Abstract
The reverse cholesterol transport mediated by high-density lipoprotein (HDL) is an important mechanism for maintaining body cholesterol, and hence, the crucial anti-atherogenic action of the lipoprotein. Recent studies, however, have shown that HDL exerts a variety of anti-inflammatory and anti-atherogenic actions independently of cholesterol metabolism. The present review provides an overview of the roles of sphingosine 1-phosphate (S1P)/S1P receptor and apolipoprotein A-I/scavenger receptor class B type I systems in the anti-atherogenic HDL actions. In addition, the physiological significance of the existence of S1P in the HDL particles is discussed.
Keywords: High-density lipoprotein, Sphingosine 1-phosphate, Scavenger receptor class B type I, Anti-atherogenic actions
INTRODUCTION
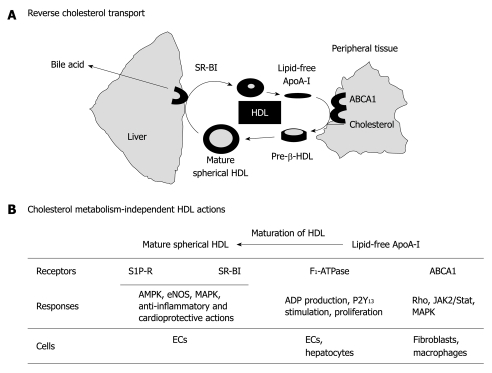
Plasma lipoprotein levels have been recognized as a crucial biomarker for the initiation and development of atherosclerosis[1-3]. Thus, high levels of low-density lipoprotein (LDL) and low levels of high-density lipoprotein (HDL) are thought to increase the risk of cardiovascular diseases, including atherosclerosis. LDL provides cholesterol to cells through LDL receptors, and HDL removes excess cholesterol from the cells, through ATP-binding cassette transporter A1 (ABCA1), in peripheral tissues, including arterial walls, and excretes it as bile acid through liver scavenger receptor class B type I (SR-BI). The so-called reverse cholesterol transport is thought to be one of the important anti-atherogenic actions of HDL[1,4] (Figure 1A). HDL particles are highly heterogeneous[5-7]. The lipid-free apolipoprotein (apo)A-I, which is newly synthesized in liver or formed by a recycling pathway through reverse cholesterol transport, is an acceptor for cholesterol and phospholipids through ABCA1, and stimulates their efflux from peripheral tissues, which results in the formation of nascent HDL particles or pre-β discoidal particles. As a consequence of the remodeling with enzymes relevant to the lipoprotein metabolism, such as lecithin:cholesterol acyltransferase (LCAT) and cholesteryl ester transfer protein, the pre-β discoidal particle grows and becomes more heterogeneous mature spherical HDL with a different size and composition. The mature spherical HDL particle is composed of enzymes, such as paraoxonase, platelet-activating factor acetylhydrolase (PAF-AH or Lp-PLA2), and LCAT, apolipoproteins (apoA-I and apoA-II), and lipid molecules, such as triglyceride, cholesterol, and phospholipids[5,8,9]. In addition, HDL has been shown to carry bioactive lipid molecules, including sphingosine 1-phosphate (S1P)[10,11] and related lysosphingolipids[12,13]. Protein components and phospholipids are present in the outer regions of the particle and triglyceride and cholesterol ester are present in the inner region. HDL-associated apoA-I also interacts with SR-BI in the liver for cholesterol extraction from HDL. Thus, apoA-I plays an important role in both transporter systems, i.e. ABCA1 and SR-BI. Reverse cholesterol transport through ABCA1 and SR-BI has been widely recognized to be a crucial mechanism of the anti-atherogenic actions of HDL[4,14]. In addition, the cholesterol metabolism-independent HDL actions have also been suggested to be important for the protection of cardiovascular system[8,9,15-20] (Figure 1B).
Figure 1.
Reverse cholesterol transport and roles of high-density lipoprotein-associated molecules in cholesterol metabolism-independent cellular activities. A: Scheme of simplified reverse cholesterol transport, in which lipid-free apolipoprotein (apo)A-I accelerates the efflux of excess cholesterol from the peripheral tissue through ATP-binding cassette transporter A1 (ABCA1), thereby forming pre-b-high-density lipoprotein (HDL). As a consequence of remodeling with enzymes relevant to the lipoprotein metabolism, such as lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein, the pre-b discoidal HDL grows and becomes heterogeneous mature spherical HDL, with a different size and composition. Finally, mature HDL supplies cholesterol to the liver through scavenger receptor class B type I (SR-BI), which results in the formation of bile acid; B: Heterogeneous products formed during the remodeling of HDL can be classified for convenience into three groups, i.e. lipid-free apoA-I, pre-β-HDL, and mature spherical HDL. These molecules stimulate endothelial cells (ECs) and hepatocytes through several cell-surface receptors (or transporters), including ABCA1, F1-ATPase, SR-BI, and sphingosine 1-phosphate (S1P) receptors. MAPK: Mitogen-activated protein kinase.
Two lines of independent research have revealed the crucial role of HDL-associated components in the anti-atherogenic actions of HDL. One line of research has focused on apoA-I. As mentioned above, SR-BI is a crucial transporter in liver for the efflux of cholesterol ester from spherical HDL. Recent studies have shown that SR-BI is also expressed in endothelial cells (ECs) and mediates HDL-associated apoA-I-induced stimulation of endothelial nitric oxide synthase (eNOS), inhibition of monocyte adhesion to ECs, vasorelaxation, and re-endothelialization following perivascular electric injury[18,21-23]. Another target of apoA-I might be F1-ATPase. Martinez et al[24] have reported that the mitochondrial-related F1-ATPase is a cell surface receptor for HDL, especially lipid-free apoA-I, and is involved in HDL endocytosis in hepatocytes through extracellular ADP production. They recently have reported that apoA-I stimulates the hydrolysis of ATP to ADP through cell surface F1-ATPase, which results in anti-apoptosis and proliferation in human umbilical vein ECs (HUVECs). These apoA-I actions are blocked by the anti-βF1-ATPase antibody independently of the scavenger receptor SR-BI and ABCA1 transporter. They have proposed that the anti-apoptotic and proliferative effects of apoA-I are mediated through F1-ATPase-catalysed ADP production and subsequent P2Y13 receptor stimulation, thus contributing to the atheroprotective functions[25]. The potential role of ABCA1 as a target of lipid-free apoA-I to couple intracellular signaling pathways has also been suggested. Cholesterol efflux initiated by apoA-I binding to a specific site of ABCA1 is associated with the activation of Rho-related small GTPases (Cdc42, Rac1, and Rho) and mitogen-activated protein kinases [MAPKs; c-jun NH2-terminal kinase, p38MAPK, and extracellular signal-regulated kinase (ERK)] in fibroblasts[26]. Haidar et al[27] have reported that apoA-I activates cAMP accumulation through the ABCA1 transporter in ABCA1-transfected CHO cells. Moreover, Tang et al[28] have shown that apoA-I activates Janus kinase 2 and STAT3, thereby inhibiting the production of inflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, in macrophages.
Another line of research has focused on HDL-associated lysolipid molecules, especially S1P. A variety of HDL actions have been demonstrated to be mediated by the lipoprotein-associated S1P in cells involved in cardiovascular systems, including ECs, smooth muscle cells (SMCs), cardiomyocytes, and macrophages, as described later. S1P is synthesized by sphingosine kinase in the cells and exported to the extracellular space; however, the mechanism of S1P export remains unknown. It should be determined whether there is any difference in biological activity between the HDL-associated S1P and other S1P that is associated with albumin or LDL. Moreover, the physiological meaning of S1P association with HDL should be explored. The present review includes a discussion of these unresolved questions.
ACCUMULATION OF S1P IN HDL FRACTIONS
Early studies by Assmann’s group have shown that HDL regulates cellular activities, including phospholipase C and DNA synthesis in fibroblasts[12] and fibrinogen binding and aggregation in platelets[29]. They also have found that HDL inhibits apoptosis of ECs through phosphoinositide 3-kinase (PI3K) and Akt activation[13]. Based on the fractionation analysis of the active components of HDL by HPLC, they have proposed that sphingosylphosphorylcholine (SPC) and lysosulfatide (LSF) mediate the HDL-induced actions[12,13]. It should be noted, however, that the existence of SPC and LSF at a high level to explain the HDL actions has been questioned[30]. DeKroon et al[31] have failed to detect LSF in HDL particles. Moreover, Liliom et al[32] have reported that the plasma SPC level is about 50 nmol/L, which is about 1/100 of the level reported by Nofer et al[12]. Sachinidis et al[33] have reported that lipid molecules closely related to SPC and S1P mediate LDL- and HDL-induced Ca2+ mobilization and ERK activation in vascular SMCs (VSMCs), on the basis of organic solvent purification and subsequent HPLC analysis.
We established a quantitative S1P measurement based on the high affinity and specificity of S1P receptors to S1P[10]. Using this method, we examined the distribution of S1P in plasma. Even though the plasma was vigorously dialyzed against PBS, the S1P content was unchanged[10], which suggests that S1P is tightly bound to plasma components. Fractionation by density gradient centrifugation and measurement of S1P in each fraction has shown that S1P is concentrated in the lipoprotein fraction with a rank order of HDL > LDL > very low density lipoprotein (VLDL), and to a lesser extent, in the lipoprotein-deficient albumin fraction when expressed as pmol/mg protein[10]. Thus, lipoproteins, especially HDL, seem to serve as carriers of plasma S1P, and hence, HDL-S1P has been proposed to mediate a variety of HDL-induced actions, as described in detail in the next chapter. When HDL-S1P is plotted against HDL-cholesterol, there is a good correlation between them[34]. This result indicates that a person with a high HDL-cholesterol level has a high HDL-S1P level, which further supports the role of S1P as a mediator of HDL-induced anti-atherogenic actions. The existence of S1P in HDL particles has been confirmed by several groups[7,35]. According to the study[7], S1P is preferentially enriched in small HDL3 vs large HDL2. It should be noted, however, that 1 mol HDL contains 15-50 mmol S1P, whereas it contains 3-4 mol apoA-I. This implies that 95%-98% of HDL is free of S1P, whereas each HDL particle contains about 3-4 molecules of apoA-I on average.
Platelets maintain high levels of S1P content due to the very low activity of the S1P-degrading enzyme, S1P lyase[36,37], therefore, it was initially thought to be a source of plasma S1P. However, Pappu et al[38] have shown that transcriptional factor NF-E2-deficent mice have normal plasma S1P concentrations despite having virtually no circulating platelets. They also have found that transferring wild-type erythrocytes to sphingosine-kinase-deficient mice, which have no detectable S1P in plasma, restored the normal plasma S1P level, and they have proposed that erythrocytes are a major S1P source in the blood[38]. The role of erythrocytes in the regulation of plasma S1P has been supported by Hänel et al[39]. Nonetheless, platelets might be important for the supply of high levels of S1P at clot loci[40]. Venkataraman et al[41] have recently shown that elimination of hematopoietic cells by chemically induced anemia and lethal whole-body irradiation, followed by reconstitution of bone marrow from sphingosine-kinase-deficient mice, fails to reduce plasma S1P, although erythrocytes decrease to more than two-thirds of the normal levels, and they have proposed that the vascular endothelium is also involved in the regulation of plasma S1P level. ABC transporters might be involved in the export of S1P[42]. Kobayashi et al[43] have shown that S1P release from platelets and erythrocytes[44] is inhibited by glibenclamide, a non-specific inhibitor of the ABC transporter. Mitra et al[45] have shown that ABCC1 is involved in S1P export from mast cells in response to albumin-conjugated antigen. Takabe et al[46] also have shown that ABCC1 and ABCG2 are involved in estradiol-induced S1P export from breast cancer cells. A transporter-like protein, Spns2, has recently been demonstrated to be an S1P transporter that is involved in myocardial precursor migration and heart development of zebrafish[47]. It remains undetermined, however, how S1P is accumulated in plasma lipoprotein fractions. As described above, ABCA1 is known to transport cholesterol and phospholipids to lipid-poor apoA-I or apoE and thereby mediate HDL formation[48-50]. In the central nervous system, S1P seems to bind to HDL-like particles[51], and there, astroglial cells are major sources of lipoproteins through ABCA1. Knockdown and knockout of the ABCA1 transporter of astroglial cells markedly attenuates S1P release from the cells, in association with the reduction of lipoprotein formation, which suggests that lipoprotein formation through ABCA1 is coupled with S1P release in astroglial cells[52]. The role of ABCA1, however, has not been proved in S1P accumulation in plasma lipoproteins.
ROLE OF HDL-ASSOCIATED S1P IN ANTI-ATHEROGENIC ACTIONS
The physiological and pathological actions of S1P and HDL-S1P in the cardiovascular system have been extensively discussed in recent reviews[17-20,53-57], therefore, the HDL actions that have been demonstrated to be mediated by S1P in the cardiovascular system are briefly summarized here.
In vitro HDL-S1P actions
ECs: Using anti-sense oligonucleotides and an RNAi strategy, we have demonstrated that S1P mediates HDL-induced cell survival through S1P1/Gi/ERK pathways and migration through the S1P1 and S1P3/Gi/PI3K/p38MAPK pathway in HUVECs[11,30]. HDL-associated S1P, through the Gi/Ras/ERK pathway, has been shown to induce angiogenesis in human coronary artery ECs[58]. HDL-like lipoproteins are also present in the follicular fluid of the ovary and induce angiogenesis through S1P in association with the activation of ERK, protein kinase C, and Akt in ECs[59]. Calcium/calmodulin-dependent protein kinase kinase (CaMKK) and serine-threonine kinase LKB1-mediated AMP-activated protein kinase (AMPK) activation seems to be involved in the HDL- and S1P-induced activation of PI3K, Akt, and eNOS in ECs[60,61]. Nofer et al[35] have reported that HDL, possibly through its associated lysosphingolipids, activated PI3K and Akt, stimulates eNOS and NO synthesis, and inhibits apoptosis[13] and TNF-α-induced E-selectin expression[62] in ECs. The role of S1P3 receptors in the HDL-induced eNOS activation has been proposed on the basis of the inhibition of S1P actions by S1P3 deficiency[35]. The receptor subtypes involved, however, are controversial. Argraves et al[63] have reported that the S1P1 receptor mediates HDL-induced Akt activation and subsequent stimulation of EC barrier integrity. The role of the S1P1 receptor in the activation of eNOS through the PI3K/Akt pathways has been reported in HUVECs[63-66], bovine aortic ECs[67], and lung microvascular ECs[68]. Stimulation of bovine aortic ECs[69] and HUVECs[70] with statins, such as pitavastatin and simvastatin, has resulted in the enhancement of HDL-induced eNOS activation. The enhancement appears to be in part due to the increase in S1P1 receptor expression[69]. Moreover, Hedrick’s group also have provided evidence that S1P1 receptors mediate eNOS-dependent inhibition of adhesion of monocytes to the endothelium in in vivo and ex vivo mouse models[71,72]. In contrast, Norata et al[73] have reported that HDL induces transforming growth factor (TGF)-β2 expression and Smad activation through PI3K/Akt, and increases expression of long pentraxin 3 (PTX3), an acute phase protein, through Gi/PI3K[74] in ECs. The siRNA experiments have suggested that HDL-induced PTX3 expression is mediated by S1P1 and S1P3 receptors.
SMCs: Sachinidis et al[33] have reported that lipid molecules closely related to SPC and S1P mediate LDL- and HDL-induced Ca2+ mobilization and ERK activation in VSMCs. Chrisman et al[75,76] have shown that HDL-associated S1P induces the desensitization of guanylyl cyclase B, a receptor for C-type natriuretic peptide (CNP), through Gi-proteins and thereby inhibits CNP-induced cGMP accumulation and proliferation in VSMCs. HDL markedly inhibits platelet-derived growth factor (PDGF)-induced migration of VSMCs through S1P2 receptors[77,78]. Monocyte chemoattractant protein-1 (MCP-1) has been shown to be involved in monocyte recruitment to the site of vascular inflammation, and to be elevated in atherosclerotic lesions. Treatment of VSMCs or isolated aortas with HDL inhibits thrombin-induced MCP-1 mRNA and protein expression, which is associated with suppression of NADPH oxidase, reactive oxygen species (ROS) production, and Rac1 activation. The HDL-induced actions are abolished by the S1P receptor antagonist, VPC23019, and mimicked by S1P and SPC. Moreover, HDL, S1P and SPC fail to inhibit MCP-1 production and ROS generation in aortas from S1P3-deficient mice[79]. These results suggest that HDL-associated S1P and SPC mediate the lipoprotein-induced inhibition of ROS and MCP-1 production through S1P3 receptors in VSMCs. González-Díez et al[80] have shown that HDL-S1P induces cyclooxygenase (COX)-2 expression and prostaglandin I2 production through S1P2 and S1P3 receptors in human VSMCs, and thereby protects the cardiovascular system. They also have shown that simvastatin potentiates the HDL- and S1P-induced COX-2 expression by enhancing expression of S1P3 receptors[80].
Cardiomyocytes: In cardiomyocytes, S1P1-4 receptors seem to be expressed[81]. HDL and S1P have been reported to protect cardiomyocytes against apoptosis in vitro[82,83]. Frias et al[84] have recently shown that HDL-S1P activates ERK and STAT3 through S1P2 receptors in rat ventricular cardiomyocytes, which might be involved in protection from apoptosis[85]. Tao et al[86] have shown that HDL protects mouse cardiomyocytes from apoptosis via S1P1 and S1P3, through mechanisms that involve the activation of ERK, PI3K, and Akt in a hypoxia/re-oxygenation model in vitro.
Monocytes and macrophages: Human monocytes and macrophages express S1P1, S1P2 and S1P4, and during differentiation of monocytes into macrophages, S1P3 is induced[87]. Although S1P actions have been extensively investigated in monocytes and macrophages[88], the roles of HDL-S1P have not been well characterized in cells. In human peripheral blood monocytes and monocyte-derived macrophages, HDL inhibits expression of chemokines, including macrophage inflammatory protein (MIP)-1α, MIP-1β, and IL-8, induced by lipopolysaccharide (LPS), a Toll-like receptor (TLR) 4 agonist; however, S1P attenuates TLR2- rather than TLR4-mediated nuclear factor (NF)-κB activation and chemokine production through S1P1 and S1P2 receptors[89]. This suggests that components other than S1P seem to mediate the HDL-induced actions.
In vivo HDL-S1P actions
Nofer et al[35] have shown that deficiency of S1P3 receptors abolishes HDL-induced vasodilation. They also have shown that intra-arterial administration of HDL and S1P lowers mean arterial blood pressure in rats. S1P has been shown to improve ischemia/reperfusion-induced injury in vivo[90]. Theilmeier et al[83] have shown that HDL and S1P dramatically attenuate infarction size in an in vivo mouse model of myocardial ischemia/reperfusion, which is associated with inhibition of inflammatory neutrophil recruitment and cardiomyocyte apoptosis in the infarcted area. They also have observed that the HDL- and S1P-induced actions are abolished by pharmacological NOS inhibition, and are completely absent in S1P3-deficient mice. Thus, HDL and its constituent, S1P, protect the heart against ischemia/perfusion injury in vivo via the S1P3-mediated and NO-dependent pathway in ECs and cardiomyocytes. The same group has previously reported that HDL administration increases myocardial perfusion in a manner dependent on eNOS activation, which reflects a vasodilatory effect on the coronary circulation in vivo. However, the vasodilatory effect of HDL is unchanged in S1P3-deficient mice, which implicates an unknown system other than S1P3 receptors in the HDL-induced eNOS/vasorelaxation effect in vivo[91].
PHYSIOLOGICAL MEANING OF THE EXISTENCE OF S1P IN HDL
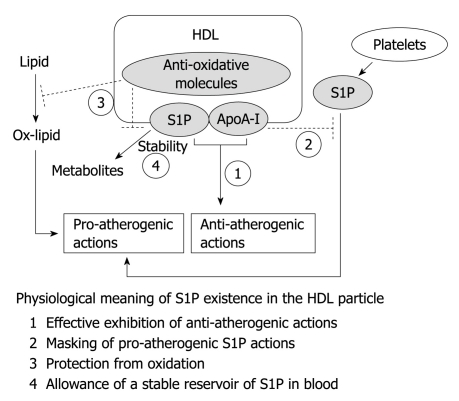
In this chapter, the physiological meaning and advantages of the existence of S1P in the HDL particle are discussed (Figure 2).
Figure 2.
Physiological meaning of sphingosine 1-phosphate in the high-density lipoprotein particle. The possible advantages of sphingosine 1-phosphate (S1P) in the high-density lipoprotein (HDL) particle are summarized. In this model, the pro-atherogenic S1P is postulated to be released from platelets at the clot locus. It is not known whether plasma albumin-associated S1P, which might be released from erythrocytes under physiological conditions, is anti- or pro-atherogenic. See the text for details. ApoA-I: Apolipoprotein A-I.
ApoA-I/SR-BI is an independent-signaling system for HDL rather than simply an anchor for HDL-S1P
Recent studies have shown that SR-BI is also expressed in ECs and mediates HDL-associated apoA-I-induced stimulation of AMPK, Akt, and eNOS activation, which results in inhibition of adhesion molecule expression and monocyte adhesion to ECs[18,21,22]. The roles of SR-BI in HDL-induced vasorelaxation in isolated aorta[92] and the repair of endothelium after injury in vivo[93] have been confirmed using SR-BI-deficient mice. In support of the role of SR-BI as a receptor that links to intracellular signaling pathways, knockdown or knockout of the adapter protein of SR-BI, termed PDZK1[94], which contains four PSD-95/Dlg/ZO-1 (PDZ) domains, has been shown to attenuate the HDL-induced activation of eNOS and subsequent inhibition of NF-κB activation and adhesion of monocytes to HUVECs[66], and carotid artery re-endothelialization following perivascular electric injury in vivo[95]. Thus, although the roles of SR-BI have been suggested by experiments using in vitro receptor knockdown and in vivo receptor knockout strategy, there is still controversy on the role of SR-BI as a receptor coupling to intracellular signaling pathways. Nofer et al[35] have proposed that SR-BI plays a role, through apoA-I, as an anchor for HDL-associated S1P and other lysolipids to interact with S1P receptors to stimulate intracellular signaling pathways. Their proposal is based on the finding that reconstituted apoA-I with cholesterol and phospholipids is ineffective to stimulate eNOS and MAPKs, whereas antibodies against apoA-I and SR-BI effectively attenuate HDL-induced enzyme activation[92]. Tölle et al[79] recently have reported that aortas from either S1P3 receptor-deficient mice or SR-BI-deficient mice have shown attenuation of the inhibitory actions of not only HDL, but also S1P on MCP-1 production, even though S1P3 receptor expression remains unchanged by SR-BI deficiency. This result raises the possibility that the S1P3 receptor signal transduction system is also damaged by SR-BI deficiency. Thus, we must interpret the results of SR-BI deficiency with caution; the reduction of the activities in the cells or tissues from SR-BI-deficient mice does not always eliminate the possible involvement of the S1P receptors in the HDL-induced actions.
However, Assanasen et al[96] have found that the reconstituted apoA-I with phospholipids, but without cholesterol or with a reduced level of cholesterol, can stimulate SR-BI, which results in activation of eNOS and MAPKs. They have speculated that cholesterol efflux from the intracellular space is necessary for SR-BI activation by apoA-I. The ability of rHDL or reconstituted apoA-I with phospholipids but without cholesterol to stimulate eNOS activation has been confirmed by our research group[66]. We have also shown that HDL-induced eNOS activation and subsequent inhibition of NF-κB-mediated adhesion molecule expression are attenuated by SR-BI or S1P receptor siRNA, and completely blocked by the combination of siRNAs against SR-BI and S1P receptors in HUVECs. Moreover, the rHDL preparation, in which no S1P exists, stimulates eNOS activation in a manner that is sensitive to SR-BI siRNA but not to S1P receptor siRNA, and S1P stimulates the enzyme activity in an opposite manner[66]. The enhancement of SR-BI expression by simvastatin results in enhancement of HDL- and rHDL-induced, but not S1P-induced, eNOS activation and subsequent inhibition of adhesion molecule expression[70], which supports the role of SR-BI in HDL-induced anti-inflammatory actions. rHDL preparation has also been shown to repair damaged endothelium[97] and to enhance ischemia-induced angiogenesis through stimulation of endothelial progenitor cells (EPCs)[98] in vivo. Moreover, rHDL increases circulating EPCs in patients with type 2 diabetes[99]. Feng et al[100] recently have reported that the transfer of apoA-I and subsequent increase in the level of circulating HDL attenuates artery-transplantation-induced neointima formation, in association with stimulation of EPC incorporation into the endothelium and acceleration of its regeneration. They also have demonstrated that attenuation of allograft vasculopathy requires expression of SR-BI in bone-marrow-derived EPCs, using SR-BI-deficient mice. These results suggest that SR-BI and S1P receptors are independently involved in the mature HDL-induced stimulation of intracellular signaling pathways in ECs.
HDL-associated apoA-I masks pro-inflammatory actions of S1P
S1P has been shown to exert diverse actions, which are beneficial and in some cases detrimental, to the cardiovascular system, depending on the expression profile of S1P receptor subtypes[18-20]. For example, in vascular ECs, S1P stimulates migration and proliferation or anti-apoptotic action in ECs, which might be important for maintaining intact ECs and repairing injured ECs. S1P also activates AMPK, Akt, and eNOS[60,61,68] and inhibits cytokine-induced adhesion molecule expression[71,101]. In rat neonatal cardiomyocytes, S1P rescues cardiomyocytes from hypoxic cell death[82]. S1P administration improves ischemia/reperfusion-induced injury[83,90]. S1P has also been shown to inhibit, through S1P2 receptors, SMC migration[78], which is suggested to be involved in the formation of neointima formation. In contrast to these beneficial effects, S1P alone has also been reported to induce the expression of adhesion molecules[101-104], which might cause the stimulation of leukocyte interaction with ECs, and leukocyte penetration into the subendothelial space or the arterial intima. Thus, adhesion of leukocytes to ECs is thought to be a crucial early step of atherogenesis. S1P has also been reported to stimulate the expression of tissue factor, an essential factor for blood coagulation through the activation of transcriptional factors NF-κB and early growth response-1 (Egr-1) in HUVECs[105], and endothelial exocytosis of Weibel-Palade bodies that contain several factors, including P-selectin, von Willebrand factor, and tissue plasminogen activator in human aortic ECs[106].
Thus, S1P has the potential to exert pro- and anti-atherogenic actions. What factors determine S1P as either a pro- or anti-atherogenic signal? As described above, although S1P stimulates adhesion of monocytes to ECs, the lysolipid simultaneously inhibits TNF-α-induced adhesion of monocytes to ECs. The stimulatory and inhibitory actions might be mediated by different S1P receptor subtypes; the stimulatory action involves NF-κB activation predominantly through the S1P3 receptor, and the inhibitory action involves PI3K/eNOS activation predominantly through the S1P1 receptor[71,101]. Apart from the question of whether S1P-induced actions are pro- or anti-atherogenic, S1P receptor subtypes mediate, in some cases, opposite actions in the cardiovascular system[18]. For example, S1P1 and S1P3 receptors stimulate proliferation and migration of SMCs, whereas S1P2 receptor inhibits these cellular activities. As for angiogenesis, the S1P1 receptor stimulates, whereas the S1P2 receptor inhibits, the EC migration and Rac activity[53]. As shown previously[101], pro-atherogenic or detrimental actions usually require a higher concentration of S1P than that required for anti-atherogenic or beneficial actions. Thus, pro-atherogenic adhesion molecule expression and vasoconstriction might occur at the platelet clot site, where a very high concentration of S1P might be present, under pathological conditions[40]. It remains unknown, however, whether lipoprotein-deficient plasma or albumin-associated S1P, which might be released from erythrocytes and bound to plasma albumin at a level as high as 50 mg/mL, is anti- or pro-atherogenic. In addition to the difference in the potency of S1P between beneficial and detrimental effects, HDL-associated apoA-I might play an important role in abolishing the detrimental actions of S1P. For example, pro-atherogenic adhesion molecule expression elicited by S1P disappears in the presence of physiological concentrations of HDL in a manner sensitive to SR-BI[66]. This result implies that, as far as S1P is associated with HDL, S1P never exerts pro-atherogenic actions. Thus, whether S1P is a pro- or anti-atherogenic signal seems to be determined by the pattern of S1P receptor subtypes expressed, the S1P concentration, the S1P sources (plasma or local cells), or the S1P binding proteins (HDL or albumin).
Anti-oxidative property of HDL
LDL contains S1P to the extent of 20%-40% of the S1P content in HDL when expressed as pmol/mg protein[10,11]. In agreement with this, LDL, similar to HDL, inhibits serum-starvation-induced EC death in association with ERK activation, and stimulates EC migration in association with p38MAPK activation[11,30,107]. However, LDL never inhibits monocyte adhesion and expression of adhesion molecules, such as vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, in ECs, whereas HDL and S1P clearly inhibit these activities[66]. As reported above, HDL and S1P inhibit PDGF-induced migration of VSMCs through S1P2 receptors. However, LDL and mildly oxidized-LDL (ox-LDL) stimulate rather than inhibit migration at even high concentrations, in which a sufficient amount of S1P exists to inhibit the migration response to PDGF[78]. The lack of LDL effects might be partly related to its susceptibility to oxidation.
The oxidative modification of LDL is a crucial step for the initiation and development of atherosclerosis[8,108]. In contrast, HDL is protective against oxidation and inhibits LDL oxidation, which is most likely due to the presence of anti-oxidants, anti-oxidative apoA-I, and anti-oxidative enzymes, such as paraoxonase, which hydrolyze ROS[5,9,109]. HDL also inhibits ox-LDL-induced inflammatory responses[9]. Spontaneous oxidation or mild oxidation with Fe2+ of LDL facilitates the production of lysophosphatidic acid (LPA)[78,110], which has been shown to be present in ox-LDL in atherosclerotic lesions[110]. Severe oxidation with Cu2+ causes the breakdown of S1P in association with LPA production in LDL[11,78]. LPA has been shown to stimulate expression of adhesion molecules in ECs[111] and migration of VSMCs[78]. Phospholipid oxidation products, such as 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine and 1-palmitoyl-2-(5,6 epoxyisoprostanoyl)-sn-glycero-3-phosphocholine, are also accumulated in ox-LDL at the site of atherosclerotic lesions and activate ECs, thereby enhancing monocyte/EC interactions[112] and stimulating migration of VSMCs[78]. HDL, through the S1P component, inhibits oxidized phospholipid-induced inflammatory cytokine production in ECs[113]. Thus, the lack of the inhibitory LDL and ox-LDL effects on adhesion molecule expression, despite the presence of S1P, could be accounted for by the accumulation of LPA and/or oxidized phospholipids, which act oppositely to S1P. Supporting this notion, when LDL-associated LPA is degraded by monoglyceride lipase, or LPA1 receptors on coronary artery SMCs are antagonized by Ki16425, an LPA1 receptor antagonist, even LDL is able to inhibit the PDGF-induced migration through S1P2 receptors[78]. These results suggest that at least LPA in the LDL particle masks the inhibitory S1P action on VSMCs, and that a balance of LPA and S1P contents in the lipoprotein is important to determine whether the lipoprotein is a positive or negative regulator of VSMC migration. It remains unknown, however, how LPA is synthesized during oxidation of LDL.
HDL allows a stable reservoir of S1P in blood
Although the plasma concentration of S1P is 200-900 nmol/L, the potency of S1P to trigger S1P receptors is around 10-100 nmol/L in in vitro assays. If plasma S1P is fully active, all the S1P receptors are saturated with S1P and fully activated. The addition of plasma that contains a low level of S1P (low-S1P plasma), which is prepared by extensive treatment of plasma with charcoal, causes a marked rightward shift of the concentration-dependent phosphatidylinositol phosphate response to S1P. For example, the addition of 10% low-S1P plasma to the assay medium increases the EC50 by about 10 times. Based on the inhibitory activity of the low-S1P plasma, we anticipated that, under physiological conditions, i.e. in the presence of 100% plasma, the active S1P content would be only 7.3 nmol/L, whereas the total S1P would be 357 nmol/L[10]. Thus, only 2% of plasma S1P is speculated to be active. As reported above, we found that S1P is bound to high-molecular-weight molecules, such as lipoproteins and albumin, in plasma with a rank order of HDL > LDL > VLDL > albumin when expressed as pmol/mg proteins[10]. However, because the total albumin content is very high, plasma S1P is roughly distributed equally (50%) between both lipoproteins and lipoprotein-deficient albumin fractions when expressed as pmol/mL plasma.
At first, we speculated that HDL is the major plasma component to inhibit the activity of S1P because the HDL traps S1P most effectively among plasma components. However, HDL-S1P is as potent as albumin-S1P (in the presence of 0.1% bovine serum albumin unless otherwise specified) to stimulate ERK[11], the migration response[30], and NOS[66,101] when plotted as total S1P content in the assay system, which suggests that HDL-S1P is biologically fully active. Therefore, we tentatively speculate that a high concentration of plasma albumin (approximately 50 mg/mL or 5%) is a major cause of the interference with S1P to interact with its receptor under physiological conditions. In contrast, when long-term functions such as cell survival are concerned, HDL-S1P is 10-30 times more potent than albumin-S1P[11]. The difference in the potency of HDL-S1P and albumin-S1P between the short-term response, such as ERK (5 min) and NOS (10 min) activation, and the long-term response, such as survival (24 h), can be explained, at least in part, by the difference in the fourfold longer half-life of HDL-associated S1P than that of albumin-S1P[11]. This suggests that binding to HDL protects S1P from degradation by ectoenzymes, such as lipid phosphate phosphohydrolases. The anti-oxidative nature of HDL might also protect S1P from oxidative degradation. Thus, HDL might allow a stable reservoir of S1P in the blood and modulate S1P actions, especially long-term anti-atherogenic actions of the lipid mediator.
CONCLUSION
HDL has long been known to exert anti-atherogenic actions through reverse cholesterol transport. However, a number of studies have emerged that have indicated that HDL also exerts a variety of actions independently of cholesterol metabolism. Two major systems have been proposed: S1P/S1P receptors and apoA-I/SR-BI. In the present review, in addition to roles of these systems in the HDL-induced anti-atherogenic actions, the merit of S1P in the HDL particle has been discussed. Although the anchoring role of SR-BI to help HDL-S1P interact with S1P receptors cannot be eliminated, SR-BI might actively mediate the HDL-apoA-I signal through PDZK1 to intracellular signaling pathways. The coexistence of apoA-I might mask the pro-atherogenic actions of S1P by stimulation of SR-BI. The anti-oxidative property of HDL is also important for inhibition of production of pro-atherogenic substances, such as LPA and oxidized phospholipids. Finally, HDL might interfere with S1P degradation, thereby allowing S1P to exhibit long-term anti-atherogenic actions.
Footnotes
Supported by Grants-in-Aid for scientific research from the Japan Society for the Promotion of Science, No. 20015008, 20054003, and 21390016
Peer reviewers: Siddhartha Das, PhD, Professor, Department of Biological Sciences, University of Texas at El Paso, El Paso, TX 79968-0519, United States; Shu-Wen Liu, PhD, Professor, School of Pharmaceutical Sciences, Southern Medical University, 1838, Guangzhou Avenue North, Guangzhou 510515, Guangdong Province, China
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am J Cardiol. 2003;92:42J–49J. doi: 10.1016/s0002-9149(03)00615-5. [DOI] [PubMed] [Google Scholar]
- 2.Assmann G, Gotto AM Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–II14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 3.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 4.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 5.Scanu AM, Edelstein C. HDL: bridging past and present with a look at the future. FASEB J. 2008;22:4044–4054. doi: 10.1096/fj.08-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 7.Kontush A, Therond P, Zerrad A, Couturier M, Négre-Salvayre A, de Souza JA, Chantepie S, Chapman MJ. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler Thromb Vasc Biol. 2007;27:1843–1849. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 8.Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 9.Negre-Salvayre A, Dousset N, Ferretti G, Bacchetti T, Curatola G, Salvayre R. Antioxidant and cytoprotective properties of high-density lipoproteins in vascular cells. Free Radic Biol Med. 2006;41:1031–1040. doi: 10.1016/j.freeradbiomed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352 Pt 3:809–815. [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, Ui M, Okajima F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 12.Nofer JR, Fobker M, Höbbel G, Voss R, Wolinska I, Tepel M, Zidek W, Junker R, Seedorf U, von Eckardstein A, et al. Activation of phosphatidylinositol-specific phospholipase C by HDL-associated lysosphingolipid. Involvement in mitogenesis but not in cholesterol efflux. Biochemistry. 2000;39:15199–15207. doi: 10.1021/bi001162a. [DOI] [PubMed] [Google Scholar]
- 13.Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, Seedorf U, Assmann G. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem. 2001;276:34480–34485. doi: 10.1074/jbc.M103782200. [DOI] [PubMed] [Google Scholar]
- 14.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 16.Nofer JR, Assmann G. Atheroprotective effects of high-density lipoprotein-associated lysosphingolipids. Trends Cardiovasc Med. 2005;15:265–271. doi: 10.1016/j.tcm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48:2325–2333. doi: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Okajima F, Sato K, Kimura T. Anti-atherogenic actions of high-density lipoprotein through sphingosine 1-phosphate receptors and scavenger receptor class B type I. Endocr J. 2009;56:317–334. doi: 10.1507/endocrj.k08e-228. [DOI] [PubMed] [Google Scholar]
- 19.Sattler K, Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res. 2009;82:201–211. doi: 10.1093/cvr/cvp070. [DOI] [PubMed] [Google Scholar]
- 20.Daum G, Grabski A, Reidy MA. Sphingosine 1-phosphate: a regulator of arterial lesions. Arterioscler Thromb Vasc Biol. 2009;29:1439–1443. doi: 10.1161/ATVBAHA.108.175240. [DOI] [PubMed] [Google Scholar]
- 21.Mineo C, Shaul PW. Role of high-density lipoprotein and scavenger receptor B type I in the promotion of endothelial repair. Trends Cardiovasc Med. 2007;17:156–161. doi: 10.1016/j.tcm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Saddar S, Mineo C, Shaul PW. Signaling by the high-affinity HDL receptor scavenger receptor B type I. Arterioscler Thromb Vasc Biol. 2010;30:144–150. doi: 10.1161/ATVBAHA.109.196170. [DOI] [PubMed] [Google Scholar]
- 23.Kimura T, Sato K, Tomura H, Okajima F. Cross-talk between exogenous statins and endogenous high-density lipoprotein in anti-inflammatory and anti-atherogenic actions. Endocr Metab Immune Disord Drug Targets. 2010;10:8–15. doi: 10.2174/187153010790827939. [DOI] [PubMed] [Google Scholar]
- 24.Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezón E, Champagne E, Pineau T, Georgeaud V, Walker JE, Tercé F, et al. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- 25.Radojkovic C, Genoux A, Pons V, Combes G, de Jonge H, Champagne E, Rolland C, Perret B, Collet X, Tercé F, et al. Stimulation of cell surface F1-ATPase activity by apolipoprotein A-I inhibits endothelial cell apoptosis and promotes proliferation. Arterioscler Thromb Vasc Biol. 2009;29:1125–1130. doi: 10.1161/ATVBAHA.109.187997. [DOI] [PubMed] [Google Scholar]
- 26.Nofer JR, Feuerborn R, Levkau B, Sokoll A, Seedorf U, Assmann G. Involvement of Cdc42 signaling in apoA-I-induced cholesterol efflux. J Biol Chem. 2003;278:53055–53062. doi: 10.1074/jbc.M305673200. [DOI] [PubMed] [Google Scholar]
- 27.Haidar B, Denis M, Marcil M, Krimbou L, Genest J Jr. Apolipoprotein A-I activates cellular cAMP signaling through the ABCA1 transporter. J Biol Chem. 2004;279:9963–9969. doi: 10.1074/jbc.M313487200. [DOI] [PubMed] [Google Scholar]
- 28.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nofer JR, Walter M, Kehrel B, Wierwille S, Tepel M, Seedorf U, Assmann G. HDL3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol 1,4,5-tris-phosphate. Arterioscler Thromb Vasc Biol. 1998;18:861–869. doi: 10.1161/01.atv.18.6.861. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler Thromb Vasc Biol. 2003;23:1283–1288. doi: 10.1161/01.ATV.0000079011.67194.5A. [DOI] [PubMed] [Google Scholar]
- 31.DeKroon RM, Mihovilovic M, Goodger ZV, Robinette JB, Sullivan PM, Saunders AM, Strittmatter WJ. ApoE genotype-specific inhibition of apoptosis. J Lipid Res. 2003;44:1566–1573. doi: 10.1194/jlr.M300097-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Liliom K, Sun G, Bünemann M, Virág T, Nusser N, Baker DL, Wang DA, Fabian MJ, Brandts B, Bender K, et al. Sphingosylphosphocholine is a naturally occurring lipid mediator in blood plasma: a possible role in regulating cardiac function via sphingolipid receptors. Biochem J. 2001;355:189–197. doi: 10.1042/0264-6021:3550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachinidis A, Kettenhofen R, Seewald S, Gouni-Berthold I, Schmitz U, Seul C, Ko Y, Vetter H. Evidence that lipoproteins are carriers of bioactive factors. Arterioscler Thromb Vasc Biol. 1999;19:2412–2421. doi: 10.1161/01.atv.19.10.2412. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Tomura H, Kuwabara A, Kimura T, Miura S, Noda K, Okajima F, Saku K. Correlation of high density lipoprotein (HDL)-associated sphingosine 1-phosphate with serum levels of HDL-cholesterol and apolipoproteins. Atherosclerosis. 2005;178:199–205. doi: 10.1016/j.atherosclerosis.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Nofer JR, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Gödecke A, Ishii I, Kleuser B, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yatomi Y. Sphingosine 1-phosphate in vascular biology: possible therapeutic strategies to control vascular diseases. Curr Pharm Des. 2006;12:575–587. doi: 10.2174/138161206775474404. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 38.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 39.Hänel P, Andréani P, Gräler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 40.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi N, Kobayashi N, Yamaguchi A, Nishi T. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J Biol Chem. 2009;284:21192–21200. doi: 10.1074/jbc.M109.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;285:10477–10486. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 48.Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol. 2005;16:19–25. doi: 10.1097/00041433-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Cavelier C, Lorenzi I, Rohrer L, von Eckardstein A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim Biophys Acta. 2006;1761:655–666. doi: 10.1016/j.bbalip.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama S. ABCA1 and biogenesis of HDL. J Atheroscler Thromb. 2006;13:1–15. doi: 10.5551/jat.13.1. [DOI] [PubMed] [Google Scholar]
- 51.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. HDL-like lipoproteins in cerebrospinal fluid affect neural cell activity through lipoprotein-associated sphingosine 1-phosphate. Biochem Biophys Res Commun. 2007;359:649–654. doi: 10.1016/j.bbrc.2007.05.131. [DOI] [PubMed] [Google Scholar]
- 52.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem. 2007;103:2610–2619. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- 53.Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim Biophys Acta. 2008;1781:483–488. doi: 10.1016/j.bbalip.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alewijnse AE, Peters SL. Sphingolipid signalling in the cardiovascular system: good, bad or both? Eur J Pharmacol. 2008;585:292–302. doi: 10.1016/j.ejphar.2008.02.089. [DOI] [PubMed] [Google Scholar]
- 56.Maceyka M, Milstien S, Spiegel S. Sphingosine-1-phosphate: the Swiss army knife of sphingolipid signaling. J Lipid Res. 2009;50 Suppl:S272–S276. doi: 10.1194/jlr.R800065-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kennedy S, Kane KA, Pyne NJ, Pyne S. Targeting sphingosine-1-phosphate signalling for cardioprotection. Curr Opin Pharmacol. 2009;9:194–201. doi: 10.1016/j.coph.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Miura S, Fujino M, Matsuo Y, Kawamura A, Tanigawa H, Nishikawa H, Saku K. High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:802–808. doi: 10.1161/01.ATV.0000066134.79956.58. [DOI] [PubMed] [Google Scholar]
- 59.von Otte S, Paletta JR, Becker S, König S, Fobker M, Greb RR, Kiesel L, Assmann G, Diedrich K, Nofer JR. Follicular fluid high density lipoprotein-associated sphingosine 1-phosphate is a novel mediator of ovarian angiogenesis. J Biol Chem. 2006;281:5398–5405. doi: 10.1074/jbc.M508759200. [DOI] [PubMed] [Google Scholar]
- 60.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 61.Kimura T, Tomura H, Sato K, Ito M, Matsuoka I, Im DS, Kuwabara A, Mogi C, Itoh H, Kurose H, et al. Mechanism and role of high density lipoprotein-induced activation of AMP-activated protein kinase in endothelial cells. J Biol Chem. 2010;285:4387–4397. doi: 10.1074/jbc.M109.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nofer JR, Geigenmüller S, Göpfert C, Assmann G, Buddecke E, Schmidt A. High density lipoprotein-associated lysosphingolipids reduce E-selectin expression in human endothelial cells. Biochem Biophys Res Commun. 2003;310:98–103. doi: 10.1016/j.bbrc.2003.08.126. [DOI] [PubMed] [Google Scholar]
- 63.Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM, Argraves WS. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem. 2008;283:25074–25081. doi: 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwon YG, Min JK, Kim KM, Lee DJ, Billiar TR, Kim YM. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J Biol Chem. 2001;276:10627–10633. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- 65.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 66.Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem. 2006;281:37457–37467. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 67.Igarashi J, Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase beta. Evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J Biol Chem. 2001;276:36281–36288. doi: 10.1074/jbc.M105628200. [DOI] [PubMed] [Google Scholar]
- 68.Morales-Ruiz M, Lee MJ, Zöllner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 69.Igarashi J, Miyoshi M, Hashimoto T, Kubota Y, Kosaka H. Statins induce S1P1 receptors and enhance endothelial nitric oxide production in response to high-density lipoproteins. Br J Pharmacol. 2007;150:470–479. doi: 10.1038/sj.bjp.0707114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimura T, Mogi C, Tomura H, Kuwabara A, Im DS, Sato K, Kurose H, Murakami M, Okajima F. Induction of scavenger receptor class B type I is critical for simvastatin enhancement of high-density lipoprotein-induced anti-inflammatory actions in endothelial cells. J Immunol. 2008;181:7332–7340. doi: 10.4049/jimmunol.181.10.7332. [DOI] [PubMed] [Google Scholar]
- 71.Bolick DT, Srinivasan S, Kim KW, Hatley ME, Clemens JJ, Whetzel A, Ferger N, Macdonald TL, Davis MD, Tsao PS, et al. Sphingosine-1-phosphate prevents tumor necrosis factor-{alpha}-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler Thromb Vasc Biol. 2005;25:976–981. doi: 10.1161/01.ATV.0000162171.30089.f6. [DOI] [PubMed] [Google Scholar]
- 72.Whetzel AM, Bolick DT, Srinivasan S, Macdonald TL, Morris MA, Ley K, Hedrick CC. Sphingosine-1 phosphate prevents monocyte/endothelial interactions in type 1 diabetic NOD mice through activation of the S1P1 receptor. Circ Res. 2006;99:731–739. doi: 10.1161/01.RES.0000244088.33375.52. [DOI] [PubMed] [Google Scholar]
- 73.Norata GD, Callegari E, Marchesi M, Chiesa G, Eriksson P, Catapano AL. High-density lipoproteins induce transforming growth factor-beta2 expression in endothelial cells. Circulation. 2005;111:2805–2811. doi: 10.1161/CIRCULATIONAHA.104.472886. [DOI] [PubMed] [Google Scholar]
- 74.Norata GD, Marchesi P, Pirillo A, Uboldi P, Chiesa G, Maina V, Garlanda C, Mantovani A, Catapano AL. Long pentraxin 3, a key component of innate immunity, is modulated by high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:925–931. doi: 10.1161/ATVBAHA.107.160606. [DOI] [PubMed] [Google Scholar]
- 75.Chrisman TD, Garbers DL. Reciprocal antagonism coordinates C-type natriuretic peptide and mitogen-signaling pathways in fibroblasts. J Biol Chem. 1999;274:4293–4299. doi: 10.1074/jbc.274.7.4293. [DOI] [PubMed] [Google Scholar]
- 76.Chrisman TD, Perkins DT, Garbers DL. Identification of a potent serum factor that causes desensitization of the receptor for C-Type natriuretic peptide. Cell Commun Signal. 2003;1:4. doi: 10.1186/1478-811X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tamama K, Tomura H, Sato K, Malchinkhuu E, Damirin A, Kimura T, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein inhibits migration of vascular smooth muscle cells through its sphingosine 1-phosphate component. Atherosclerosis. 2005;178:19–23. doi: 10.1016/j.atherosclerosis.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 78.Damirin A, Tomura H, Komachi M, Liu JP, Mogi C, Tobo M, Wang JQ, Kimura T, Kuwabara A, Yamazaki Y, et al. Role of lipoprotein-associated lysophospholipids in migratory activity of coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2513–H2522. doi: 10.1152/ajpheart.00865.2006. [DOI] [PubMed] [Google Scholar]
- 79.Tölle M, Pawlak A, Schuchardt M, Kawamura A, Tietge UJ, Lorkowski S, Keul P, Assmann G, Chun J, Levkau B, et al. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol. 2008;28:1542–1548. doi: 10.1161/ATVBAHA.107.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.González-Díez M, Rodríguez C, Badimon L, Martínez-González J. Prostacyclin induction by high-density lipoprotein (HDL) in vascular smooth muscle cells depends on sphingosine 1-phosphate receptors: effect of simvastatin. Thromb Haemost. 2008;100:119–126. doi: 10.1160/TH07-11-0675. [DOI] [PubMed] [Google Scholar]
- 81.Karliner JS. Lysophospholipids and the cardiovascular system. Biochim Biophys Acta. 2002;1582:216–221. doi: 10.1016/s1388-1981(02)00174-9. [DOI] [PubMed] [Google Scholar]
- 82.Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J Cardiovasc Pharmacol. 2009;53:189–197. doi: 10.1097/FJC.0b013e3181926706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 84.Frias MA, James RW, Gerber-Wicht C, Lang U. Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: role of sphingosine-1-phosphate. Cardiovasc Res. 2009;82:313–323. doi: 10.1093/cvr/cvp024. [DOI] [PubMed] [Google Scholar]
- 85.Frias MA, Lang U, Gerber-Wicht C, James RW. Native and reconstituted HDL protect cardiomyocytes from doxorubicin-induced apoptosis. Cardiovasc Res. 2010;85:118–126. doi: 10.1093/cvr/cvp289. [DOI] [PubMed] [Google Scholar]
- 86.Tao R, Hoover HE, Honbo N, Kalinowski M, Alano CC, Karliner JS, Raffai R. High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia-reoxygenation through lipoprotein-associated sphingosine 1-phosphate. Am J Physiol Heart Circ Physiol. 2010;298:H1022–H1028. doi: 10.1152/ajpheart.00902.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duong CQ, Bared SM, Abu-Khader A, Buechler C, Schmitz A, Schmitz G. Expression of the lysophospholipid receptor family and investigation of lysophospholipid-mediated responses in human macrophages. Biochim Biophys Acta. 2004;1682:112–119. doi: 10.1016/j.bbalip.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Weigert A, Weis N, Brüne B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology. 2009;214:748–760. doi: 10.1016/j.imbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Dueñas AI, Aceves M, Fernández-Pisonero I, Gómez C, Orduña A, Crespo MS, García-Rodríguez C. Selective attenuation of Toll-like receptor 2 signalling may explain the atheroprotective effect of sphingosine 1-phosphate. Cardiovasc Res. 2008;79:537–544. doi: 10.1093/cvr/cvn087. [DOI] [PubMed] [Google Scholar]
- 90.Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 91.Levkau B, Hermann S, Theilmeier G, van der Giet M, Chun J, Schober O, Schäfers M. High-density lipoprotein stimulates myocardial perfusion in vivo. Circulation. 2004;110:3355–3359. doi: 10.1161/01.CIR.0000147827.43912.AE. [DOI] [PubMed] [Google Scholar]
- 92.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 93.Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 94.Kocher O, Krieger M. Role of the adaptor protein PDZK1 in controlling the HDL receptor SR-BI. Curr Opin Lipidol. 2009;20:236–241. doi: 10.1097/MOL.0b013e32832aee82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu W, Saddar S, Seetharam D, Chambliss KL, Longoria C, Silver DL, Yuhanna IS, Shaul PW, Mineo C. The scavenger receptor class B type I adaptor protein PDZK1 maintains endothelial monolayer integrity. Circ Res. 2008;102:480–487. doi: 10.1161/CIRCRESAHA.107.159079. [DOI] [PubMed] [Google Scholar]
- 96.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tso C, Martinic G, Fan WH, Rogers C, Rye KA, Barter PJ. High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler Thromb Vasc Biol. 2006;26:1144–1149. doi: 10.1161/01.ATV.0000216600.37436.cf. [DOI] [PubMed] [Google Scholar]
- 98.Sumi M, Sata M, Miura S, Rye KA, Toya N, Kanaoka Y, Yanaga K, Ohki T, Saku K, Nagai R. Reconstituted high-density lipoprotein stimulates differentiation of endothelial progenitor cells and enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:813–818. doi: 10.1161/01.ATV.0000259299.38843.64. [DOI] [PubMed] [Google Scholar]
- 99.van Oostrom O, Nieuwdorp M, Westerweel PE, Hoefer IE, Basser R, Stroes ES, Verhaar MC. Reconstituted HDL increases circulating endothelial progenitor cells in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol. 2007;27:1864–1865. doi: 10.1161/ATVBAHA.107.143875. [DOI] [PubMed] [Google Scholar]
- 100.Feng Y, van Eck M, Van Craeyveld E, Jacobs F, Carlier V, Van Linthout S, Erdel M, Tjwa M, De Geest B. Critical role of scavenger receptor-BI-expressing bone marrow-derived endothelial progenitor cells in the attenuation of allograft vasculopathy after human apo A-I transfer. Blood. 2009;113:755–764. doi: 10.1182/blood-2008-06-161794. [DOI] [PubMed] [Google Scholar]
- 101.Kimura T, Tomura H, Mogi C, Kuwabara A, Ishiwara M, Shibasawa K, Sato K, Ohwada S, Im DS, Kurose H, et al. Sphingosine 1-phosphate receptors mediate stimulatory and inhibitory signalings for expression of adhesion molecules in endothelial cells. Cell Signal. 2006;18:841–850. doi: 10.1016/j.cellsig.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 102.Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shimamura K, Takashiro Y, Akiyama N, Hirabayashi T, Murayama T. Expression of adhesion molecules by sphingosine 1-phosphate and histamine in endothelial cells. Eur J Pharmacol. 2004;486:141–150. doi: 10.1016/j.ejphar.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 104.Lee H, Lin CI, Liao JJ, Lee YW, Yang HY, Lee CY, Hsu HY, Wu HL. Lysophospholipids increase ICAM-1 expression in HUVEC through a Gi- and NF-kappaB-dependent mechanism. Am J Physiol Cell Physiol. 2004;287:C1657–C1666. doi: 10.1152/ajpcell.00172.2004. [DOI] [PubMed] [Google Scholar]
- 105.Takeya H, Gabazza EC, Aoki S, Ueno H, Suzuki K. Synergistic effect of sphingosine 1-phosphate on thrombin-induced tissue factor expression in endothelial cells. Blood. 2003;102:1693–1700. doi: 10.1182/blood-2002-11-3607. [DOI] [PubMed] [Google Scholar]
- 106.Matsushita K, Morrell CN, Lowenstein CJ. Sphingosine 1-phosphate activates Weibel-Palade body exocytosis. Proc Natl Acad Sci USA. 2004;101:11483–11487. doi: 10.1073/pnas.0400185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, Kuwabara A, Kanda T, Kobayashi I, Ohta H, et al. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J. 2000;348 Pt 1:71–76. [PMC free article] [PubMed] [Google Scholar]
- 108.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 109.Kovanen PT, Pentikäinen MO. Circulating lipoproteins as proinflammatory and anti-inflammatory particles in atherogenesis. Curr Opin Lipidol. 2003;14:411–419. doi: 10.1097/01.mol.0000092615.86399.07. [DOI] [PubMed] [Google Scholar]
- 110.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci USA. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010;285:12536–12542. doi: 10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berliner JA, Subbanagounder G, Leitinger N, Watson AD, Vora D. Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc Med. 2001;11:142–147. doi: 10.1016/s1050-1738(01)00098-6. [DOI] [PubMed] [Google Scholar]
- 113.Gharavi NM, Gargalovic PS, Chang I, Araujo JA, Clark MJ, Szeto WL, Watson AD, Lusis AJ, Berliner JA. High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. Arterioscler Thromb Vasc Biol. 2007;27:1346–1353. doi: 10.1161/ATVBAHA.107.141283. [DOI] [PubMed] [Google Scholar]