Abstract
Cell signaling is a very complex network of biochemical reactions triggered by a huge number of stimuli coming from the external medium. The function of any single signaling component depends not only on its own structure but also on its connections with other biomolecules. During prokaryotic-eukaryotic transition, the rearrangement of cell organization in terms of diffusional compartmentalization exerts a deep change in cell signaling functional potentiality. In this review I briefly introduce an intriguing ancient relationship between pathways involved in cell responses to chemical agonists (growth factors, nutrients, hormones) as well as to mechanical forces (stretch, osmotic changes). Some biomolecules (ion channels and enzymes) act as “hubs”, thanks to their ability to be directly or indirectly chemically/mechanically co-regulated. In particular calcium signaling machinery and arachidonic acid metabolism are very ancient networks, already present before eukaryotic appearance. A number of molecular “hubs”, including phospholipase A2 and some calcium channels, appear tightly interconnected in a cross regulation leading to the cellular response to chemical and mechanical stimulations.
Keywords: Arachidonic acid, Calcium signaling, Evolution, Mechanosensitivity, Phospholipase A2
Ca2+: AN ION FOR ALL FUNCTIONS
Calcium is the fifth most abundant element in the earth’s crust (3.5%) and is present in the oceans (0.04%). In addition to its extracellular functions as a major material used for the mineralization of bones and shells in Metazoa, Ca2+ has been recruited as a ubiquitous and universal intracellular messenger since the beginning of life. Its interaction with biomolecules exhibits flexible coordination chemistry, high affinity for carboxylate oxygen (the most frequent motif in amino acids) and rapid binding kinetics[1]. The overall consequence is that cell biochemistry and physiology are Ca2+-dependent. On the other hand, when free cytosolic Ca2+ concentration (Cac) is too high, it is cytotoxic for both prokaryotes and eukaryotes, triggering aggregation of proteins and nucleic acids, precipitation of phosphates and affects the integrity of lipid membranes[1,2]. This is probably the reason why an energy-consuming homeostatic system for Cac regulation has been established and fixed since the very beginning of biological evolution[1]. Cac is usually very low (nmol/L to μmol/L range) compared with the extracellular Cac (mmol/L) and the electrochemical gradient (the driving force) is huge, its maintenance demanding substantial energy consumption. Cac homeostasis is generally a steady state due to the balance between passive and active mechanisms, respectively, following and against the electrochemical gradient. They are mediated by a number of different membrane proteins, including ion channels, exchangers and pumps expressed by both prokaryotes and eukaryotes[3-7]. The components of the machinery initially acting (at least primarily) on cell survival allowed the evolution of the current Cac signaling system through a long history of adaptations and exaptations[1,2,8-12]. The rise of eukaryotic cell organization and the following evolutionary events - the acquisition of polarity, differentiation and multicellularity - correlated with the increasing complexity of biological systems. Consequently, a more efficient signaling apparatus was required to account for coding, control and integration of the biochemical processes in space and time.
Theoretically speaking, we can identify a number of steps in which Ca2+ might have played a significant role and, in turn, Cac signaling has been drastically modified[1].
The transition from prokaryotic to eukaryotic cell structure led to an increase in intracellular spatial compartmentalization due to the rise in membrane-delimited organelles (nucleus, endoplasmic reticulum-ER, mitochondria, vesicles). ER and mitochondria became Ca2+ stores, dynamic intracellular sources for the ion. This new mode of Cac regulation added to the preexisting Ca2+ entry from the extracellular medium. We know that the so-called Ca2+ microdomains, highly localized Ca2+ hot spots identified in all cell types, often require the interplay between Ca2+ entry (as well as calcium release from ER) and mitochondrial buffering[13,14]. Moreover, new modes for calcium wave generation evolved through the interplay between Ca2+ fluxes occurring in the plasma membrane and ER. This is the case for calcium-induced calcium release and store-dependent calcium entry[13,14].
The following evolutionary step, cell polarization, can be viewed as another very intriguing step concerning the modification of tridimensional cell shape, surface/volume ratio as well as of the structural anisotropy. From the diffusional point of view, Ca2+ wave propagation within the cell was more dishomogeneous and articulated. Highly polarized epithelial cells from secreting and adsorbing epithelia exhibit a polarized arrangement of Ca2+ channel membrane distribution and Ca2+-dependent machinery. A good example is provided by exocrine acinar cells in pancreatic and salivary glands. They evolved sophisticated and complex Cac signaling mechanisms that control the secretory events occurring across the apical plasma membrane bordering the gland lumen. In these cells the receptor-evoked Cac wave starts at the apical pole, where specific calcium channels are located, and propagates to the basal pole[15,16].
The third step, multicellularity and cell differentiation, definitively strengthened and fixed the great potentiality of Cac signaling. Indeed, its omnipresence and versatility was very useful as a framework in cell-cell communication which was required for the functional integrity of multicellular organisms. For this reason, it is not surprising that in Metazoa highly diverse extracellular agonists - including hormones, neurotransmitters and growth factors - trigger changes in Cac that function as a chemical signal to transduce information for survival, proliferation, motility, differentiation and death in both physiological and pathological conditions. The appearance and diversification of a truly versatile class of “Ca2+ effector/sensor” molecules (Ca2+-binding proteins), considered absent in prokaryota until recently, have been claimed as a possible solution to this demand. The most ubiquitous Ca2+ sensors are the so-called EF-hand containing proteins, among which the calmodulin family is the best known. EF-hand Ca2+ sensors are abundant in yeasts and ubiquitous in multicellular organisms, where they represent 120 families with more than 270 members[1,2,17-19].
The evolutionary association between eukaryotic cell evolution and Ca2+ sensors diversification is intriguing, but recent findings do not support this idea. In bacteria, Ca2+ is implicated in a wide variety of cellular processes, including the cell cycle and cell division[5-7]. Moreover, a recent analysis of the prokaryotic protein sequences available in the databases revealed the presence of several calmodulin-like proteins. They contain two or more authentic EF-hand motifs, suggesting that calmodulin-like proteins could be involved in Cac signaling in bacteria[5].
The same argument is true for eukaryotic transition from single cell to multicellular organization. It is generally believed that the complex Cac signaling “toolkit” has arisen from the ancestral multicellular organisms to fit unique physiological roles of specialized cell types. However, the expression of a surprisingly extensive Ca2+ signaling “toolkit” in the unicellular choanoflagellate Monosiga brevicollis has been reported[12]. Choanoflagellates possess homologues of some animal plasma membrane calcium channels, inositol 1,4,5-trisphosphate receptors, plasma membrane and sarco/endoplasmic reticulum Ca2+ ATPases and cation/Ca2+ exchanger families. Therefore, a complex Cac signaling machinery might have evolved before the emergence of multicellular animals[12]. It may be interesting to investigate the proteome and genome of other unicellular eukaryotes in order to extend and confirm this hypothesis.
These experimental observations prompted us to provide more articulated evolutionary explanations in order to account for a Ca2+-dependent role in the increased complexity related to prokaryota-eukaryota and single-cell/Metazoa transitions. The complex structure-function relationship of proteins suggests that new functions do not necessarily arise from structural modifications of preexisting proteins or “de-novo” expression, but also from insertion of the same or a similar protein in a different microenvironment (prokaryotic vs eukaryotic cell). The impressive increase in eukaryotic spatial compartmentalization would have driven the reassignment of preexisting proteins into new intracellular sites. Indeed, new functions of Ca2+ sensor proteins already expressed and functional in prokaryota may be recruited in the new context of eukaryotic and multicellular framework, leading to a functional enlargement of Ca2+ machinery potentiality.
THE TIGHT CONNECTIONS BETWEEN MECHANICAL STIMULI, CALCIUM SIGNALING AND LIPIDS
Mechanical forces are universal and their sensing by living beings, termed mechanosensation, underlies critical physiological processes including osmotic regulation, hearing, touch, balance, proprioception and blood-pressure monitoring[20].
Cell transduction of mechanical stimuli depends on mechanosensitive biomolecules that integrate information into the complex networking biochemical pathways. Such cell components can be considered as functional “hubs”. Here we focus our interest on the pivotal role of two well-known and ancient “hubs”: Phospholipase A2 (PLA2) with its lipid products and some Ca2+-permeable channels belonging to the superfamily of transient receptor potential (TRP) proteins.
Since the middle of 1990, several authors have reported on the ability of PLA2-related membrane lipids to modulate the activity of Ca2+-permeable channels in different cell types. In particular, arachidonic acid (AA) and some of its metabolites activate a number of TRP channels[21-29] (discussed below). Interestingly, some of these channels are directly or indirectly involved in cell response to mechanical stimuli (mechanotransduction), a very ancient feature of both prokaryotes and eukaryotes. Besides external forces, the composition of the lipid bilayer itself can affect its internal forces and different lipids can change channel gating[20].
Starting from these introductory highlights, the question is: did an intriguing complex partnership between calcium signaling, AA metabolism and mechanotransduction occur since the very beginning of cell evolution? Let us try to dissect this intriguing issue.
PLA2: a mechano- and chemo-regulated intracellular hub for AA release
AA, a 20-carbon omega-6 polyunsaturated fatty acid, is present in the cell membranes of both prokaryotes and eukaryotes[30-34].
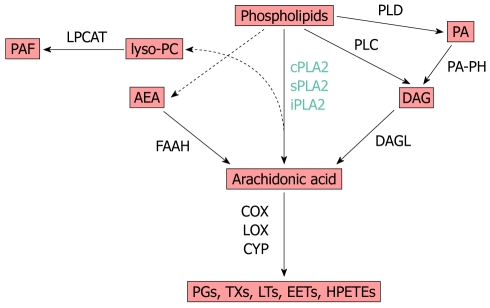
In eukaryotic membrane phospholipids, AA is esterified to glycerol. Three phospholipases, A2, C and D, mediate the deacylation reaction that releases the free fatty acid (Figure 1). Free AA interacts with several proteins (ion channels, enzymes) in the same cell and freely diffuses giving rise to a paracrine pathway in the neighboring cells. It has a short half-life being the substrate of cyclooxygenases, lipoxygenases and cytochrome P450 monooxygenases, yielding a great number of bioactive lipids and eicosanoids, that act as autocrine/paracrine regulators of a variety of functions in physiological and pathological conditions[35,36]. AA diffusion is also facilitated by its binding to fatty acid binding proteins, a superfamily of ancient proteins expressed in both prokaryotes and eukaryotes[37,38].
Figure 1.
Simplified scheme showing arachidonic acid metabolism. COX: Cyclooxygenase; LOX: Lipoxygenase; CYP: Cytochrome P450 epoxygenase; PGs: Prostaglandins; TXs: Thromboxanes; LTs: Leukotrienes; EETs: Epoxyeicosatrienoic acids; HPETEs: Hydroperoxyeicosatetraenoic acids; DAG: Diacylglycerol; DAGL: DAG lipase; PA: Phosphatidic acid; PLD: Phospholipase D; sPLA2: Secretory phospholipase A2; cPLA2: Cytosolic phospholipase A2; iPLA2: Ca2+-independent phospholipase A2; PLC: Phospholipase C; PA-PH: Phosphatidic acid phosphatase; lyso-PC: Lysophosphatidylcoline; PAF: Platelet activating factor; LPCAT: LPC acetyltransferase; AEA: Arachidonoylethanolamide (anandamide); FAAH: Fatty acid amidohydrolase.
While PLA2s release arachidonate in a single-step reaction, phospholipase C and phospholipase D produce the AA-containing lipid products, diacylglycerol (DAG) and phosphatidic acid, respectively (Figure 1)[29,39,40].
PLA2s are a superfamily of diverse intracellular and secreted enzymes, whose activity was first reported in snake venom, more than a century ago (Table 1). They are very ancient proteins also expressed in prokaryotes and viruses[36,41-46]. An intriguing feature of some PLA2 isoforms is their mechanosensitivity, used by both prokaryotes and eukaryotes for the transduction of external mechanical stimuli into the cell[36,41,42,44,47,48]. Several investigations have focused on the structural basis responsible for PLA2 mechanosensitivity[48-51]. A striking feature of lipolytic enzymes, including PLA2, is the so-called interfacial activation[51]. Compared to the hydrolysis of monomeric substrates, their activity is dramatically enhanced when reacting with phospholipid interfaces, such as those present in micelles, monolayers, and bilayers. The activity of PLA2 is modulated by the lipid composition and the phase state of the substrate phospholipids. Accordingly, a number of environmental factors affect the catalytic rate of PLA2. They include negatively charged phospholipids, vicinity to the main phase transition temperature of the substrate, fluid-gel phase coexistence, packing defects, lipid lateral packing density, lipid protrusions, and membrane curvature[50]. A pivotal study reported that in large unilamellar liposomes, osmotic stretching can modulate the sensitivity of the bilayer phospholipids to the action of PLA2s[48]. More recently it has been shown in a number of cell types that cell swelling promotes PLA2 activation (usually cPLA2, see below)[52,53]. Ankyrin repeats, suggested to be involved in the mechanosensitivity of some TRP channels (see below), might play a similar role in PLA2 regulation[44].
Table 1.
| Group | Location | Size (kDa) | Common sources | Calcium requirement |
| I | Secreted | 13-15 | Cobra, human | mmol/L |
| II | Secreted | 13-15 | Viper, mouse, human | mmol/L |
| III | Secreted | 16-18 | Bee, lizard, scorpion, human | mmol/L |
| IV | Cytosolic | 85 | Human | < μmol/L |
| V | Secreted | 14 | Human | No |
| VI | Cytosolic | 80-85 | Human | No |
| VII | Cytosolic | 40 | Human, bovine | No |
| Secreted | 45 | Human, bovine | No | |
| VIII | Cytosolic | 29 | Human | No |
| IX | Secreted | 14 | Marine snail | < mmol/L |
| X | Secreted | 14 | Human | mmol/L |
| XI | Secreted | 12-13 | Green rice | mmol/L |
| Other | Secreted | 14-15 | Streptomyces (Bacteria) | mmol/L |
| Cytosolic | 93 | Sporothrix schenckii (Fungi) | < μmol/L | |
| Secreted | 10 | Parvovirus | mmol/L |
PLA2 isoforms are classified into groups which differ in structure, intracellular localization, regulation, calcium dependence and pharmacological inhibition (Table 1)[54,55]. They are also usually distinguished into secretory PLA2, cytosolic PLA2 (cPLA2) and Ca2+-independent PLA2. In particular, the cPLA2s (cPLA2α, cPLA2β, cPLA2γ) are high-molecular weight (60-110 kDa), intracellular PLA2s[36,43,44]. cPLA2α, the best characterized isoform, has a catalytic domain at the C-terminal of the protein and a Ca2+-binding domain in the N-terminal portion. cPLA2α displays high selectivity for glycerophospholipids with choline as the polar head group and the polyunsaturated AA in the sn-2 position[36,43,44]. The catalytic action of cPLA2α is Ca2+-independent, but a submicromolar Cac is required for translocation of the enzyme from the cytosol to cell membranes. A key catalytic regulatory step for cPLA2 is due to phosphorylation by members of the mitogen-activated protein kinase (MAPK) family. MAPKs constitute a large family of multifunctional proteins, usually acting as a convergence point for intracellular pathways starting from different membrane receptors. A multitude of paralogous MAPK isoforms has been found in yeast, vertebrates, and other eukaryotes[56,57]. The activity of most MAPKs expressed in animal cells is modulated during osmotic stress and it is likely that MAPKs are evolutionary ancient transducers of osmosensory signals. This hypothesis is further strengthened by the fact that the physiological capacity for osmosensory signal transduction is a highly conserved function not only of animal MAPKs, but also of certain plant and fungal MAPKs[58]. Mechanosensitive PLA2s, already expressed in prokaryotes, might therefore have acquired new regulation by a mechanotransductory MAPK cascade in eukaryotes. This event could account for the increase in complexity in the integration between chemically- and mechanically-stimulated responsivity of eukaryotes, particular of Metazoa.
AA and calcium signaling: an ancient association
As mentioned before, several calcium-permeable channels in the plasma membrane of eukaryotic cells are regulated by AA and its metabolites[29,39,59].
TRP proteins are cation channels, mostly calcium-permeable, and function as universal polymodal cellular sensors in all eukaryota. Most TRPs can be activated by various chemical or physical stimuli, such as ligand binding, temperature, changes in osmolarity, cell volume, mechanical forces, or voltage[21,23,24,60-66]. The polymodality of TRPs is very ancient as suggested by the biophysical properties of the budding yeast Saccharomyces cerevisiae TRPY1. This large conductance cation channel is curiously not located in the plasma membrane but in the vacuolar membrane where it is activated by hyperosmotic stimuli. The subsequent calcium release from the vacuole into the cytoplasm initiates osmotic defense responses[67-70]. Even if the intracellular distribution of TRPY1 appears to be unexpected, it has to be noted that a number of mammalian TRP channels studied thus far, are also found in intracellular membranes. Intracellular TRP channels actively participate in regulating membrane traffic, signal transduction, and vesicular ion homeostasis[71].
Like its animal counterparts, TRPY1 is polymodal, being not only a direct mechanosensor but also a chemosensor activated by indole and other aromatic compounds. Intriguingly, TRPY1 gating is calcium-dependent[62].
First cloned in Drosophila photoreceptors, TRP channels are now classified in 7 subfamilies[60,61,63,72]. Database searches have not (yet) revealed TRP-related genes in eubacteria, archaebacteria and plants (Table 2). TRP-related genes can be found in Paramecium, Tetrahymena, Dictyostelium, Trypanosoma, Leishmania, and other protists[68-70]. In single-celled fungi (yeasts), one TRP-related gene has been identified (encoding the afore-mentioned TRPY1), which cannot be assigned to any of the known TRP subfamilies in animals[68-70]. The unicellular choanoflagellate Monosiga brevicollis displays homologues of 5 mammalian TRP channel families, including TRPC, TRPV, TRPM, TRPML, and TRPA[12]. In animalia, a plethora of TRP-related genes has now been identified (Table 2).
Table 2.
| Organism | TRP |
| Arabidopsis (Angiospermae) | 0 |
| Chlamydomonas (Chlorophyta) | 19 |
| Fungi (unicellular and multicellular) | 3 |
| Monosiga brevicollis (Choanoflagellates) | 5 |
| Caenorabditis elegans (Nematoda) | 17 |
| Drosophila melanogaster (Insecta) | 13 |
| Ciona intestinalis (sea squirt, Ascidiacea) | 27 |
| Fugu rubripes (Puffer fish) | 25 |
| Danio rerio (Zebrafish) | 27 |
| Mus musculus (Mammalia) | 28 |
| Homo sapiens (Mammalia) | 27 |
Transient receptor potential (TRP) genes are also found in the genomes of a number of protists not shown in table (see text).
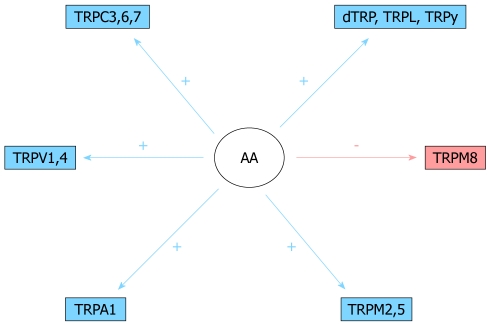
As discussed below, some TRPs are directly activated by lipid intracellular messengers, including DAG and AA (Figure 2). TRPV4, TRPV1, as well as TRPC3 and TRPC6, are substrates for AA and its metabolites[22,26,28,73]. Other calcium channels, evolutionary and structurally unrelated to TRP proteins, are also targets for AA-dependent regulation[74].
Figure 2.
Scheme showing transient receptor potential channels modulated by arachidonic acid and its metabolites. AA: Arachidonic acid.
These calcium-permeable AA-dependent channels provide a mechanistic link between mechanical stimulation and calcium signaling, through the involvement of PLA2, its product AA (and its metabolites) and regulation of calcium channels[21-23,60].
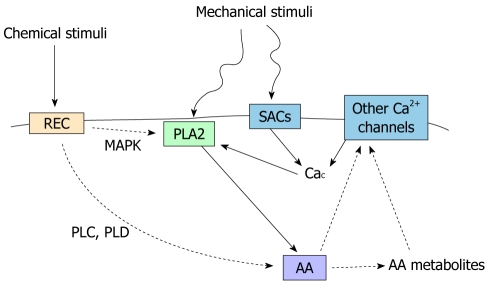
Interestingly, the functional interactions between PLA2 and TRPs are reciprocal (Figure 3): calcium entry following AA-dependent TRP activation can eventually act as a modulator of the calcium-dependent isoform of PLA2 providing a non-linear network.
Figure 3.
Simplified scheme showing the interplay between mechanical stimuli, arachidonic acid metabolism and calcium signaling. AA: Arachidonic acid; REC: Membrane receptors; SACs: Stretch activated calcium channels; PLD: Phospholipase D; PLC: Phospholipase C; MAPK: Mitogen-activated protein kinase; Cac: Ca2+ concentration; PLA2: Phospholipase A2.
Mechanosensitivity of calcium channels
Mechanosensitive (MS) channels are widespread and universal. While some channels are directly mechanically gated, many others acquire mechanosensitivity indirectly through their interaction with true primary mechanosensors including membrane receptors and associated second messengers[75-77].
The bacterial MscL channel was the first stretch-activated channel (SAC) to be cloned and reconstituted into artificial bilayers. It can be directly activated by tension coming from the lipid bilayer itself as well as from the tethered extracellular matrix and/or cytoskeleton[75,78].
Several calcium-permeable eukaryotic channels are sensitive to mechanical stimuli and a number of TRP channels play a key role in animal sensory mechanotransduction. This function is very ancient and shared by Metazoa[20,60,68-70,77,79,80].
In eukaryotes, several members of the TRP family are involved in mechanosensation and are recognized as promising candidate MS channels. In the nematode Caenorhabditis elegans, Osm-9 is required for nose touch and hypertonic-stress response. In the fly Drosophila melanogaster, Nanchung and Inactive (Nan/Iav) of the TRPV subfamily function in hearing, while Nan and water witch (wtrw) of the TRPA subfamily mediate hygrosensation. The vertebrate homologue, TRPV4, plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli. TRPV4 is expressed in DRG neurons, keratinocytes, and inner-ear hair cells where it can play a critical role in hearing, in addition to the afore-mentioned TRPA1[60,81]. Cell swelling activates TRPV4 by means of the PLA2-dependent formation of AA, and its subsequent metabolization to 5,6-epoxyeicosatrienoic acid (5,6 EET) (Figure 1)[64]. Moreover, in response to vasoactive factors or shear stress, sustained TRPV4-dependent Ca2+ entry into endothelial cells contributes to the Cac signaling requested for the synthesis and release of vasoactive compounds such as nitric oxide and prostaglandins[82]. Such events globally contribute to the relaxant effects on vascular tone[25,82-84]. This is a paradigmatic example of the interesting complex role of PLA2s in eukaryotic cells. Indeed, in addition to its osmosensitivity, PLA2 enzymatic activity can also be chemically modulated, following cell exposure to growth factors, proinflammatory agents, hormones and neurotransmitters[43,85].
Many other TRPs are activated either by osmotonicity or stress (or both). They include TRPV1, TRPV2, TRPC1,3,6, TRPP1, TRPM4,7[25,64,81,83,84]. In human kidney, TRPP1 and TRPP2 sense fluid flow and their mutations result in polycystic kidney disease[86]. Recently, it was claimed that TRPC1 and TRPC6 may encode the mammalian SACs, although other reports warrant more detailed evidence and discussion[76,87].
CONCLUSION
Comparative analysis of genomic, proteomic and lipidomic databases allows us to investigate the evolutionary paths of cell transduction machinery. We usually distinguish cell responses to chemical agonists (growth factor, nutrients, hormones) from those triggered by mechanical stimuli due to stretch, pressure and osmotic changes. Nevertheless, we know that several intracellular actors in signal transduction are common and some proteins are directly on indirectly chemically/mechanically co-regulated (Figure 3). In particular Cac signaling machinery and AA metabolism are very ancient networks, expressed and functional in both prokaryotes and eukaryotes. Intriguingly, they are regulated and mediate the cellular response to both chemical and mechanical stimulations. Along with the increase in spatiotemporal complexity during prokaryotic-eukaryotic and unicellular-multicellular transitions, new functions have been assumed by these intracellular pathways through a diversification of isoforms, but also (and perhaps more importantly) via a rearrangement of the interactions and cross-regulations inside and among the signaling networks. The evolutionary history of calcium signaling and AA metabolism represents a useful conceptual and experimental model to investigate the idea that the structure-function relationship of single biomolecules, as well as of the networks including a number of biomolecules, is critically constrained by the micro/macro environment in which they work.
Footnotes
Peer reviewers: Christopher M Norris, PhD, Associate Professor of Molecular and Biomedical, Pharmacology and the Sanders-Brown Center on Aging, 131 Sanders-Brown Building, University of Kentucky College of Medicine, Lexington, KY 40536, United States; Shangwei Hou, Senior Research Investigator, D100 Richards, Department of Physiology, University of Pennsylvania, 3700 Hamilton Walk, Philadelphia, PA 19104, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium. 2007;42:345–350. doi: 10.1016/j.ceca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Gangola P, Rosen BP. Maintenance of intracellular calcium in Escherichia coli. J Biol Chem. 1987;262:12570–12574. [PubMed] [Google Scholar]
- 4.Herbaud ML, Guiseppi A, Denizot F, Haiech J, Kilhoffer MC. Calcium signalling in Bacillus subtilis. Biochim Biophys Acta. 1998;1448:212–226. doi: 10.1016/s0167-4889(98)00145-1. [DOI] [PubMed] [Google Scholar]
- 5.Michiels J, Xi C, Verhaert J, Vanderleyden J. The functions of Ca(2+) in bacteria: a role for EF-hand proteins? Trends Microbiol. 2002;10:87–93. doi: 10.1016/s0966-842x(01)02284-3. [DOI] [PubMed] [Google Scholar]
- 6.Torrecilla I, Leganés F, Bonilla I, Fernández-Piñas F. Use of recombinant aequorin to study calcium homeostasis and monitor calcium transients in response to heat and cold shock in cyanobacteria. Plant Physiol. 2000;123:161–176. doi: 10.1104/pp.123.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez DC. Calcium signalling in bacteria. Mol Microbiol. 2004;54:291–297. doi: 10.1111/j.1365-2958.2004.04276.x. [DOI] [PubMed] [Google Scholar]
- 8.Berridge MJ. Calcium signal transduction and cellular control mechanisms. Biochim Biophys Acta. 2004;1742:3–7. doi: 10.1016/j.bbamcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Cole K, Kohn E. Calcium-mediated signal transduction: biology, biochemistry, and therapy. Cancer Metastasis Rev. 1994;13:31–44. doi: 10.1007/BF00690417. [DOI] [PubMed] [Google Scholar]
- 10.McAinsh MR, Pittman JK. Shaping the calcium signature. New Phytol. 2009;181:275–294. doi: 10.1111/j.1469-8137.2008.02682.x. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal JK. Calcium - how and why? J Biosci. 2001;26:357–363. doi: 10.1007/BF02703745. [DOI] [PubMed] [Google Scholar]
- 12.Cai X. Unicellular Ca2+ signaling 'toolkit' at the origin of metazoa. Mol Biol Evol. 2008;25:1357–1361. doi: 10.1093/molbev/msn077. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 14.Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca(2+) signals. J Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- 15.Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic. 2011;12:232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 17.Friedberg F, Rhoads AR. Evolutionary aspects of calmodulin. IUBMB Life. 2001;51:215–221. doi: 10.1080/152165401753311753. [DOI] [PubMed] [Google Scholar]
- 18.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 19.Hoeflich KP, Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–742. doi: 10.1016/s0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 20.Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 21.Beech DJ, Bahnasi YM, Dedman AM, Al-Shawaf E. TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium. 2009;45:583–588. doi: 10.1016/j.ceca.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilius B, Mahieu F, Karashima Y, Voets T. Regulation of TRP channels: a voltage-lipid connection. Biochem Soc Trans. 2007;35:105–108. doi: 10.1042/BST0350105. [DOI] [PubMed] [Google Scholar]
- 24.Raghu P, Hardie RC. Regulation of Drosophila TRPC channels by lipid messengers. Cell Calcium. 2009;45:566–573. doi: 10.1016/j.ceca.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 26.Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 27.Hardie RC. Regulation of TRP channels via lipid second messengers. Annu Rev Physiol. 2003;65:735–759. doi: 10.1146/annurev.physiol.65.092101.142505. [DOI] [PubMed] [Google Scholar]
- 28.Hardie RC, Muallem S. Lipids in Ca2+ signalling--an introduction. Cell Calcium. 2009;45:517–520. doi: 10.1016/j.ceca.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Meves H. Arachidonic acid and ion channels: an update. Br J Pharmacol. 2008;155:4–16. doi: 10.1038/bjp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronan JE, Thomas J. Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol. 2009;459:395–433. doi: 10.1016/S0076-6879(09)04617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bigogno C, Khozin-Goldberg I, Boussiba S, Vonshak A, Cohen Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry. 2002;60:497–503. doi: 10.1016/s0031-9422(02)00100-0. [DOI] [PubMed] [Google Scholar]
- 32.Scheurbrandt G, Bloch K. Unsaturated fatty acids in microorganisms. J Biol Chem. 1962;237:2064–2068. [PubMed] [Google Scholar]
- 33.Bajpai PK, Bajpai P, Ward OP. Arachidonic Acid production by fungi. Appl Environ Microbiol. 1991;57:1255–1258. doi: 10.1128/aem.57.4.1255-1258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang YM, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 35.Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259:315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Rossum DB, Patterson RL. PKC and PLA2: probing the complexities of the calcium network. Cell Calcium. 2009;45:535–545. doi: 10.1016/j.ceca.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Ganfornina MD, Gutiérrez G, Bastiani M, Sánchez D. A phylogenetic analysis of the lipocalin protein family. Mol Biol Evol. 2000;17:114–126. doi: 10.1093/oxfordjournals.molbev.a026224. [DOI] [PubMed] [Google Scholar]
- 38.Shepard W, Haouz A, Graña M, Buschiazzo A, Betton JM, Cole ST, Alzari PM. The crystal structure of Rv0813c from Mycobacterium tuberculosis reveals a new family of fatty acid-binding protein-like proteins in bacteria. J Bacteriol. 2007;189:1899–1904. doi: 10.1128/JB.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munaron L. Intracellular calcium, endothelial cells and angiogenesis. Recent Pat Anticancer Drug Discov. 2006;1:105–119. doi: 10.2174/157489206775246502. [DOI] [PubMed] [Google Scholar]
- 40.Munaron L, Antoniotti S, Fiorio Pla A, Lovisolo D. Blocking Ca2+entry: a way to control cell proliferation. Curr Med Chem. 2004;11:1533–1543. doi: 10.2174/0929867043365008. [DOI] [PubMed] [Google Scholar]
- 41.Sugiyama M, Ohtani K, Izuhara M, Koike T, Suzuki K, Imamura S, Misaki H. A novel prokaryotic phospholipase A2. Characterization, gene cloning, and solution structure. J Biol Chem. 2002;277:20051–20058. doi: 10.1074/jbc.M200264200. [DOI] [PubMed] [Google Scholar]
- 42.Valentín-Berríos S, González-Velázquez W, Pérez-Sánchez L, González-Méndez R, Rodríguez-Del Valle N. Cytosolic phospholipase A2: a member of the signalling pathway of a new G protein alpha subunit in Sporothrix schenckii. BMC Microbiol. 2009;9:100. doi: 10.1186/1471-2180-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50 Suppl:S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert IH, Pedersen SF, Poulsen KA. Activation of PLA2 isoforms by cell swelling and ischaemia/hypoxia. Acta Physiol (Oxf) 2006;187:75–85. doi: 10.1111/j.1748-1716.2006.01557.x. [DOI] [PubMed] [Google Scholar]
- 45.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 46.Zádori Z, Szelei J, Lacoste MC, Li Y, Gariépy S, Raymond P, Allaire M, Nabi IR, Tijssen P. A viral phospholipase A2 is required for parvovirus infectivity. Dev Cell. 2001;1:291–302. doi: 10.1016/s1534-5807(01)00031-4. [DOI] [PubMed] [Google Scholar]
- 47.Snijder HJ, Dijkstra BW. Bacterial phospholipase A: structure and function of an integral membrane phospholipase. Biochim Biophys Acta. 2000;1488:91–101. doi: 10.1016/s1388-1981(00)00113-x. [DOI] [PubMed] [Google Scholar]
- 48.Lehtonen JY, Kinnunen PK. Phospholipase A2 as a mechanosensor. Biophys J. 1995;68:1888–1894. doi: 10.1016/S0006-3495(95)80366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 50.Code C, Domanov Y, Jutila A, Kinnunen PK. Amyloid-type fiber formation in control of enzyme action: interfacial activation of phospholipase A2. Biophys J. 2008;95:215–224. doi: 10.1529/biophysj.108.128710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winget JM, Pan YH, Bahnson BJ. The interfacial binding surface of phospholipase A2s. Biochim Biophys Acta. 2006;1761:1260–1269. doi: 10.1016/j.bbalip.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Basavappa S, Pedersen SF, Jørgensen NK, Ellory JC, Hoffmann EK. Swelling-induced arachidonic acid release via the 85-kDa cPLA2 in human neuroblastoma cells. J Neurophysiol. 1998;79:1441–1449. doi: 10.1152/jn.1998.79.3.1441. [DOI] [PubMed] [Google Scholar]
- 53.Thoroed SM, Lauritzen L, Lambert IH, Hansen HS, Hoffmann EK. Cell swelling activates phospholipase A2 in Ehrlich ascites tumor cells. J Membr Biol. 1997;160:47–58. doi: 10.1007/s002329900294. [DOI] [PubMed] [Google Scholar]
- 54.Sitkiewicz I, Stockbauer KE, Musser JM. Secreted bacterial phospholipase A2 enzymes: better living through phospholipolysis. Trends Microbiol. 2007;15:63–69. doi: 10.1016/j.tim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 57.Choi HS, Kim JR, Lee SW, Cho KH. Why have serine/threonine/tyrosine kinases been evolutionarily selected in eukaryotic signaling cascades? Comput Biol Chem. 2008;32:218–221. doi: 10.1016/j.compbiolchem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Kültz D. Evolution of osmosensory MAP kinase signaling pathways. Amer Zool. 2001;41:743–757. [Google Scholar]
- 59.Munaron L, Fiorio Pla A. Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Curr Med Chem. 2009;16:4691–4703. doi: 10.2174/092986709789878210. [DOI] [PubMed] [Google Scholar]
- 60.Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol. 2008;18:R880–R889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 61.Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol. 2006;68:719–736. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- 62.Su Z, Zhou X, Loukin SH, Haynes WJ, Saimi Y, Kung C. The use of yeast to understand TRP-channel mechanosensitivity. Pflugers Arch. 2009;458:861–867. doi: 10.1007/s00424-009-0680-0. [DOI] [PubMed] [Google Scholar]
- 63.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao X, Kwan HY, Huang Y. Regulation of TRP channels by phosphorylation. Neurosignals. 2005;14:273–280. doi: 10.1159/000093042. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 67.Chang Y, Schlenstedt G, Flockerzi V, Beck A. Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett. 2010;584:2028–2032. doi: 10.1016/j.febslet.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 68.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 69.Martinac B, Saimi Y, Kung C. Ion channels in microbes. Physiol Rev. 2008;88:1449–1490. doi: 10.1152/physrev.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saimi Y, Zhou X, Loukin SH, Haynes WJ, Kung C. Microbial TRP channels and their mechanosensitivity. Curr Top Membr. 2007;58:311–327. [Google Scholar]
- 71.Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J Neurochem. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 74.Shuttleworth TJ. Arachidonic acid, ARC channels, and Orai proteins. Cell Calcium. 2009;45:602–610. doi: 10.1016/j.ceca.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blount P, Moe PC. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 1999;7:420–424. doi: 10.1016/s0966-842x(99)01594-2. [DOI] [PubMed] [Google Scholar]
- 76.Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honoré E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflugers Arch. 2010;460:571–581. doi: 10.1007/s00424-010-0847-8. [DOI] [PubMed] [Google Scholar]
- 77.Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- 78.Sukharev S. Mechanosensitive channels in bacteria as membrane tension reporters. FASEB J. 1999;13 Suppl:S55–S61. doi: 10.1096/fasebj.13.9001.s55. [DOI] [PubMed] [Google Scholar]
- 79.Myers BR, Saimi Y, Julius D, Kung C. Multiple unbiased prospective screens identify TRP channels and their conserved gating elements. J Gen Physiol. 2008;132:481–486. doi: 10.1085/jgp.200810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sachs F. Stretch-activated ion channels: what are they? Physiology (Bethesda) 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 82.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 83.Nilius B, Watanabe H, Vriens J. The TRPV4 channel: structure-function relationship and promiscuous gating behaviour. Pflugers Arch. 2003;446:298–303. doi: 10.1007/s00424-003-1028-9. [DOI] [PubMed] [Google Scholar]
- 84.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 85.Dennis EA. Phospholipase A2 in eicosanoid generation. Am J Respir Crit Care Med. 2000;161:S32–S35. doi: 10.1164/ajrccm.161.supplement_1.ltta-7. [DOI] [PubMed] [Google Scholar]
- 86.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 87.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 88.Wheeler GL, Brownlee C. Ca2+ signalling in plants and green algae--changing channels. Trends Plant Sci. 2008;13:506–514. doi: 10.1016/j.tplants.2008.06.004. [DOI] [PubMed] [Google Scholar]