu ZL, Shin YA, Yang JM, DiDonato JA, Ballard DW. IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. J Biol Chem. 1999;274:15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- 70.Harhaj EW, Sun SC. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 71.Xiao G, Harhaj EW, Sun SC. Domain-specific interaction with the I kappa B kinase (IKK)regulatory subunit IKK gamma is an essential step in tax-mediated activation of IKK. J Biol Chem. 2000;275:34060–34067. doi: 10.1074/jbc.M002970200. [DOI] [PubMed] [Google Scholar]
- 72.Wu X, Sun SC. Retroviral oncoprotein Tax deregulates NF-kappaB by activating Tak1 and mediating the physical association of Tak1-IKK. EMBO Rep. 2007;8:510–515. doi: 10.1038/sj.embor.7400931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harhaj NS, Sun SC, Harhaj EW. Activation of NF-kappa B by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of I kappa B kinase. J Biol Chem. 2007;282:4185–4192. doi: 10.1074/jbc.M611031200. [DOI] [PubMed] [Google Scholar]
- 74.Harhaj EW, Good L, Xiao G, Uhlik M, Cvijic ME, Rivera-Walsh I, Sun SC. Somatic mutagenesis studies of NF-kappa B signaling in human T cells: evidence for an essential role of IKK gamma in NF-kappa B activation by T-cell costimulatory signals and HTLV-1 Tax protein. Oncogene. 2000;19:1448–1456. doi: 10.1038/sj.onc.1203445. [DOI] [PubMed] [Google Scholar]
- 75.Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, Sun SC. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 2001;20:6805–6815. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Journo C, Filipe J, About F, Chevalier SA, Afonso PV, Brady JN, Flynn D, Tangy F, Israël A, Vidalain PO, et al. NRP/Optineurin Cooperates with TAX1BP1 to potentiate the activation of NF-kappaB by human T-lymphotropic virus type 1 tax protein. PLoS Pathog. 2009;5:e1000521. doi: 10.1371/journal.ppat.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang J, Ren T, Guan H, Jiang Y, Cheng H. HTLV-1 Tax is a critical lipid raft modulator that hijacks IkappaB kinases to the microdomains for persistent activation of NF-kappaB. J Biol Chem. 2009;284:6208–6217. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- 78.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shembade N, Parvatiyar K, Harhaj NS, Harhaj EW. The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling. EMBO J. 2009;28:513–522. doi: 10.1038/emboj.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shembade N, Harhaj NS, Liebl DJ, Harhaj EW. Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. EMBO J. 2007;26:3910–3922. doi: 10.1038/sj.emboj.7601823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shembade N, Harhaj NS, Parvatiyar K, Copeland NG, Jenkins NA, Matesic LE, Harhaj EW. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat Immunol. 2008;9:254–262. doi: 10.1038/ni1563. [DOI] [PubMed] [Google Scholar]
- 82.Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 83.Jin DY, Teramoto H, Giam CZ, Chun RF, Gutkind JS, Jeang KT. A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor alpha. J Biol Chem. 1997;272:25816–25823. doi: 10.1074/jbc.272.41.25816. [DOI] [PubMed] [Google Scholar]
- 84.Gachon F, Peleraux A, Thebault S, Dick J, Lemasson I, Devaux C, Mesnard JM. CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J Virol. 1998;72:8332–8337. doi: 10.1128/jvi.72.10.8332-8337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iha H, Peloponese JM, Verstrepen L, Zapart G, Ikeda F, Smith CD, Starost MF, Yedavalli V, Heyninck K, Dikic I, et al. Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-kappaB activation. EMBO J. 2008;27:629–641. doi: 10.1038/emboj.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shembade N, Harhaj NS, Yamamoto M, Akira S, Harhaj EW. The human T-cell leukemia virus type 1 Tax oncoprotein requires the ubiquitin-conjugating enzyme Ubc13 for NF-kappaB activation. J Virol. 2007;81:13735–13742. doi: 10.1128/JVI.01790-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol Cell Biol. 2005;25:10391–10406. doi: 10.1128/MCB.25.23.10391-10406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nasr R, Chiari E, El-Sabban M, Mahieux R, Kfoury Y, Abdulhay M, Yazbeck V, Hermine O, de Thé H, Pique C, et al. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-kappaB activation. Blood. 2006;107:4021–4029. doi: 10.1182/blood-2005-09-3572. [DOI] [PubMed] [Google Scholar]
- 89.Peloponese JM Jr, Iha H, Yedavalli VR, Miyazato A, Li Y, Haller K, Benkirane M, Jeang KT. Ubiquitination of human T-cell leukemia virus type 1 tax modulates its activity. J Virol. 2004;78:11686–11695. doi: 10.1128/JVI.78.21.11686-11695.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bex F, Murphy K, Wattiez R, Burny A, Gaynor RB. Phosphorylation of the human T-cell leukemia virus type 1 transactivator tax on adjacent serine residues is critical for tax activation. J Virol. 1999;73:738–745. doi: 10.1128/jvi.73.1.738-745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Durkin SS, Ward MD, Fryrear KA, Semmes OJ. Site-specific phosphorylation differentiates active from inactive forms of the human T-cell leukemia virus type 1 Tax oncoprotein. J Biol Chem. 2006;281:31705–31712. doi: 10.1074/jbc.M607011200. [DOI] [PubMed] [Google Scholar]
- 92.Yu Q, Minoda Y, Yoshida R, Yoshida H, Iha H, Kobayashi T, Yoshimura A, Takaesu G. HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem Biophys Res Commun. 2008;365:189–194. doi: 10.1016/j.bbrc.2007.10.172. [DOI] [PubMed] [Google Scholar]
- 93.Kfoury Y, Nasr R, Favre-Bonvin A, El-Sabban M, Renault N, Giron ML, Setterblad N, Hajj HE, Chiari E, Mikati AG, et al. Ubiquitylated Tax targets and binds the IKK signalosome at the centrosome. Oncogene. 2008;27:1665–1676. doi: 10.1038/sj.onc.1210804. [DOI] [PubMed] [Google Scholar]
- 94.Chiari E, Lamsoul I, Lodewick J, Chopin C, Bex F, Pique C. Stable ubiquitination of human T-cell leukemia virus type 1 tax is required for proteasome binding. J Virol. 2004;78:11823–11832. doi: 10.1128/JVI.78.21.11823-11832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lodewick J, Lamsoul I, Polania A, Lebrun S, Burny A, Ratner L, Bex F. Acetylation of the human T-cell leukemia virus type 1 Tax oncoprotein by p300 promotes activation of the NF-kappaB pathway. Virology. 2009;386:68–78. doi: 10.1016/j.virol.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nyborg JK, Egan D, Sharma N. The HTLV-1 Tax protein: Revealing mechanisms of transcriptional activation through histone acetylation and nucleosome disassembly. Biochim Biophys Acta. 2009:Epub ahead of print. doi: 10.1016/j.bbagrm.2009.09.002. [DOI] [PubMed] [Google Scholar]