Abstract
The International Harmonization Project defined complete response (CR) after treatment for Hodgkin disease (HD) by absence of fluorodeoxyglucose avidity, regardless of the size of residual masses. Residual avidity after initial treatment is known to predict inferior survival. In the setting of retrieval therapy, early PET scans may improve assessment of treatment efficacy. Retrospective analysis after two cycles of gemcitabine and vinorelbine for refractory HD revealed six CR among 13 patients by PET and one CR in 13 by CT. No relationship between PET response and event-free or overall survival could be discerned, presumably because of the heterogeneity of subsequent therapies.
Keywords: gemcitabine, vinorelbine, Hodgkin disease, Phase II trial, positron emission tomography, treatment response
Introduction
Children’s Oncology Group protocol AHOD0321 was a phase 2 trial to test the safety and efficacy of weekly gemcitabine and vinorelbine (GV) for patients with relapsed or refractory HD. Measurable responses (defined by reduction in size of target lesions on computed tomography and determined by each patient’s institution) were seen in 19 of 25 evaluable patients (76%; 95% exact binomial CI: 55%, 91%), including 6 complete responses (CR), 11 very good partial responses (VGPR), and 2 partial responses (PR).1
After the study opened, the International Harmonization Project (IHP) presented revised definitions of response to treatment for patients with lymphoma,2,3 placing greater importance on [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET). A CR is defined for patients whose pretreatment disease is FDG-avid as PET negativity after treatment, regardless of the size of residual masses seen on conventional imaging modalities such as computed tomography. This definition is supported by studies demonstrating that residual FDG avidity after early cycles of multiagent chemotherapy is strongly predictive of relapse risk.4,5 However, the predictive value of this CR definition has not yet been proven among patients treated for relapsed/refractory disease. We therefore conducted a retrospective analysis of patients treated on AHOD0321, using the revised response criteria.
Methods
Children’s Oncology Group protocol AHOD0321, “A Phase 2 Study of Weekly Gemcitabine and Vinorelbine for Children with Recurrent or Refractory Hodgkin Disease” enrolled pediatric patients with relapsed or primary refractory HD between January 2005 and April 2007. Treatment details, responses and toxicity have been previously reported.1 Prior to treatment, and after every two 21-day treatment cycles, all patients on AHOD0321 were required to have CT and either Gallium or FDG-PET scans. Selection between Gallium and FDG-PET was determined by institutional availability. Because all patients were required to have dedicated CT scans of involved regions in addition to Gallium or FDG-PET, those patients on AHOD0321 who had combined PET/CT scans also had full-dose CT scans. Copies of all imaging studies and corresponding reports were submitted to the Quality Assurance Review Center (QARC), a repository for storage of clinical and imaging data on multi-center trials. Central review of images at QARC was done to validate data reporting for quality assurance, but did not directly influence protocol therapy for patients enrolled on AHOD0321.
In this retrospective central review, treatment response among those patients evaluated by FDG-PET was determined following the IHP recommendations.2,3 Specifically, the designation of CR requires (1) complete disappearance of all detectable clinical evidence of disease and disease-related symptoms, (2) negative bone marrow, (3) absence of splenomegaly or splenic nodules, and (4) PET negativity. A post-treatment residual mass of any size is permitted. PET positivity was interpreted visually, following the Consensus recommendations of the Imaging Subcommittee of the International Harmonization Project in Lymphoma.6 In brief, a positive scan is defined as focal or diffuse FDG uptake above background in a location incompatible with normal anatomy or physiology, without any specific standardized uptake value cutoff.
Statistical methods
Event-free survival (EFS) and overall survival (OS) were determined using the methods of Kaplan and Meier.7 EFS and OS for patients who were PET negative after two cycles of GV were compared with those who were still PET positive using log-rank analysis. The number of total cycles received by patients with negative vs positive PET after two cycles were compared using Wilcoxon rank sum test.
Results
PET scans at baseline and after two cycles of GV were available to review for 13 of the 30 eligible patients enrolled on AHOD0321. Seventeen of the 26 scans reviewed (65%) were combined PET/CT and 9 (35%) were PET only. As noted in the methods, all patients who had combined PET/CT were also required to have dedicated full-dose CT scans of involved regions. All 13 were FDG avid at baseline. Six of 13 were PET negative after 2 cycles of GV, meeting the IHP definition for CR (46%; 95% exact binomial CI: 19–75%; Figures 1 and 2). Five of these six patients with negative PET had measurable disease on CT at this time point, determined in the central review of images, including one who met CT size criteria for VGPR, 2 with PRs and two who had stable disease (Table 1).
Figure 1. Responses among patients on AHOD0321 evaluated by FDG-PET.
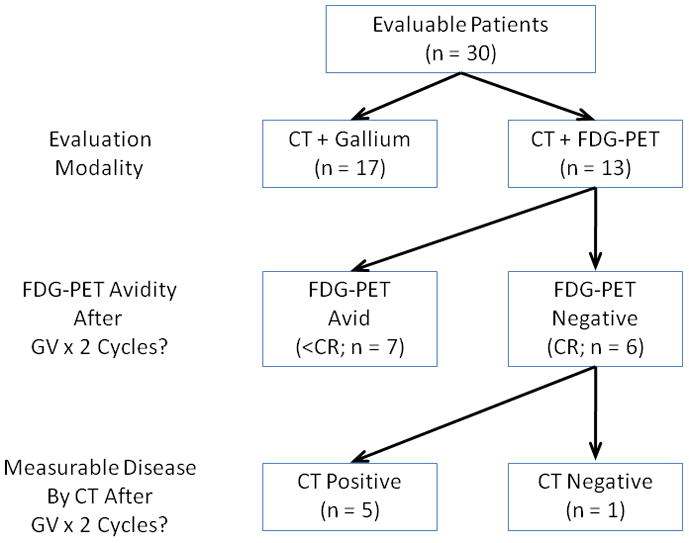
Figure 2. Complete PET response after two cycles of gemcitabine and vinorelbine.
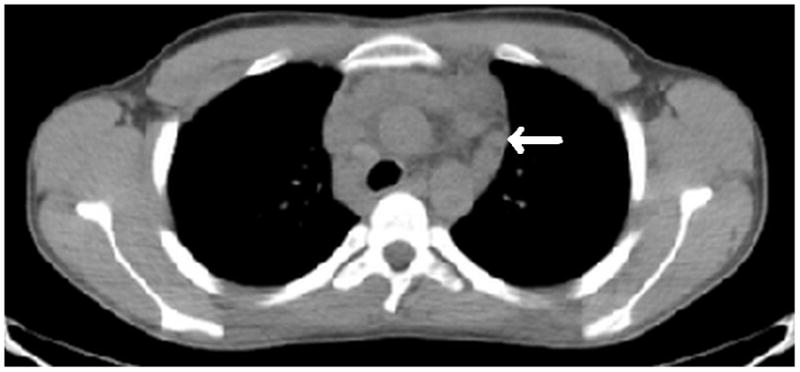
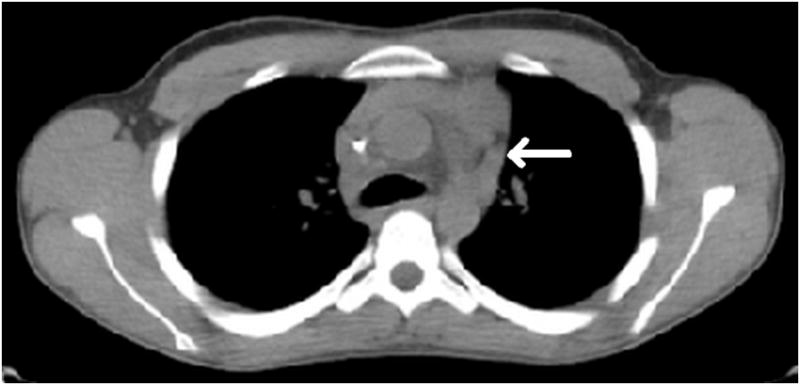
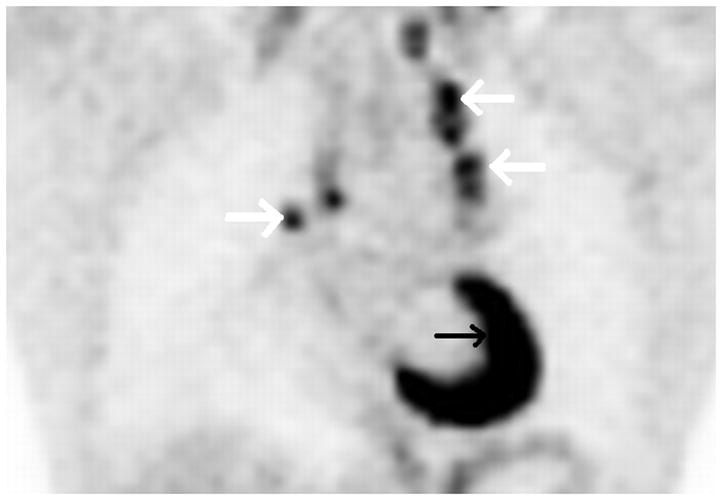
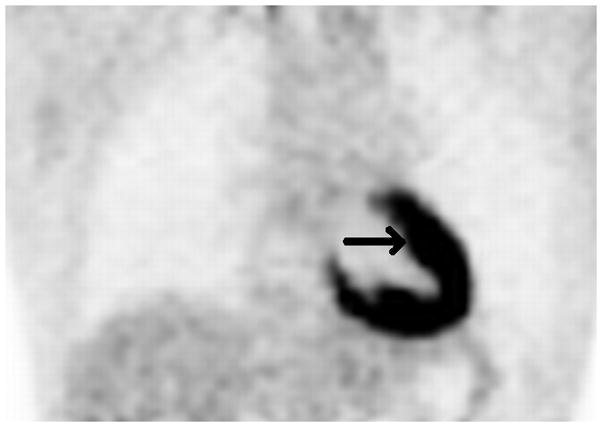
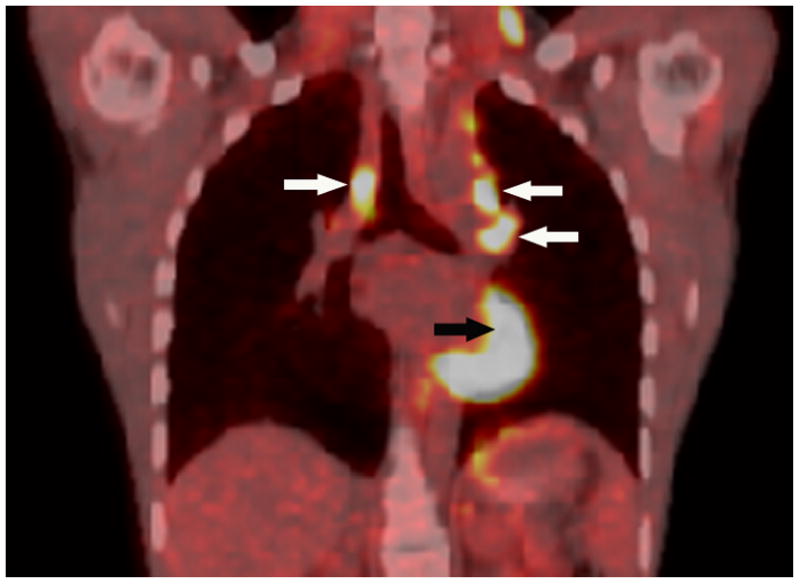
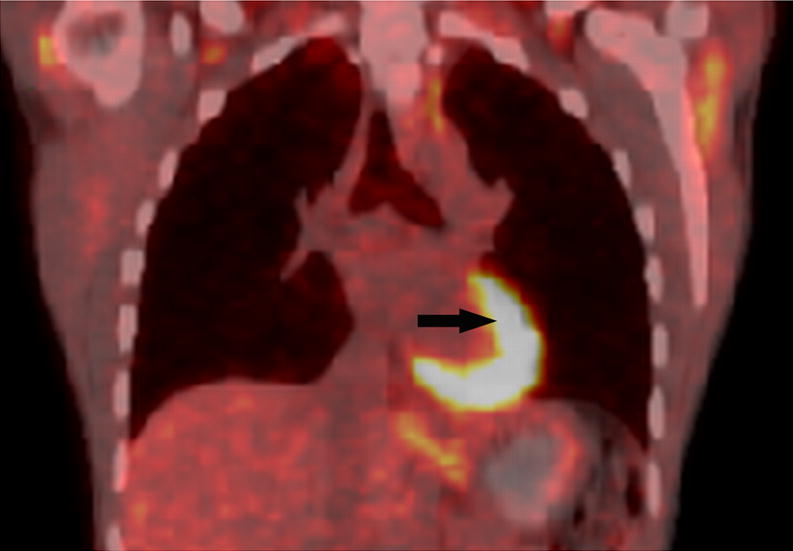
Axial low dose non contrast CT images of the mediastinum obtained as part of the PET scan for attenuation correction show very little change in size of the left superior mediastinal adenopathy (white arrows) from the pre-treatment (Fig. 2A) to post-treatment (Fig. 2B) scans. PET activity is seen to resolve in bilateral superior mediastial adenopathy (white arrows) between pre (Fig, 2C) and post (Fig. 2D) treatment coronal Maximum Intensity Projection (MIP) images. This change is confirmed on the corresponding pre- and post-treatment fused coronal PET/CT images (Figs. 2E and 2F, respectively). Normal cardiac PET activity is denoted by black arrows on both MIP and fused sequences. These representative images are from patient #1 in Table 1.
Table 1. Pretreatment Characteristics and Response after Two Cycles of Gemcitabine and Vinorelbine, as determined on central review.
Abbreviations: ASCT, autologous stem cell transplantation; GV, gemcitabine and vinorelbine; CT, computed tomography; PET, positron emission tomography; SD, stable disease; PR, partial response; VGPR, very good partial response; CR, complete response.
| Pretreatment Characteristics | Response after GV × 2 cycles | Subsequent Therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| Relapsed or Primary Refractory | Prior Gemcitabine | Prior Vinorelbine | Prior ASCT | CT-based response | CR by PET | total GV cycles | Subsequent SCT | |
| 1 | Relapsed | No | No | Yes | SD | Yes | 3 | Yes |
| 2 | Refractory | No | Yes | No | SD | Yes | 2 | Yes |
| 3 | Relapsed | No | No | Yes | PR | Yes | 3 | Yes |
| 4 | Relapsed | No | No | Yes | PR | Yes | 10 | No |
| 5 | Relapsed | No | Yes | Yes | VGPR | Yes | 15 | No |
| 6 | Relapsed | No | Yes | Yes | CR | Yes | 6 | Yes |
| 7 | Refractory | No | Yes | Yes | SD | No | 2 | Yes |
| 8 | Refractory | No | Yes | Yes | SD | No | 3 | No |
| 9 | Relapsed | No | Yes | No | SD | No | 4 | Yes |
| 10 | Refractory | No | Yes | No | PR | No | 2 | Yes |
| 11 | Relapsed | Yes | No | Yes | VGPR | No | 4 | No |
| 12 | Relapsed | No | No | Yes | VGPR | No | 3 | No |
| 13 | Relapsed | No | No | Yes | VGPR | No | 4 | No |
Of the 7 patients with VGPR or PR, as determined by CT scanning, only 3 achieved a PET defined CR (Table 1). Conversely, two of the 5 patients with stable disease on CT achieved CR by PET. These data suggest considerable variability in response assessment between PET and CT based criteria.
Although 6 of 13 had CRs by PET criteria, only one of these 13 (7.7%) met criteria for CR on central review, using the CT-based response evaluation defined in the protocol, which required greater than 80% reduction in the size of all masses. This proportion is similar to the rate of CRs after 2 cycles using CT-based criteria, as determined by each patient’s institution, among the full cohort of eligible patients who received 2 cycles of GV on AHOD0321 (3 CRs among 28 eligible patients; 11%; 95% exact binomial CI 2–28%).1
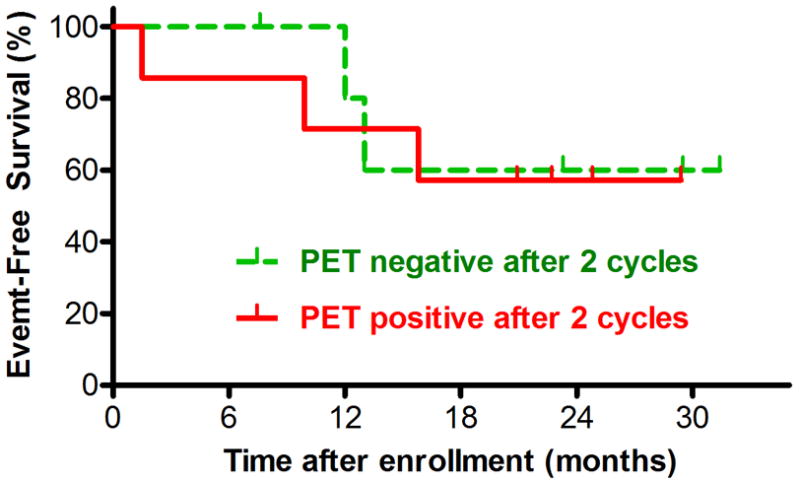
With median follow up of 20.9 months, there was no significant difference in EFS (Figure 3) or overall survival (data not shown) between the six patients with negative PET scans after two cycles of GV and the seven who were still positive. Differences in subsequent therapy (eg. use of SCT) may confound this analysis.
Figure 3. Event-Free Survival after Entry on COG-AHOD0321, by FDG-PET positivity after Two Cycles of Gemcitabine and Vinorelbine.
Discussion
Patients with primary refractory HD or early recurrence typically have poor long-term disease free survival. High-dose therapy with autologous stem cell transplantation (ASCT) is now an accepted approach for this population that may improve long-term outcomes,8–10 especially if a CR can be achieved with reinduction therapy prior to ASCT.11 However, it is not yet clear whether CR, in this particular context, should be defined by disappearance of all measurable disease on CT or disappearance of FDG avidity.
By either criteria, weekly GV appears to be a useful reinduction regimen. As we previously published, 1 76% of patients experienced measurable responses, including 6 CRs among 25 evaluable patients (24%; 95% exact binomial CI 9–45%), as determined by each patient’s institution using the conventional, CT-based assessment. It is possible that the CR rate would have been higher if those patients with PRs and VGPRs who were taken off study treatment to pursue ASCT had continued on GV therapy.
PET may be superior to conventional imaging for evaluation of treatment response if it can distinguish between residual masses that contain viable tumor and those that are fibrotic.3 Early CR as defined by PET may prove to be a better measure of efficacy of a given treatment regimen and the likelihood of cure with SCT. Here we show that using the PET-based response definitions proposed by the IHP, six of 13 patients (46%) with heavily pretreated refractory HD experienced CRs after only two cycles of GV.
Five of 12 patients (42%) who had residual masses after two cycles of GV detected by conventional imaging modalities on central review, were PET negative. This observation is consistent with published reports describing the frequent discrepancy between conventional imaging and PET among patients undergoing initial treatment of HD. Furth, et al., for example, noted recently that among 40 children treated for HD the proportion of PET-negative patients was significantly higher than those with complete disappearance of all masses on conventional imaging at each response assessment.12
In agreement with earlier reports,4,5 Furth, et al. further noted that PET evaluation after early cycles of initial treatment for HD had a high degree of predictive value for subsequent treatment outcome. In contrast, we found no relationship between EFS or OS and PET response after two cycles of retrieval therapy on this phase 2 trial. Our ability to detect an outcome difference was clearly limited by the small number of patients on this trial evaluated by PET scans at all time points. Furthermore, the number of cycles of GV given varied significantly (Table 1), as investigators were allowed to pull patients off treatment for ASCT after any measurable response. In this cohort, patients who were PET positive after two cycles tended to receive less total GV (median: 3 cycles; range: 2–4) than those who were PET negative (median: 4.5 cycles; range: 2–15), but this difference is not statistically significant. Clarification of the predictive value of PET response in the setting of retrieval therapy will require prospective analysis in a larger trial.
Four of seven patients who remained PET positive after two cycles of GV went off study before receiving the minimum of four cycles recommended in the protocol and none continued beyond four cycles. Although the proportion who would eventually achieve CRs by PET based definition would likely increase if those who had not yet achieved PET-negativity continued on treatment, this early response may be more predictive of long term outcome and of treatment efficacy than that defined by CT. Larger studies with less heterogeneity in subsequent treatment may address this issue.
Conclusions
Nearly half of 13 patients with heavily pretreated refractory HD became PET negative after only two cycles of GV, including five who still had residual masses. Testing whether early PET response after therapy for refractory HD predicts long-term EFS should be included as an objective of subsequent cooperative group phase 1 and 2 trials for patients with lymphomas.
Acknowledgments
Research is supported by the Chair’s Grant U10 CA98543 and the Statistics and Data Center Grant U10 CA98413 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncoogygroup.org/admin/grantinfo.htm
References
- 1.Cole PD, Schwartz CL, Drachtman RA, et al. Phase 2 Study of Weekly Gemcitabine and Vinorelbine for Children with Recurrent or Refractory Hodgkin Disease, A Children’s Oncology Group Report. Journal of Clinical Oncology. 2009;27:1456–61. doi: 10.1200/JCO.2008.20.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheson BD. New Staging and Response Criteria for Non-Hodgkin Lymphoma and Hodgkin Lymphoma. Radiologic Clinics of North America. 2008;46:213–223. doi: 10.1016/j.rcl.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Pfistner B, Juweid ME, et al. Revised Response Criteria for Malignant Lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 4.Gallamini A, Rigacci L, Merli F, et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica. 2006;91:475–81. [PubMed] [Google Scholar]
- 5.Zinzani PL, Tani M, Fanti S, et al. Early positron emission tomography (PET) restaging: a predictive final response in Hodgkin’s disease patients. Annals of Oncology. 2006;17:1296–300. doi: 10.1093/annonc/mdl122. [DOI] [PubMed] [Google Scholar]
- 6.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. Journal of Clinical Oncology. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–81. [Google Scholar]
- 8.Lieskovsky YE, Donaldson SS, Torres MA, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin’s disease: results and prognostic indices. Journal of Clinical Oncology. 2004;22:4532–40. doi: 10.1200/JCO.2004.02.121. [DOI] [PubMed] [Google Scholar]
- 9.Lavoie JC, Connors JM, Phillips GL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005;106:1473–8. doi: 10.1182/blood-2004-12-4689. [DOI] [PubMed] [Google Scholar]
- 10.Josting A. Autologous transplantation in relapsed and refractory Hodgkin’s disease. European Journal of Haematology Supplementum. 2005:141–5. doi: 10.1111/j.1600-0609.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 11.Hagemeister FB, Tannir N, McLaughlin P, et al. MIME chemotherapy (methyl-GAG, ifosfamide, methotrexate, etoposide) as treatment for recurrent Hodgkin’s disease. Journal of Clinical Oncology. 1987;5:556–61. doi: 10.1200/JCO.1987.5.4.556. [DOI] [PubMed] [Google Scholar]
- 12.Furth C, Steffen IG, Amthauer H, et al. Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin’s lymphoma: analysis of a prospective multicenter trial. Journal of Clinical Oncology. 2009;27:4385–91. doi: 10.1200/JCO.2008.19.7814. [DOI] [PubMed] [Google Scholar]


