Abstract
3-phosphoinositide-dependent protein kinase-1 (PDK1) is a central mediator of cellular signaling between phosphoinositide-3 kinase and various intracellular serine/threonine kinases, including protein kinase B, p70 ribosomal S6 kinase, serum and glucocorticoid-inducible kinase, and protein kinase C. PDK1 activates members of the AGC family of protein kinases by phosphorylating serine/threonine residues in the activation loop. Here, we review the regulatory mechanisms of PDK1 and its roles in cancer. PDK1 is activated by autophosphorylation in the activation loop and other serine residues, as well as by phosphorylation of Tyr-9 and Tyr-373/376. Src appears to recognize PDK1 following tyrosine phosphorylation. The role of heat shock protein 90 in regulating PDK1 stability and PDK1-Src complex formation are also discussed. Furthermore, we summarize the subcellular distribution of PDK1. Finally, an important role for PDK1 in cancer chemotherapy is proposed. In conclusion, a better understanding of its molecular regulatory mechanisms in various signaling pathways will help to explain how PDK1 acts as an oncogenic kinase in various cancers, and will contribute to the development of novel cancer chemotherapies.
Keywords: 3-phosphoinositide-dependent protein kinase-1, Protein kinase B, Oncogenic kinase, Cell signaling, Cancer therapy
INTRODUCTION
The regulation of individual protein components of signaling cascades provides biological specificity and flexibility, and allows cells to respond quickly to extracellular stimuli in a physiologically appropriate manner[1]. Protein phosphorylation is the most common and universal mode of regulating protein function in eukaryotes. Since its discovery over a decade ago, 3-phosphoinositide-dependent protein kinase-1 (PDK1) has emerged as a master regulator of the AGC family of protein kinases, which also includes protein kinase B (PKB)/Akt, p70 ribosomal S6 kinase (S6K1), serum and glucocorticoid-inducible kinase (SGK), and protein kinase C (PKC)[2]. Initially, PDK1 was identified by its ability to phosphorylate Thr-308 on PKBα[3-8], which has been shown to play a crucial role in normal and pathophysiological conditions (e.g. diabetes and cancer; reviewed in[9-11]). PDK1 was named for its kinase activity, which is dependent on phosphatidylinositol 3,4,5 trisphosphate [PtdIns(3,4,5)P3) or phosphatidylinositol 3,4 bisphosphate [PtdIns(3,4)P2][12,13]. Activation of PDK1 has been established to regulate cell survival and growth, cell cycle progression, gene expression, and differentiation[2].
PDK1 recognizes substrate kinases in each signaling pathway through a distinct regulatory mechanism. In the case of PKB, this recognition appears to be facilitated by the pleckstrin homology (PH) domain, which mediates recruitment of both PKB and PDK1 to the plasma membrane to promote phosphorylation of PKB[3,6,14]. The C-terminal PH domain of PDK1 has been shown to bind the phospholipid second messengers PtdIns(3,4,5)P3 and PtdIns(3,4)P2, which target PDK1 to the plasma membrane[13,15]. The N-terminal lobe of the catalytic domain of PDK1 contains a docking site that recognizes the non-catalytic C-terminal hydrophobic motifs of substrate kinases[16]. Therefore, it has been proposed that PDK1 and SGK/p90RSK/p70S6K associate transiently via the PDK1-interacting fragment (PIF) motif, thereby leading to subsequent phosphorylation by PDK1[17].
SECONDARY STRUCTURE OF PDK1
PDK1, which is 63 kDa, consists of an N-terminal kinase domain (amino acids 71-359) and a C-terminal PH domain (amino acids 459-550), which binds PtdIns(3,4,5)P3 and PtdIns(3,4)P2[18-21] (Figure 1A). Identification of the PH domain as a specialized lipid-binding module has been a crucial clue in understanding the mechanism by which membrane-bound lipids convey signals to the cytoplasm[7,22]. Deletion of the PH domain prevents PDK1 recruitment to the plasma membrane and affects the activation and membrane localization of PKB[23-25]. Binding of PDK1 to PtdIns(3,4,5)P3 induces a major conformational change that is likely required for the activation of substrates[15]. However, PtdIns(3,4,5)P3 binding to the PH domain of PDK1 does not affect the activity of PDK1 directly[3].
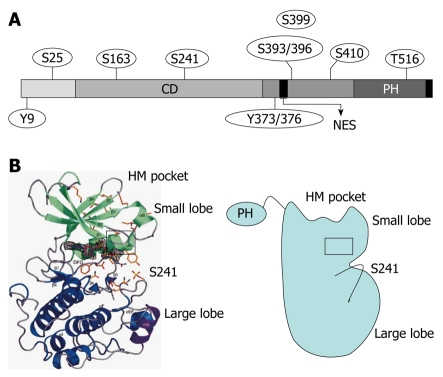
Figure 1.
Secondary structure and phosphorylation sites of 3-phosphoinositide-dependent protein kinase-1. A: 3-phosphoinositide-dependent protein kinase-1 (PDK1) consists of an N-terminal kinase catalytic domain (CD; amino acids 71-359) and a C-terminal pleckstrin homology (PH) domain (amino acids 459-550). The nuclear export sequence (NES) (amino acids 379-388) is essential for exporting PDK1 into the cytoplasm from the nucleus. The phosphorylation sites of PDK1 include Ser-241 and Tyr-373/376, which is dependent on Tyr-9 and required for PDK1 catalytic activity; B: Crystal structure of the human PDK1 kinase domain. Residues 71-163 are green (small lobe) and residues 164-358 are blue (large lobe). The αC-helix (amino acids 124-136) is boxed in black and encompasses residues 287-295, which are purple. The hydrophobic motif (HM) pocket with the Ser-241 in the activation loop is also shown. This figure was adopted from Biondi et al[26], 2002.
As an AGC protein kinase, PDK1 belongs to the same subfamily of protein kinases as its substrates. Like all members of this family, the catalytic core of PDK1 possesses an N-terminal lobe that consists mainly of a β-sheet and a predominantly α-helical C-terminal lobe[26] (Figure 1B). Unlike other AGC kinases, PDK1 does not possess a hydrophobic motif (HM) C-terminal in its catalytic domain. Instead, it has been proposed that PDK1 possesses an HM pocket in the small lobe of its catalytic motif[26]. The αC-helix (residues 124-136), located in the small lobe of the kinase domain, is a key regulatory domain because it links a substrate-interacting site (HM pocket) with Ser-241 in the activation loop. The HM pocket in the kinase domain of PDK1 has been termed the PIF pocket after the first discovery that the C terminus of PKC-related kinase-2, which contains an HM motif, interacts with the kinase domain of PDK1[22]. Subsequent studies have indicated that this PIF pocket in PDK1 functions as a docking site, which enables the kinase to interact with some of its physiological substrates[26]. The crystal structure of PDK1 reveals that phosphorylation of Ser-241 results in a hydrogen bond interaction with four residues, namely Arg-204 and Lys-228 from the C-terminal lobe, and Tyr-126 and Arg-129 from the αC-helix in the N-terminal lobe[26]. The highly conserved Arg-204, which immediately precedes the catalytic Arg-205, is connected directly to the catalytic machinery due to its position within the catalytic loop. Arg-204 controls the folding of the activation loop after interaction with phosphorylated Ser-241. Lys-228 might also play a role in aligning catalytic site residues including Arg-223, which interacts with Mg2+[27].
REGULATORY MECHANISM OF PDK1 ACTIVITY
Regulation of PDK1 activity mediated by phosphorylation events
Protein phosphorylation, which plays a key regulatory role in nearly every aspect of eukaryotic cell biology, is a reversible and dynamic process that is mediated by kinases and phosphatases[17]. PDK1 is thought to be a constitutively active kinase that can use distinct mechanisms to phosphorylate different substrates within cells[17]. PDK1 undergoes autophosphorylation and growth-factor-induced phosphorylation at different sites, and its activity is correlated with its phosphorylation status. Therefore, understanding the mechanism of PDK1 phosphorylation could lead to greater knowledge of its function.
Ser-241 is required for PDK1 catalytic activity
Autophosphorylation in the activation loop is required for PDK1 kinase activity[28-30] (Figure 1A). The phosphorylation level of each serine is unaffected by stimulation with insulin growth factor-1 (IGF-1). However, S241A mutation abolished PDK1 catalytic activity completely. The binding of 14-3-3 to PDK1 negatively regulates its kinase activity through the autophosphorylation site at Ser-241[28]. Activation of mouse PDK1 (mPDK1) requires phosphorylation in the activation loop at Ser-244, which corresponds to Ser-241 in humans[29]. Kinase-defective mPDK1 (K108M) was phosphorylated in intact cells whereas another kinase defective mPDK1 (S244A) remained unphosphorylated[29], which suggests that Ser-241 is a major active site of PDK1. mPDK1 also possesses Ser-163, which corresponds to Ser-160 in humans, and is located in the hinge region between the large and small lobes of the kinase domain. The residue that corresponds to Ser-163 of mPDK1 in other AGC kinases is glutamate, which is negatively charged. Substitution of this serine residue with glutamate (S163E) leads to a twofold increase in mPDK1 activity[29].
Reports have also indicated that IGF-1 stimulates PDK1 phosphorylation at Ser-396[31]. Alanine substitution of Ser-396 reduces IGF-1-stimulated PDK1 nuclear localization. These results suggest that mitogen-stimulated phosphorylation of PDK1 at Ser-396 provides a means for regulating PDK1 subcellular trafficking with a potential implication for PDK1 signaling. It is noteworthy that Ser-396 resides in close proximity to the nuclear export signal of PDK1.
Autophosphorylation of mPDK1 occurs at multiple sites through cis and trans mechanisms, which indicates that dimerization and trans-phosphorylation might serve as mechanisms to regulate PDK1 activity in cells[29]. As expected, trans-autophosphorylation of mPDK1 occurs mainly on Ser-244, as demonstrated by phospho-amino acid analysis and phospho-peptide mapping. In contrast, Ser-399 and Thr-516, two recently identified autophosphorylation sites of mPDK1, are phosphorylated primarily through a cis mechanism[29]. mPDK1 undergoes dimerization in cells and this self-association is enhanced by kinase inactivation. Deletion of the extreme C-terminal region disrupts mPDK1 dimerization and Ser-244 trans-phosphorylation, which suggests that dimerization is important for mPDK1 trans-phosphorylation.
RAFTK/Pyk2 and Src are essential for regulating tyrosine phosphorylation of PDK1
The candidate kinases that phosphorylate Tyr-9 in PDK1 have been suggested by two independent groups. However, much less is known about the role and regulation of PDK1 phosphorylation of tyrosine residues[32]. There is evidence to show that insulin induces tyrosine phosphorylation of PDK1[23]. Insulin binds to the extracellular α subunit of the insulin receptor (IR), which is a heterotetramer that consists of two α and two β subunits. Binding of insulin to the IR transduces signals across a series of intramolecular trans-phosphorylation reactions in which one β subunit phosphorylates its adjacent partner on a specific tyrosine residue[33-35]. The IR binds to and phosphorylates PDK1 on tyrosine residues in response to insulin, thereby leading to PDK1 activation. Membrane anchoring of PDK1 through the C-terminal 1340-1382 region of the IR is a crucial step in insulin metabolic action and can affect PDK1 signaling in response to other hormones as well[36].
It has been proposed that rearranged in transformation/papillary thyroid carcinomas (RET/PTC) acts as a thyroid-specific oncogenic kinase in the development of spontaneous and post-radiation papillary thyroid cancer[32]. Co-localization of RET/PTC and PDK1 in the cytoplasm leads to Tyr-9 phosphorylation of PDK1, which is independent of phosphoinositide 3-kinase (PI3K) or Src activity[32]. Studies have shown that RET/PTC3 enhances insulin-stimulated PKB activity via PI3K[37]. Consistent with this, the levels of total and phosphorylated IR substrate 2 (IRS2) protein increases, PDK1 activation is observed, and IRS2-p85 interactions are enhanced in RET/PTC3-expressing cells[38].
Furthermore, the calcium-activated tyrosine kinase RAFTK/Pyk2 acts as a scaffold for Src-dependent phosphorylation of PDK1 on Tyr-9[39]. The tyrosine phosphatase SH2 domain-2 (SHP-2) is recruited to SH2 domain-containing protein tyrosine phosphatase substrate-1 and associates with RAFTK/Pyk2 in a PI3K-dependent manner[40]. Compared to Tyr-9 phosphorylation of PDK1, the mechanism of Tyr-373/376 phosphorylation has not yet been proposed. Tyr-373/376 phosphorylation, which is important for PDK1 catalytic activity, is dependent on Tyr-9 phosphorylation[23,39]. In this regard, it is necessary to elucidate the SH2-containing protein that binds to PDK1 and is dependent on Tyr-9 phosphorylation for Tyr-373/376 phosphorylation. Src, an SH2 domain-containing protein, has been identified to further activate PDK1 by mediating phosphorylation at Tyr-9, Tyr-373, and Tyr-376 residues[41]. Recently it has been proposed that Tyr-9 and Tyr-376 are binding sites for SHP-1 (presumably via its tandem SH2 domains), whereas Tyr-333 and Tyr-373 are potential catalytic targets[42]. In addition, tumor suppressor candidate 4 (TUSC4; also known as nitrogen permease regulator-like 2) has been suggested as a novel regulator of PDK1 by using Escherichia-coli-based two-hybrid screening[43]. TUSC4 forms a complex with PDK1 and suppresses Src-dependent tyrosine phosphorylation of PDK1 in vitro and in vivo. Furthermore, TUSC4 inhibits PDK1 downstream signaling, including PKB and S6K1, and increases cancer cell sensitivity to several anticancer drugs[43].
Roles of Src in PDK1 tyrosine phosphorylation
Src, a non-receptor tyrosine kinase, is the prototypic member of the Src family of kinases (SFKs). SFKs are involved in multiple signaling pathways, with roles that are crucial to tumor development, including proliferation, invasion, adhesion, angiogenesis and survival[44-47]. Src contains an N-terminal 14-carbon myristoyl group, an SH4 domain, a poorly conserved unique domain, an SH3 domain, an SH2 domain, a tyrosine kinase domain, and a C-terminal regulatory tail[48]. The SH2 domain of Src, Crk, and GTPase activating protein recognizes tyrosine-phosphorylated PDK1 in vitro. Src binds to Tyr-9 and Tyr-373/376 in vivo and phosphorylation of PDK1 on Tyr-9, distinct from Tyr-373/376, is important for PDK1/Src complex formation, which leads to PDK1 activation[41]. Furthermore, overexpression of heat shock protein 90 (Hsp90) enhances the binding affinity of PDK1 and Src, increases PDK1 tyrosine phosphorylation, and promotes PDK1 downstream kinase activity[23,41]. In addition, the screening of drugs, which could interfere with the PKB signaling pathway, has revealed that Hsp90 inhibitors (e.g. geldanamycin, radicicol, and its analogs) induce PKB dephosphorylation, which results in its inactivation and apoptotic cell death[49]. Hsp90 inhibitors do not affect PKB kinase activity directly in vitro, but destabilize PDK1 without affecting its activity[49-51]. These results suggest that Hsp90 plays an important role in the PDK1/PKB survival pathway.
Hsp90-mediated stabilization of PDK1
The function of Hsp90 might be to form complexes with client proteins and thus to stabilize their functional structures[50]. Hsp90 exerts its chaperone activity together with a number of co-chaperones (e.g. cell-division-cycle 37 homolog: Cdc37/p50, activator of heat shock 90 kDa protein ATPase, p23, cyclophilin 40, and protein phosphatase 5). In particular, Cdc37 facilitates the interaction of Hsp90 and kinase, which leads to the stabilization of kinase clients[50]. Cdc37 has been shown to have molecular-chaperone-like activity for substrates including kinases[50], which indicates that Cdc37 performs more tasks than simply functioning as a stable bridge between kinases and Hsp90[52].
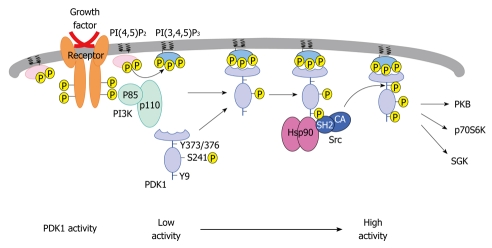
Intracellular PKB is associated with Hsp90 and Cdc37 in a complex in which PKB is active and regulated by PI3K[49]. Inhibition of Hsp90 function causes dephosphorylation and proteasome-dependent ubiquitination of PKB, which shortens the half-life of this kinase from 36 to 12 h and reduces its expression by 80%[49]. Hsp90 inhibitors do not affect PKB kinase activity directly in vitro and decrease the quantity of PDK1 by occupying the binding sites of Hsp90 with PDK1, which results in proteasome targeting[51]. In addition, Hsp90 inhibitors also decrease the levels of mutant PDK1 that possess phenylalanine substitutions for tyrosine residues (i.e. Y9F and Y373/376F), which indicates that PDK1 stability is independent of Tyr-9 and Tyr-373/376. These data are consistent with previous observations that show that PDK1 binds Hsp90 in an expression-dependent manner. Thus, the binding is not affected by the Tyr-9 and Tyr-373/376 residues[41]. PDK1-Y9F does not respond to the treatment of cells with pervanadate[23], and overexpression of this mutant completely blocks Tyr-373/376 phosphorylation. However, Tyr-9 phosphorylation is still detected in bound PDK1-Y373F/Y376F[41]. Furthermore, PDK1-Y9F appears to inhibit vascular smooth muscle cell migration significantly[53], and to block focal adhesion formation[39]. As illustrated in Figure 2, growth factor binding to its cognate receptor activates PI3K, which results in the generation of PtdIns(3,4,5)P3. PDK1 is then recruited to the plasma membrane and phosphorylated by the IR, RET/PTC, and Pyk2 on the Tyr-9 residue. This phosphorylated amino acid then acts as a docking site for Src, which leads to Tyr-373 phosphorylation and activation of PDK1. In this context, Hsp90 serves as an adaptor molecule that enhances PDK1 stability and PDK1-Src complex formation.
Figure 2.
Proposed mechanism for the regulation of 3-phosphoinositide-dependent protein kinase-1 phosphorylation and stability. 3-phosphoinositide-dependent protein kinase-1 (PDK1) autophosphorylates itself on Ser-241. In the presence of pervanadate or insulin, PDK1 is phosphorylated on Tyr-9 and Tyr-373/376 with the help of Src and heat shock protein 90 (Hsp90). Tyrosine phosphorylation further increases PDK1 catalytic activity. In the absence of Hsp90 interaction, PDK1 is promoted towards proteasome-dependent degradation. This figure was adopted from Yang et al[41], 2008. PI3K: Phosphoinositide 3-kinase; PI(4,5)P2: Phosphatidylinositol 3,4 bisphosphate; PI(3,4,5)P3: Phosphatidylinositol 3,4,5 trisphosphate; CA: Constitutively active; PH: Pleckstrin homology; PKB: Protein kinase B; SGK: Serum and glucocorticoid-inducible kinase.
SUBCELLULAR LOCALIZATION OF PDK1
PDK1 is localized in the cytoplasm and membranes in unstimulated cells and can shuttle between these compartments[31,54-57] (Figure 3A). Although the mechanisms of translocation to the plasma membrane are well-established for PI3K, PDK1, and PKB[58], it remains unknown whether these proteins accumulate in specific micro-domains of the plasma membrane. Specific tyrosine residues in PDK1 contribute to its activation as well as to its ability to localize to the plasma membrane[23]. Significant amounts of Src also are translocated to the plasma membrane under these conditions. Overexpression of either constitutively active Src (Src-CA) or Hsp90 leads to membrane translocation of PDK1 in serum-starved conditions[41], which clearly shows that Src-CA and Hsp90 play important roles in regulating PDK1 subcellular localization (Figure 3B).
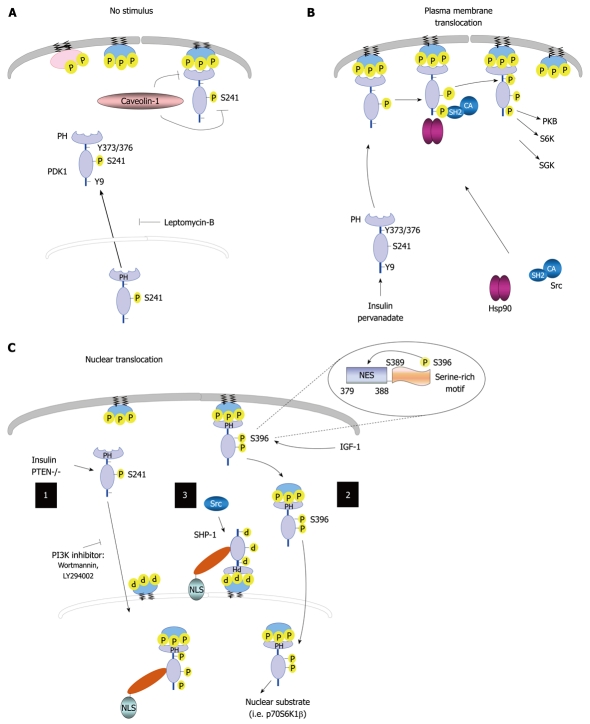
Figure 3.
Regulation of 3-phosphoinositide-dependent protein kinase-1 cellular localization. A: 3-phosphoinositide-dependent protein kinase-1 (PDK1) resides in the cytoplasm and membranes in unstimulated cells, and can shuttle between these two compartments. The treatment of cells with leptomycin-B results in PDK1 accumulation in the nucleus. The localization of this kinase to the plasma membrane is disrupted by caveolin-1; B: PDK1 can translocate to the plasma membrane following treatment with pervanadate or insulin, which leads to PDK1 phosphorylation on Tyr-9 and Tyr-373/376; C: Nucleus-cytoplasm shuttle. (1) Phosphoinositide 3-kinase (PI3K) signaling regulates PDK1 nuclear translocation; (2) Insulin growth factor-1 (IGF-1) induces Ser-396 phosphorylation, thereby placing the serine-rich motif (amino acids Ser-389-396) close to its nuclear export sequence (NES) region, which leads to PDK1 nuclear translocation; and (3) Tyrosine phosphatase SH2 domain-1 (SHP-1) binds to PDK1 following tyrosine phosphorylation. The SHP-1/PDK1 complex is recruited to the nuclear membrane by binding to perinuclear phosphatidylinositol 3,4,5 trisphosphate, where SHP-1 and its nuclear localization signal (NLS) facilitates active import. In addition, Src promotes the association of SHP-1 to PDK1. Nuclear PDK1 regulates its nuclear substrates and activates corresponding signaling pathways. PTEN: Phosphatase and tensin homolog; CA: Constitutively active; PH: Pleckstrin homology; PKB: Protein kinase B; SGK: Serum and glucocorticoid-inducible kinase; Hsp90: Heat shock protein 90.
PDK1 associates with caveolin-1, the principal 22-kDa integral membrane protein that is crucial to the structural and regulatory component of caveolar membranes[14]. PDK1 localization to the plasma membrane can be disrupted by caveolin-1 binding. In transient transfection experiments, the interaction of caveolin-1 with PDK1 inhibits serine/threonine phosphorylation of PDK1 in vivo[25]. Lim and colleagues have shown that PDK1 can localize to the nucleus during specific signaling events[56]. Mutation or deletion of the nuclear export sequence (NES), which is required to bind chromosome region maintenance 1, also leads to constitutive PDK1 nuclear localization, similar to the effects of leptomycin-B, a nuclear export inhibitor[56]. These results suggest that the NES has an important role in PDK1 export from the nucleus. Reports indicate that growth factors (e.g. insulin, nerve growth factor, and IGF-1) not only promote PDK1 tyrosine phosphorylation, but also stimulate its translocation into the nucleus[31,56]. However, the physiological significance of PDK1 nuclear translocation in response to insulin remains to be addressed. Insulin-induced accumulation of PDK1 into the nucleus can be enhanced in phosphatase and tensin homolog (PTEN)-deficient embryonic fibroblasts and blocked by PI3K inhibition using wortmannin and LY294002[42,56]. This finding indicates that PDK1 nuclear import is regulated by the availability of PtdIns(3,4,5)P3.
A recent study using PDK1 that lacked its nuclear localization signal suggested a mechanism for PDK1 nuclear import. In this mechanism, the SHP-1/PDK1 complex is recruited to the nuclear membrane following binding to perinuclear PtdIns(3,4,5)P3[42]. SHP-1 and its nuclear localization signal (NLS) facilitate active import, whereas export from the nucleus relies on PDK1 and its NES. Expression of activated Src kinase in C6 glioblastoma cells promotes the association of tyrosine-phosphorylated PDK1 with the NLS-containing tyrosine phosphatase SHP-1, as well as the nuclear localization of both proteins[42]. However, the role of SHP-1-mediated nuclear localization of PDK1 in the physiological and pathophysiological environment should be further investigated. In addition, deletion mapping and mutagenesis studies have further revealed a functional NES in mPDK1 between the kinase and PH domains. Mutation of Ser-396 to alanine disrupts IGF-1-induced phosphorylation of PDK1, thereby reducing nuclear localization. Ser-396 phosphorylation places the serine-rich motif (Ser-389 to Ser-396) proximal to the putative NES region, which suggests that Ser-396 phosphorylation provides a means for directed PDK1 subcellular trafficking[31] (Figure 3C). Constitutive nuclear localization of PDK1 does not dampen its kinase activity. However, the ability of constitutively nuclear PDK1 to promote anchorage-independent growth and protect against UV-induced apoptosis is impaired[56]. Although PDK1 nuclear localization might sequester the kinase from activating cytosolic signaling pathways (e.g. PKB), it might also position PDK1 near nuclear substrates (e.g. p70S6K1β), which enable the activation of other signaling pathways[56]. Taking these results together, PDK1 subcellular trafficking provides another means for understanding the potential implications of PDK1 signaling in disease.
PDK1: A TARGET FOR CANCER CHEMOTHERAPY
PDK1 mediates diverse and important cellular functions and contributes to many human diseases such as cancer and diabetes[42]. Further investigation into PDK1 regulation will probably establish this kinase as a promising anticancer target for the prevention of tumors. There is increasing evidence that PDK1 is involved in cancer progression and invasion[59]. Tissue microarray analysis of human invasive breast cancer has revealed that phosphorylation of PDK1 on Ser-241 was strongly enhanced in 90% of the samples tested[59]. Immunohistochemical analysis using anti-phospho-Tyr-9 antibodies has shown that the level of Tyr-9 phosphorylation is increased markedly in diseased lung, liver, colon, and breast tissue compared to normal tissue[41]. Studies have shown that angiotensin-II-induced focal adhesion formation is inhibited by infection with Adeno-PDK1-Y9F (adenovirus that expresses PDK1-Y9F mutant) via paxillin[39]. This regulation of focal adhesion suggests that PDK1 participates in integrating signals that control cell growth, apoptosis, and migration.
Increased expression of PDK1 has been detected in various invasive cancers[59-62]. In breast cancer cells, PDK1 plays a crucial role in metastasis[59]. This kinase mediates mammary epithelial cell growth and invasion in the transformed phenotype, in part, by membrane type 1-matrix metalloproteinase (MMP) induction, which in turn activates MMP-2 and modulates the extracellular matrix proteins decorin and collagen[59]. Knockdown of PDK1 inhibits spontaneous migration and epidermal-growth-factor-induced chemotaxis in breast cancer cells[42]. In severe combined immunodeficiency mice, PDK1-depleted human breast cancer cells form tumors more slowly and are defective in extravasation to the lungs after intravenous injection[42]. These results indicate that PDK1 plays an important role in regulating malignancy in breast cancer cells. Moreover, reducing PDK1 expression in PTEN+/- mice protects these animals from developing a wide range of tumors[63], thereby providing genetic evidence that PDK1 is a key effector in mediating neoplasia that result from loss of PTEN. These results also validate PDK1 as an anticancer target[63]. Recently, it has been revealed that PDK1 regulates Rho-associated, coiled-coil-containing protein kinase 1 (ROCK1) positively at the plasma membrane, by opposing the inhibitory effect of RhoE, thereby promoting ameboid cell motility. This mode of ROCK1 regulation is not required for PDK1 kinase activity, but is instead involved in direct binding of PDK1 to ROCK1 at the plasma membrane[64]. Evidence accumulated over the past several years suggests an important role for PDK1 in cancer progression and mobility, in addition to its function in PI3K signaling.
Accumulating reports have suggested that PDK1 can be considered as a promising target for anticancer drugs, because PDK1 plays a key role in cancer cell growth and survival and tumor angiogenesis[11,65]. Various classes of small-molecule PDK1 inhibitors have been proposed[66]. Novel small-molecule inhibitors of PDK1 have also been suggested, including BX-795, BX-912, BX-320 and OSU03012[65,67,68]. BX-320 inhibits the growth of LOX melanoma tumors in the lungs of nude mice after injection of tumor cells into the tail vein[65]. OSU03012 induces mitochondrial-dependent apoptosis of medulloblastoma cells and inhibits the growth of established medulloblastoma xenograft tumors in a dose-dependent manner[68]. The effect of BX-320 and OSU03012 on cancer cell growth in vitro and in vivo indicates that PDK1 inhibitors have clinical utility as anticancer agents. These findings demonstrate the importance of PDK1 and rationalize PDK1 as a therapeutic target in treatment of cancer.
CONCLUSION
PDK1 has been well-characterized as a kinase. In the field of cancer therapy, much research on PDK1 has focused on its involvement in signaling pathways such as PI3K, PKB and mammalian target of rapamycin. However, PDK1 is also a key anticancer target[69]. In our opinion, identification of a novel role for PDK1 in cancer has significant benefits. Therefore, further investigation into PDK1 function will reveal the potential of PDK1 in cancer therapy. Thus far, the regulation of PDK1 activity, its subcellular localization, and its interactions with other proteins have been intense areas of investigation. PDK1 mutation or dysregulation results in the pathogenesis of many human diseases, including cancer and diabetes. A better understanding of its molecular regulatory mechanisms in various signaling pathways will help to explain its diverse and important cellular functions. Furthermore, PDK1 is a promising target for the development of novel cancer chemotherapies.
Footnotes
Supported by National Research Foundation of Korea grant funded by the Korea Government (MEST), No. 2010-0001356; and by a grant from the National R and D Program for Cancer Control funded by Ministry of Health and Welfare, Republic of Korea, No. 0720560
Peer reviewers: Wolfgang Link, PhD, Experimental Therapeutics, Spanish National Cancer Centre, C/ Melchor Fdez Almagro, 3, 28029 Madrid, Spain; Satish Raina, Professor, Research Center Borstel, Leibniz Center for Medicine, Parkallee 22, 23845, Borstel, Germany
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 2.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 4.Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 5.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 6.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayascas JR, Wullschleger S, Sakamoto K, García-Martínez JM, Clacher C, Komander D, van Aalten DM, Boini KM, Lang F, Lipina C, et al. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol Cell Biol. 2008;28:3258–3272. doi: 10.1128/MCB.02032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denley A, Gymnopoulos M, Kang S, Mitchell C, Vogt PK. Requirement of phosphatidylinositol(3,4,5)trisphosphate in phosphatidylinositol 3-kinase-induced oncogenic transformation. Mol Cancer Res. 2009;7:1132–1138. doi: 10.1158/1541-7786.MCR-09-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr Opin Cell Biol. 2009;21:256–261. doi: 10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA. Protein Kinase B (PKB/Akt), a Key Mediator of the PI3K Signaling Pathway. Curr Top Microbiol Immunol. 2010:Epub ahead of print. doi: 10.1007/82_2010_58. [DOI] [PubMed] [Google Scholar]
- 11.Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 2010;16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- 12.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 13.McManus EJ, Collins BJ, Ashby PR, Prescott AR, Murray-Tait V, Armit LJ, Arthur JS, Alessi DR. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. 2004;23:2071–2082. doi: 10.1038/sj.emboj.7600218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmings BA. PtdIns(3,4,5)P3 gets its message across. Science. 1997;277:534. doi: 10.1126/science.277.5325.534. [DOI] [PubMed] [Google Scholar]
- 16.Stockman BJ, Kothe M, Kohls D, Weibley L, Connolly BJ, Sheils AL, Cao Q, Cheng AC, Yang L, Kamath AV, et al. Identification of allosteric PIF-pocket ligands for PDK1 using NMR-based fragment screening and 1H-15N TROSY experiments. Chem Biol Drug Des. 2009;73:179–188. doi: 10.1111/j.1747-0285.2008.00768.x. [DOI] [PubMed] [Google Scholar]
- 17.Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klarlund JK, Guilherme A, Holik JJ, Virbasius JV, Chawla A, Czech MP. Signaling by phosphoinositide-3,4,5-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- 19.Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Ali H, Ragan TJ, Gao X, Harris TK. Reconstitution of modular PDK1 functions on trans-splicing of the regulatory PH and catalytic kinase domains. Bioconjug Chem. 2007;18:1294–1302. doi: 10.1021/bc070055r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Kwon SH, Vogel WK, Filtz TM. PI(3,4,5)P3 potentiates phospholipase C-beta activity. J Recept Signal Transduct Res. 2009;29:52–62. doi: 10.1080/10799890902729449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes CP, Alessi DR. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 23.Park J, Hill MM, Hess D, Brazil DP, Hofsteenge J, Hemmings BA. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J Biol Chem. 2001;276:37459–37471. doi: 10.1074/jbc.M105916200. [DOI] [PubMed] [Google Scholar]
- 24.Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 2000;275:40400–40406. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- 25.Chun J, Kwon T, Lee EJ, Hyun S, Hong SK, Kang SS. The subcellular localization of 3-phosphoinositide-dependent protein kinase is controlled by caveolin-1 binding. Biochem Biophys Res Commun. 2005;326:136–146. doi: 10.1016/j.bbrc.2004.10.208. [DOI] [PubMed] [Google Scholar]
- 26.Biondi RM, Komander D, Thomas CC, Lizcano JM, Deak M, Alessi DR, van Aalten DM. High resolution crystal structure of the human PDK1 catalytic domain defines the regulatory phosphopeptide docking site. EMBO J. 2002;21:4219–4228. doi: 10.1093/emboj/cdf437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komander D, Kular G, Deak M, Alessi DR, van Aalten DM. Role of T-loop phosphorylation in PDK1 activation, stability, and substrate binding. J Biol Chem. 2005;280:18797–18802. doi: 10.1074/jbc.M500977200. [DOI] [PubMed] [Google Scholar]
- 28.Sato S, Fujita N, Tsuruo T. Regulation of kinase activity of 3-phosphoinositide-dependent protein kinase-1 by binding to 14-3-3. J Biol Chem. 2002;277:39360–39367. doi: 10.1074/jbc.M205141200. [DOI] [PubMed] [Google Scholar]
- 29.Wick MJ, Ramos FJ, Chen H, Quon MJ, Dong LQ, Liu F. Mouse 3-phosphoinositide-dependent protein kinase-1 undergoes dimerization and trans-phosphorylation in the activation loop. J Biol Chem. 2003;278:42913–42919. doi: 10.1074/jbc.M304172200. [DOI] [PubMed] [Google Scholar]
- 30.Kikani CK, Dong LQ, Liu F. "New"-clear functions of PDK1: beyond a master kinase in the cytosol? J Cell Biochem. 2005;96:1157–1162. doi: 10.1002/jcb.20651. [DOI] [PubMed] [Google Scholar]
- 31.Scheid MP, Parsons M, Woodgett JR. Phosphoinositide-dependent phosphorylation of PDK1 regulates nuclear translocation. Mol Cell Biol. 2005;25:2347–2363. doi: 10.1128/MCB.25.6.2347-2363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DW, Hwang JH, Suh JM, Kim H, Song JH, Hwang ES, Hwang IY, Park KC, Chung HK, Kim JM, et al. RET/PTC (rearranged in transformation/papillary thyroid carcinomas) tyrosine kinase phosphorylates and activates phosphoinositide-dependent kinase 1 (PDK1): an alternative phosphatidylinositol 3-kinase-independent pathway to activate PDK1. Mol Endocrinol. 2003;17:1382–1394. doi: 10.1210/me.2002-0402. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya S, Dey D, Roy SS. Molecular mechanism of insulin resistance. J Biosci. 2007;32:405–413. doi: 10.1007/s12038-007-0038-8. [DOI] [PubMed] [Google Scholar]
- 34.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiory F, Alberobello AT, Miele C, Oriente F, Esposito I, Corbo V, Ruvo M, Tizzano B, Rasmussen TE, Gammeltoft S, et al. Tyrosine phosphorylation of phosphoinositide-dependent kinase 1 by the insulin receptor is necessary for insulin metabolic signaling. Mol Cell Biol. 2005;25:10803–10814. doi: 10.1128/MCB.25.24.10803-10814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung HS, Kim DW, Jo YS, Chung HK, Song JH, Park JS, Park KC, Park SH, Hwang JH, Jo KW, et al. Regulation of protein kinase B tyrosine phosphorylation by thyroid-specific oncogenic RET/PTC kinases. Mol Endocrinol. 2005;19:2748–2759. doi: 10.1210/me.2005-0122. [DOI] [PubMed] [Google Scholar]
- 38.Miyagi E, Braga-Basaria M, Hardy E, Vasko V, Burman KD, Jhiang S, Saji M, Ringel MD. Chronic expression of RET/PTC 3 enhances basal and insulin-stimulated PI3 kinase/AKT signaling and increases IRS-2 expression in FRTL-5 thyroid cells. Mol Carcinog. 2004;41:98–107. doi: 10.1002/mc.20042. [DOI] [PubMed] [Google Scholar]
- 39.Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003;23:8019–8029. doi: 10.1128/MCB.23.22.8019-8029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koziak K, Kaczmarek E, Park SY, Fu Y, Avraham S, Avraham H. RAFTK/Pyk2 involvement in platelet activation is mediated by phosphoinositide 3-kinase. Br J Haematol. 2001;114:134–140. doi: 10.1046/j.1365-2141.2001.02894.x. [DOI] [PubMed] [Google Scholar]
- 41.Yang KJ, Shin S, Piao L, Shin E, Li Y, Park KA, Byun HS, Won M, Hong J, Kweon GR, et al. Regulation of 3-phosphoinositide-dependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding. J Biol Chem. 2008;283:1480–1491. doi: 10.1074/jbc.M706361200. [DOI] [PubMed] [Google Scholar]
- 42.Sephton CF, Zhang D, Lehmann TM, Pennington PR, Scheid MP, Mousseau DD. The nuclear localization of 3'-phosphoinositide-dependent kinase-1 is dependent on its association with the protein tyrosine phosphatase SHP-1. Cell Signal. 2009;21:1634–1644. doi: 10.1016/j.cellsig.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Kurata A, Katayama R, Watanabe T, Tsuruo T, Fujita N. TUSC4/NPRL2, a novel PDK1-interacting protein, inhibits PDK1 tyrosine phosphorylation and its downstream signaling. Cancer Sci. 2008;99:1827–1834. doi: 10.1111/j.1349-7006.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards J. Src kinase inhibitors: an emerging therapeutic treatment option for prostate cancer. Expert Opin Investig Drugs. 2010;19:605–614. doi: 10.1517/13543781003789388. [DOI] [PubMed] [Google Scholar]
- 45.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 46.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 47.Edwards A, Pallone TL. Ouabain modulation of cellular calcium stores and signaling. Am J Physiol Renal Physiol. 2007;293:F1518–F1532. doi: 10.1152/ajprenal.00251.2007. [DOI] [PubMed] [Google Scholar]
- 48.Summy JM, Qian Y, Jiang BH, Guappone-Koay A, Gatesman A, Shi X, Flynn DC. The SH4-Unique-SH3-SH2 domains dictate specificity in signaling that differentiate c-Yes from c-Src. J Cell Sci. 2003;116:2585–2598. doi: 10.1242/jcs.00466. [DOI] [PubMed] [Google Scholar]
- 49.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 50.Miyata Y, Nishida E. CK2 binds, phosphorylates, and regulates its pivotal substrate Cdc37, an Hsp90-cochaperone. Mol Cell Biochem. 2005;274:171–179. doi: 10.1007/s11010-005-2949-8. [DOI] [PubMed] [Google Scholar]
- 51.Fujita N, Sato S, Ishida A, Tsuruo T. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2002;277:10346–10353. doi: 10.1074/jbc.M106736200. [DOI] [PubMed] [Google Scholar]
- 52.MacLean M, Picard D. Cdc37 goes beyond Hsp90 and kinases. Cell Stress Chaperones. 2003;8:114–119. doi: 10.1379/1466-1268(2003)008<0114:cgbhak>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 54.Andjelković M, Maira SM, Cron P, Parker PJ, Hemmings BA. Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase. Mol Cell Biol. 1999;19:5061–5072. doi: 10.1128/mcb.19.7.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, Cohen P, Alessi DR, Lucocq J. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(Pt 3):575–583. [PMC free article] [PubMed] [Google Scholar]
- 56.Lim MA, Kikani CK, Wick MJ, Dong LQ. Nuclear translocation of 3'-phosphoinositide-dependent protein kinase 1 (PDK-1): a potential regulatory mechanism for PDK-1 function. Proc Natl Acad Sci USA. 2003;100:14006–14011. doi: 10.1073/pnas.2335486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bayascas JR. Dissecting the role of the 3-phosphoinositide-dependent protein kinase-1 (PDK1) signalling pathways. Cell Cycle. 2008;7:2978–2982. doi: 10.4161/cc.7.19.6810. [DOI] [PubMed] [Google Scholar]
- 58.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 59.Xie Z, Yuan H, Yin Y, Zeng X, Bai R, Glazer RI. 3-phosphoinositide-dependent protein kinase-1 (PDK1) promotes invasion and activation of matrix metalloproteinases. BMC Cancer. 2006;6:77. doi: 10.1186/1471-2407-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Z, Cox-Hipkin MA, Windsor WT, Boyapati A. 3-phosphoinositide-dependent protein kinase-1 regulates proliferation and survival of cancer cells with an activated mitogen-activated protein kinase pathway. Mol Cancer Res. 2010;8:421–432. doi: 10.1158/1541-7786.MCR-09-0179. [DOI] [PubMed] [Google Scholar]
- 61.Finlay DK, Sinclair LV, Feijoo C, Waugh CM, Hagenbeek TJ, Spits H, Cantrell DA. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J Exp Med. 2009;206:2441–2454. doi: 10.1084/jem.20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maurer M, Su T, Saal LH, Koujak S, Hopkins BD, Barkley CR, Wu J, Nandula S, Dutta B, Xie Y, et al. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res. 2009;69:6299–6306. doi: 10.1158/0008-5472.CAN-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/-) mice. Curr Biol. 2005;15:1839–1846. doi: 10.1016/j.cub.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 64.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127–137. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 65.Feldman RI, Wu JM, Polokoff MA, Kochanny MJ, Dinter H, Zhu D, Biroc SL, Alicke B, Bryant J, Yuan S, et al. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2005;280:19867–19874. doi: 10.1074/jbc.M501367200. [DOI] [PubMed] [Google Scholar]
- 66.Peifer C, Alessi DR. Small-molecule inhibitors of PDK1. ChemMedChem. 2008;3:1810–1838. doi: 10.1002/cmdc.200800195. [DOI] [PubMed] [Google Scholar]
- 67.Sargeant AM, Klein RD, Rengel RC, Clinton SK, Kulp SK, Kashida Y, Yamaguchi M, Wang X, Chen CS. Chemopreventive and bioenergetic signaling effects of PDK1/Akt pathway inhibition in a transgenic mouse model of prostate cancer. Toxicol Pathol. 2007;35:549–561. doi: 10.1080/01926230701338966. [DOI] [PubMed] [Google Scholar]
- 68.Baryawno N, Sveinbjörnsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 2010;70:266–276. doi: 10.1158/0008-5472.CAN-09-0578. [DOI] [PubMed] [Google Scholar]
- 69.Peifer C, Alessi DR. New anti-cancer role for PDK1 inhibitors: preventing resistance to tamoxifen. Biochem J. 2009;417:e5–e7. doi: 10.1042/BJ20082243. [DOI] [PubMed] [Google Scholar]