Abstract
A novel selenium-containing compound, selenoneine, has been isolated as the major form of organic selenium in the blood and tissues of tuna. Selenoneine harbors a selenium atom in the imidazole ring, 2-selenyl-Nα, Nα, Nα-trimethyl-L-histidine, and is a selenium analog of ergothioneine. This selenium compound has strong antioxidant capacity and binds to heme proteins, such as hemoglobin and myoglobin, to protect them from iron auto-oxidation, and it reacts with radicals and methylmercury (MeHg). The organic cations/carnitine transporter OCTN1 transports selenoneine and MeHg, regulates Se-enhanced antioxidant activity, and decreases MeHg toxicity. Thus, the dietary intake of selenoneine, by consuming fish, might decrease the formation of reactive oxygen radicals that could oxidize nucleotides in DNA, and thereby inhibit carcinogenesis, chronic diseases, and aging.
Keywords: Selenium-containing compound, Antioxidant, Methylmercury, Organic cations/carnitine transporter OCTN1, Ergothioneine, Glutathione peroxidase, Tuna, Fish
INTRODUCTION
Selenium is an essential micronutrient for human health[1-3]. Food sources include fish and other seafood, red meat, eggs, chicken, and liver[4]. Selenium is incorporated as a selenocysteine residue in protein synthesis to produce antioxidant enzymes, such as glutathione peroxidase (GPx)[5,6], thioredoxin reductase[7], and selenoprotein P[8]. The entire selenoprotein gene population, designated the “selenoproteome,” has been identified in the human genome[9]. The antioxidant function of selenium plays protective roles in 50 human diseases, including prostate, lung, and intestine/colon cancer, immunodeficiency, and heart diseases[10-14]. Diseases of domestic animals and birds, such as myopathy, exudative diathesis, and pancreatic degeneration, are caused by selenium deficiency[10]. Additionally, selenium is thought to protect against mercury toxicity in humans and animals[15-18].
Previously, various selenium compounds have been designed to mimic natural GPx and the selenium redox cycle[10,19]. However, to the best of our knowledge, no biologically functional antioxidant selenium compound has been identified in fish or other animal cells or tissues.
Recently, a novel selenium-containing compound, selenoneine, was identified as the major form of organic selenium in the muscle and other tissues of tuna[20]. This compound has a unique selenoketone structure and strong antioxidant capacity (Figure 1). The dietary intake of selenoneine by consuming fish might be important in free radical detoxification mechanisms. Thus, many of the nutritional effects of selenium can be explained by its antioxidant roles in animal cells.
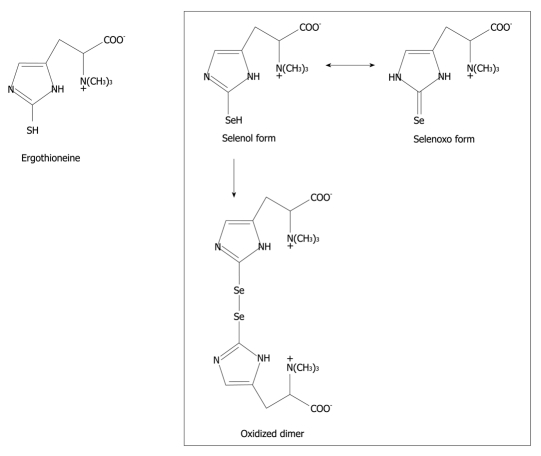
Figure 1.
Chemical structure of ergothioneine and selenoneine. The monomeric form of the selenium compound is unstable under cold and room temperature conditions. The extracted material that contains the selenoketone of the selenium compound from the tuna tissues with acetonitrile/tetrahydrofuran (1:1 in volume) accelerates selenoxo-selenol tautomerization and formation of the oxidized dimeric form.
BIOAVAILABILITY OF DIETARY ORGANIC SELENIUM
The maximum expression of selenoprotein requires daily dietary selenium levels of 0.1-0.2 mg/kg for animals and no more than 55 μg for humans[21]. From human clinical trials and epidemiological studies, inorganic and organic selenium compounds are considered to be anticarcinogenic, at doses greater than those required to support the maximum expression of the selenoenzymes, which are generally regarded as being responsible for the complete nutritional effects of the element[22,23]. Combs and colleagues have suggested that antioxidant selenium compounds present in foods are metabolized to yield selenide or methylated selenides, which might have anticarcinogenic activity[10,22-24].
Selenium bioavailability depends on the food source. Thus, characterizing the selenium bioavailability from various food sources is important for the establishment of dietary guidelines for improved selenium intake. Fish selenium is a highly bioavailable source of dietary selenium. Several studies have examined the bioavailability of selenium from fish and other food sources. Coastal fishermen and inland men were examined in Latvia[25]. The number of fish meals per month correlated with plasma selenium, selenoprotein P, and GPx levels. The mean plasma selenium level in those with a high fish intake (21-50 fish meals/mo) was 81% higher than in those with the lowest fish intake (0-3 fish meals/mo). The bioavailability of selenium from cooked and raw trout in humans was also estimated by comparing the apparent absorption and retention of selenium in fish with labeled selenate and brewers’ yeast, labeled with selenium[26]. Cooking did not affect the apparent absorption or retention of selenium. Compared to fish, selenium from yeast was less bioavailable. In another study, wheat, garlic and cod were intrinsically labeled with stable 77Se or 82Se isotopes, and were fed in random order to 14 adults, with a minimum washout period of 6 wk between each test meal[27]. Selenium absorption from wheat and garlic was higher than from fish with negligible individual variances. These findings suggest that the chemical form of selenium and the food source are the key determinants of post-absorptive metabolism.
Under physiological conditions, most selenite is transported as mixed thiols with glutathione (GSH) and other tissue thiol compounds[24,28-30]. Selenium amino acids are transported by corresponding transfer systems with specificity for analogous sulfur-containing amino acids. Any bioavailability evaluation must consider their metabolic retention and toxicity indices[30].
Selenoneine is an ergothioneine selenium analog in which the sulfur of ergothioneine is replaced by selenium[20]. Selenoneine is easily incorporated into cultured human and fish cells, and shows cell proliferation ability (Figure 2), which suggests the presence of its specific metabolic pathways in animal cells. Organic cation transporter OCTN1, which is an ergothioneine transporter, imports selenoneine and ergothioneine[31-33]. Both selenoneine and ergothioneine can be incorporated into tissues and cells from food, and act as important antioxidants in the redox cycle. Selenoneine can be produced from ergothioneine as a substrate in erythrocytes and other animal cells that are rich in GSH and ergothioneine.
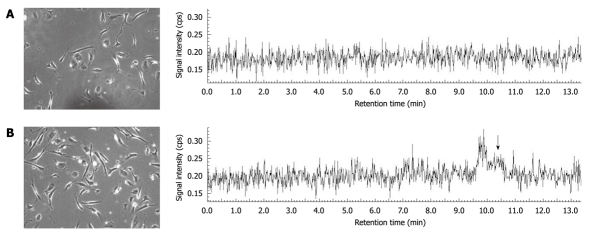
Figure 2.
Effect of selenoneine on cell growth. A: Control; B: Selenoneine. Human umbilical vein endothelial cells (HUVECs) were cultured by supplementation with 10% calf serum in the presence of selenoneine (50 μmol/L) or its absence in MCDB131 medium at 37°C for 24 h. Cell extracts with distilled water were subjected to liquid chromatography inductively coupled plasma mass spectroscopy (LC-ICP-MS) by monitoring 82Se. The arrow shows a peak of selenoneine detected in the HUVEC extract with distilled water at a retention time of 10.5 min. Thus, selenoneine was incorporated into the cells and promoted cell growth.
Biochemical properties of other selenium compounds have been well characterized. Selenite and methylated and hydrogen selenides produce reactive oxygen species (ROS) in vivo, whereas l-selenomethionine has ROS-scavenging activity[24]. However, l-selenomethionine is contained only in plant-derived foods and selenium dietary supplements[21]. Additionally, l-selenomethionine mimics l-methionine in protein turnover, and substitution of l-methionine by l-selenomethionine can affect protein activity and stability[24].
Selenium compounds, such as selenocysteine (LD50 35.8 mg/kg), selenomethionine (LD50 4.3 mg/kg), and selenite (LD50 3.5 mg/kg), can be highly toxic[34-36]. Tuna tissues contain selenoneine at 5-40 μg Se/g[20,37]. Selenoneine may be a non-toxic, strong antioxidant in animal tissues and cells. The chemical structure of inorganic and organic selenium and the biological origin (e.g. plant or animal) play an important role in the regulation of post-absorptive metabolism of selenium compounds. The metabolic pathways involved in the regulation of the synthesis and decomposition of selenoneine and other organic selenium compounds in human and animal cells require further characterization.
SPECIATION ANALYSIS OF ORGANIC SELENIUM IN FISH TISSUES
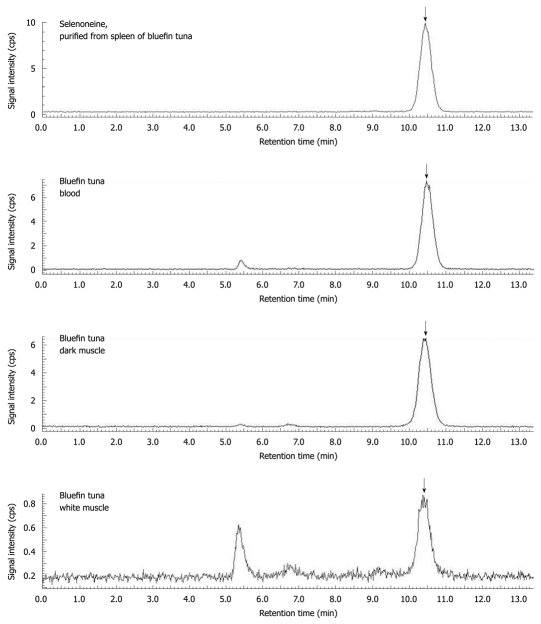
To determine the tissue distribution of selenoneine and other organic selenium compounds, a speciation analysis method was used for organic selenium in animal tissues. This method is based on monitoring 82Se using liquid chromatography inductively coupled plasma mass spectroscopy (LC-ICP-MS) with a GPC column[20]. Since selenoneine was associated with the gel matrix, it was eluted 10.5 min after the bed volume of the column (Figure 3). Tuna red muscle contained selenoneine at 190 nmol Se/g and selenoproteins at 4.5 nmol Se/g. Almost all of the organic selenium (98%) was present as selenoneine in the tuna muscle. Tuna and mackerel blood contained high levels of selenium as selenoneine, 430-437 nmol Se/g. Other tissues, such as the spleen, hepatopancreas, heart, white muscle, and blood, also contained selenoneine above 11.5 nmol Se/g. In contrast, tilapia blood, porcine kidney, chicken heart, gizzard and liver, and squid hepatopancreas contained low levels of selenoneine and selenoproteins. Furthermore, porcine liver contained only selenoproteins and not selenoneine. In summary, selenoneine was found to be widely distributed in various animal tissues and occurred at especially high levels in tuna tissues. These findings indicate that the chemical forms of organic selenium are very different between fish and terrestrial animals.
Figure 3.
Speciation analysis of organic selenium in the various tissues of bluefin tuna by LC-ICP-MS. Selenium compounds were separated with an Ultrahydrogel 120 (7.8 mm × 250 mm, Nihon Waters, Tokyo, Japan), equilibrated with 100 mmol/L ammonium formate buffer[30]. The mobile phase was delivered at 1 mL/min isocratically, and selenium was detected using online LC-ICP-MS (ELAN DRC II, PerkinElmer, Waltham, MA, USA) monitoring 82Se. GPx, selenite, selenocysteine, selenomethionine, and selenoneine were eluted at retention times of 5.4, 7.4, 7.8, 9.8, and 10.5 min, respectively, and the selenium concentration was determined using the respective compounds as standards. The arrow shows the elution of selenoneine. Selenoproteins including GPx were eluted near the void volume of the column at 5.4 min.
BIOLOGICAL FUNCTIONS OF SELENONEINE IN FISH
Selenoneine has high antioxidant ability and binds to heme proteins, such as hemoglobin and myoglobin, thus protecting them from iron auto-oxidation[20]. The selenoneine concentration in purified myoglobin from bluefin tuna and sperm whale was estimated at a Se:Fe molar ratio of 3:1000, whereas it was not detected in bovine or horse proteins using LC-ICP-MS.
A recent study has reported that ‘burnt’ meat, which lacks a bright red meat color and has a more watery, softer texture, is often seen in tuna and mackerel under stress conditions when fish are caught during the spawning period in summer[37]. The oxidative stress is caused by selenium deficiency and hypoxia. After catching, extensive apoptosis and autophagy occur in white muscle, and hemolysis occurs in fish that contain low selenium levels (< 1 μg Se/g in blood)[37]. Thus, the antioxidant capacity of selenoneine is essential in fish for adaptation and survival in low-oxygen marine environments. White muscle disease that is identified in selenium-deficient animals is considered to be similar to this disorder[21]. According to selenoproteome analysis, evolution from fish to mammals is accompanied by decreased use of selenocysteine by selenocysteine/cysteine transitions[38]. It is therefore possible that adequate selenium intake in tuna and other marine animals is very different from that of terrestrial animals.
Intracellular methylmercury (MeHg) tends to reduce the amount of selenium that is biologically available for normal selenoenzyme synthesis, especially as Hg:Se molar ratios approach or exceed a 1:1 stoichiometry[39]. Dietary selenium is postulated to protect against mercury toxicity and the mercury-dependent inhibition of selenoenzyme activity. Additional dietary selenium has been shown to offset the selenium sequestered by mercury and thereby maintain normal antioxidant activity of brain selenoenzymes[39-44]. Increasing blood Hg:Se ratios are indicative of increasing risk or harm, therefore, assessment of MeHg exposure should involve the evaluation of blood Hg:Se ratios, rather than mercury levels alone. Impaired growth and disruption of motor function are readily observed indications of severe MeHg toxicity[39-44]. Additionally, more sensitive biomarkers are needed to assess the effects of low MeHg exposure[39-44]. Selenium supplementation in the nutritionally relevant range counteracts MeHg toxicity[39-44]. Selenium tends to be abundant in fish. MeHg exposure alone therefore may not provide an accurate index of the risk of fish consumption[39].
It should also be noted that selenoneine regulates the detoxification and metabolism of MeHg. When MeHg toxicity was assessed using zebrafish embryos, abnormal morphogenesis was observed in the brain and in cardiac development upon treatment of the culture medium with MeHg-Cys at 10-100 ng Hg/mL. MeHg toxicity was however reduced in the presence of selenoneine. OCTN1, which transports ergothioneine and carnitine[32,33], regulates the incorporation and excretion of selenoneine. OCTN1-deficient embryos treated with an antisense morpholino oligo and MeHg had decreased selenium and increased mercury levels. In zebrafish development, OCTN1 is expressed in erythrocytes and is suggested to act in heme metabolism[45]. Thus, this transporter might transport the selenoneine-MeHg complex, regulate selenium-enhanced antioxidant activity, and decrease MeHg toxicity. Since stress-induced apoptosis is triggered by depletion of GSH[46], reduced selenoneine and GSH might induce apoptosis and produce ROS under oxidative stress conditions, including MeHg exposure.
POSSIBLE ROLES OF SELENONEINE AS AN ANTIOXIDANT IN ANIMAL CELLS
Selenoneine has strong radical-scavenging activity against 1,1-diphenyl-2-picrylhydrazyl in vitro[20]. Thus, selenoneine could play an important role in the redox cycle in animal cells. Selenoneine binds to the heme moiety of hemoglobin and myoglobin to protect them from auto-oxidation by iron ions under hypoxic conditions[20]. GPx and other selenoproteins, which are induced by selenium intake, have been postulated to enhance antioxidant ability in animal tissues and cells[1-6]. However, it should be noted, that selenoneine is the predominant chemical form in tuna. Thus, it is possible that selenoneine derived from a dietary intake of fish is the major radical scavenger in the physiological and nutritional functions described in these previously published studies[1-14]. For example, Keshan disease, which results in an enlarged heart and poor heart function, occurs in selenium-deficient children in areas with selenium-poor soil, such as high altitudes in China[10,21,47]. Relationships between nutrition and viral infection have also been studied, and selenium deficiency can alter a viral genome such that a normally benign or mildly pathogenic virus becomes highly virulent in the deficient, oxidatively stressed host[48]. In addition, protective roles of selenoneine as a radical scavenger in the heart and blood cells in humans might also be essential for the adaptation to low-oxygen environments at high altitudes.
Coronary heart disease, cancer, and aging involve actions by ROS. Lipid peroxidation reactions yield ROS, including •OH and peroxides, which can function as DNA-reactive genotoxins and as promoters[49]. A generous intake of vegetables, fruits, soy products, red wine, and tea rich in antioxidants, which can eliminate ROS, is protective against cancer and heart disease[49]. Dietary intake of selenoneine might decrease the formation of ROS and the oxidation of nucleotides in DNA, and thereby inhibit carcinogenesis, chronic diseases, and aging (Figure 4).
Figure 4.
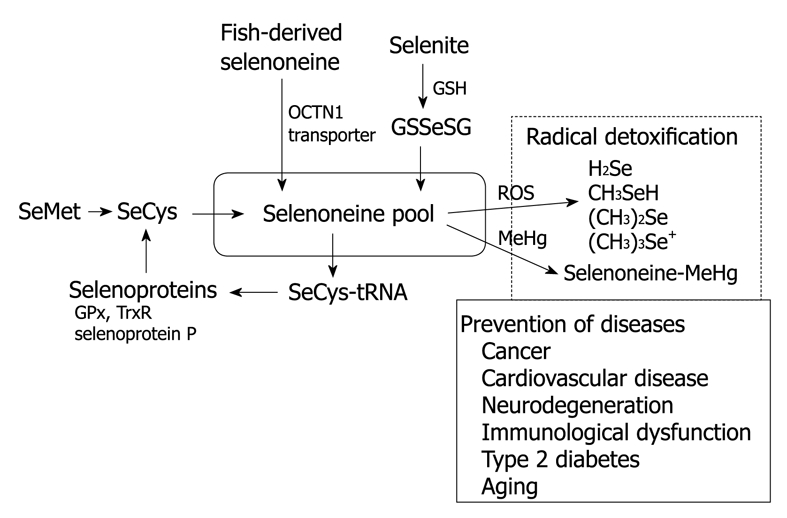
Proposed model of selenium metabolism and radical detoxification.
A related dietary inhibitor of oxidative damage, α-tocopherol, prevents fatty acid hydroperoxide formation and protects against hemolysis in cellular membranes[6,50]. Subcellular fractionation has localized selenoneine to the cytosolic fraction in erythrocytes and other animal cell types. Thus, selenoneine might act as an antioxidant in the cytosol. Membrane-bound α-tocopherol and cytosolic selenoneine are thought to protect against oxidant damage in distinct subcellular compartments. Additionally, in response to such dual antioxidant systems, different types of GPx are involved in peroxide destruction. Selenoneine or total selenium might therefore serve as useful serum biomarkers. The measurement of selenoneine or total selenium and α-tocopherol levels in the blood could help to determine the level of oxidative stress for pathological diagnoses and epidemiological surveillance.
CONCLUSION
Although selenium is an important micronutrient that is linked to a variety of human and animal diseases, the organic selenium compound responsible for the antioxidant and redox mechanisms has never been identified and characterized. Selenoneine is considered to play the major role as a strong radical scavenger in a variety of physiological and nutritional functions. We believe that characterization of this compound will accelerate biochemical and biomedical research on cancer, cardiovascular diseases, neurodegeneration, immunological dysfunction, type 2 diabetes, MeHg toxicity, and aging, which could be related to selenium deficiency.
Footnotes
Supported by In part grants from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Rural Biomass Research Project, BM-D2300) and Fisheries Research Agency
Peer reviewer: Duygu D Harrison-Findik, DVM, PhD, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE 68198-5820, United States
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Himeno S, Imura N. New aspects of physiological and pharmacological roles of selenium. J Health Sci. 2000;46:1–6. [Google Scholar]
- 2.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 3.Gerald F Jr, Combs SB. The Role of Selenium in Nutrition. New York: Academic Press; 1986. [Google Scholar]
- 4.Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int J Environ Res Public Health. 2009;6:1894–1916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 6.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 7.Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346 Pt 1:1–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Burk RF, Hill KE. Selenoprotein P. A selenium-rich extracellular glycoprotein. J Nutr. 1994;124:1891–1897. doi: 10.1093/jn/124.10.1891. [DOI] [PubMed] [Google Scholar]
- 9.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 10.Hatfield DL, Berry MJ, Gladyshev VN. Selenium: its molecular biology and role in human health. 2nd ed. New York: Springer; 2006. [Google Scholar]
- 11.Kiremidjian-Schumacher L, Roy M. Effect of selenium on the immunocompetence of patients with head and neck cancer and on adoptive immunotherapy of early and established lesions. Biofactors. 2001;14:161–168. doi: 10.1002/biof.5520140121. [DOI] [PubMed] [Google Scholar]
- 12.Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet. 1982;2:175–179. doi: 10.1016/s0140-6736(82)91028-5. [DOI] [PubMed] [Google Scholar]
- 13.Ghose A, Fleming J, Harrison PR. Selenium and signal transduction: roads to cell death and anti-tumour activity. Biofactors. 2001;14:127–133. doi: 10.1002/biof.5520140117. [DOI] [PubMed] [Google Scholar]
- 14.El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutat Res. 2001;475:123–139. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 15.Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;175:1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- 16.Cuvin-Aralar ML, Furness RW. Mercury and selenium interaction: a review. Ecotoxicol Environ Saf. 1991;21:348–364. doi: 10.1016/0147-6513(91)90074-y. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Imura N, Clarkson TW. Advances in Mercury Toxicology. New York: Plenum Press; 1991. [Google Scholar]
- 18.Raymond LJ, Ralston NVC. Mercury: selenium interactions and health implications. SMDJ Seychelles Niger Med Dent J. 2004;7:72–77. doi: 10.1016/j.neuro.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 19.De Silva V, Woznichak MM, Burns KL, Grant KB, May SW. Selenium redox cycling in the protective effects of organoselenides against oxidant-induced DNA damage. J Am Chem Soc. 2004;126:2409–2413. doi: 10.1021/ja037294j. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Yamashita M. Identification of a novel selenium-containing compound, selenoneine, as the predominant chemical form of organic selenium in the blood of bluefin tuna. J Biol Chem. 2010;285:18134–18138. doi: 10.1074/jbc.C110.106377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietary Supplement Fact Sheet: Selenium. National Institute of Health, 2009. Available from: http://ods.od.nih.gov/factsheets/selenium.asp.
- 22.Combs GF Jr. Impact of selenium and cancer-prevention findings on the nutrition-health paradigm. Nutr Cancer. 2001;40:6–11. doi: 10.1207/S15327914NC401_4. [DOI] [PubMed] [Google Scholar]
- 23.Combs GF Jr. Current evidence and research needs to support a health claim for selenium and cancer prevention. J Nutr. 2005;135:343–347. doi: 10.1093/jn/135.2.343. [DOI] [PubMed] [Google Scholar]
- 24.Jackson MI, Combs GF Jr. Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care. 2008;11:718–726. doi: 10.1097/MCO.0b013e3283139674. [DOI] [PubMed] [Google Scholar]
- 25.Hagmar L, Persson-Moschos M, Akesson B, Schütz A. Plasma levels of selenium, selenoprotein P and glutathione peroxidase and their correlations to fish intake and serum levels of thyrotropin and thyroid hormones: a study on Latvian fish consumers. Eur J Clin Nutr. 1998;52:796–800. doi: 10.1038/sj.ejcn.1600649. [DOI] [PubMed] [Google Scholar]
- 26.Fox TE, Van den Heuvel EG, Atherton CA, Dainty JR, Lewis DJ, Langford NJ, Crews HM, Luten JB, Lorentzen M, Sieling FW, et al. Bioavailability of selenium from fish, yeast and selenate: a comparative study in humans using stable isotopes. Eur J Clin Nutr. 2004;58:343–349. doi: 10.1038/sj.ejcn.1601787. [DOI] [PubMed] [Google Scholar]
- 27.Fox TE, Atherton C, Dainty JR, Lewis DJ, Langford NJ, Baxter MJ, Crews HM, Fairweather-Tait SJ. Absorption of selenium from wheat, garlic, and cod intrinsically labeled with Se-77 and Se-82 stable isotopes. Int J Vitam Nutr Res. 2005;75:179–186. doi: 10.1024/0300-9831.75.3.179. [DOI] [PubMed] [Google Scholar]
- 28.McAdam PA, Orville LA. Chronic toxicity and retention of dietary selenium fed to rats as D- or L-selenomethionine, selenite, or selenate. Nutr Res. 1987;7:601–610. [Google Scholar]
- 29.Alfthan G, Aro A, Arvilommi H, Huttunen JK. Selenium metabolism and platelet glutathione peroxidase activity in healthy Finnish men: effects of selenium yeast, selenite, and selenate. Am J Clin Nutr. 1991;53:120–125. doi: 10.1093/ajcn/53.1.120. [DOI] [PubMed] [Google Scholar]
- 30.Schrauzer GN. Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr. 2000;130:1653–1656. doi: 10.1093/jn/130.7.1653. [DOI] [PubMed] [Google Scholar]
- 31.Tamai I, Yabuuchi H, Nezu J, Sai Y, Oku A, Shimane M, Tsuji A. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett. 1997;419:107–111. doi: 10.1016/s0014-5793(97)01441-5. [DOI] [PubMed] [Google Scholar]
- 32.Gründemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, Schömig E. Discovery of the ergothioneine transporter. Proc Natl Acad Sci USA. 2005;102:5256–5261. doi: 10.1073/pnas.0408624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber GJ, Choe SE, Dooley KA, Paffett-Lugassy NN, Zhou Y, Zon LI. Mutant-specific gene programs in the zebrafish. Blood. 2005;106:521–530. doi: 10.1182/blood-2004-11-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilber CG. Toxicology of selenium: a review. Clin Toxicol. 1980;17:171–230. doi: 10.3109/15563658008985076. [DOI] [PubMed] [Google Scholar]
- 35.Sayato Y, Hasegawa T, Taniguchi S, Maeda H, Ozaki K, Narama I, Nakamuro K. Acute and subacute oral toxicity of selenocystine in mice. Jpn J Toxicol Environ Health. 1993;39:289–296. [Google Scholar]
- 36.World Health Organization (WHO) Environmental Health Criteria 58, selenium. Geneva: World Health Organization Publication, 1987. Available from: http://www.who.int/water_sanitation_health/dwq/chemicals/en/selenium.pdf.
- 37.Yamashita M. Stress Responses of Fish during Catching Process. In: Konno K, Ochiai Y, Fukuda Y, editors. Quality control of tuna meat by optimization of fishing and handling. Tokyo: Koseisha-Koseikaku; 2010. pp. 81–94. [Google Scholar]
- 38.Lobanov AV, Hatfield DL, Gladyshev VN. Reduced reliance on the trace element selenium during evolution of mammals. Genome Biol. 2008;9:R62. doi: 10.1186/gb-2008-9-3-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ralston NV, Ralston CR, Blackwell JL 3rd, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology. 2008;29:802–811. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Prohaska JR, Ganther HE. Interactions between selenium and methylmercury in rat brain. Chem Biol Interact. 1977;16:155–167. doi: 10.1016/0009-2797(77)90125-9. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe C, Yoshida K, Kasanuma Y, Kun Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environ Res. 1999;80:208–214. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol Teratol. 1999;21:83–88. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 43.Ralston NV, Blackwell JL 3rd, Raymond LJ. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol Trace Elem Res. 2007;119:255–268. doi: 10.1007/s12011-007-8005-7. [DOI] [PubMed] [Google Scholar]
- 44.Yang DY, Chen YW, Gunn JM, Belzile N. Selenium and mercury in organisms: Interactions and mechanisms. Environ Rev. 2008;16:71–92. [Google Scholar]
- 45.Nilsson R, Schultz IJ, Pierce EL, Soltis KA, Naranuntarat A, Ward DM, Baughman JM, Paradkar PN, Kingsley PD, Culotta VC, et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009;10:119–130. doi: 10.1016/j.cmet.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yabu T, Imamura S, Yamashita M, Okazaki T. Identification of Mg2+ -dependent neutral sphingomyelinase 1 as a mediator of heat stress-induced ceramide generation and apoptosis. J Biol Chem. 2008;283:29971–29982. doi: 10.1074/jbc.M805402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu BQ. Pathology of Keshan disease. A comprehensive review. Chin Med J (Engl) 1983;96:251–261. [PubMed] [Google Scholar]
- 48.Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133:1463S–1467S. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 49.Weisburger JH. Antimutagenesis and anticarcinogenesis, from the past to the future. Mutat Res. 2001;480-481:23–35. doi: 10.1016/s0027-5107(01)00166-x. [DOI] [PubMed] [Google Scholar]
- 50.Rotruck JT, Pope AL, Ganther HE, Hoekstra WG. Prevention of oxidative damage to rat erythrocytes by dietary selenium. J Nutr. 1972;102:689–696. doi: 10.1093/jn/102.5.689. [DOI] [PubMed] [Google Scholar]