Abstract
Purpose
The suitability of fexofenadine as a probe substrate to assess hepatobiliary transport function in humans was evaluated by pharmacokinetic modeling/simulation and in vitro/in situ studies using chemical modulators.
Methods
Simulations based on a pharmacokinetic model developed to describe fexofenadine disposition in humans were conducted to examine the impact of altered hepatobiliary transport on fexofenadine disposition. The effect of GF120918 on fexofenadine disposition was evaluated in human sandwich-cultured hepatocytes (SCH). Additionally, the effect of GF120918, bosentan, and taurocholate on fexofenadine disposition in perfused livers from TR− Wistar rats was examined.
Results
Based on modeling/simulation, fexofenadine systemic exposure was most sensitive to changes in the hepatic uptake rate constant, and did not reflect changes in hepatic exposure due to altered hepatic efflux. GF120918 did not impair fexofenadine biliary excretion in SCH. GF120918 coadministration significantly decreased Cl’biliary to 27.5% of control in perfused livers.
Conclusions
Simulations were in agreement with perfused liver data which predicted changes in fexofenadine systemic exposure primarily due to altered hepatic uptake. Fexofenadine is not a suitable probe to assess hepatic efflux function based on systemic concentrations. GF120918-sensitive protein(s) mediate fexofenadine biliary excretion in rat liver, whereas in human hepatocytes multiple efflux proteins are involved, in fexofenadine hepatobiliary disposition.
Keywords: Fexofenadine, Hepatic Transport, Rat Perfused Liver, Human Pharmacokinetics, Sandwich-Cultured Hepatocytes
Introduction
Fexofenadine is an orally administered nonsedating H1-receptor antagonist commonly used in the treatment of allergic rhinitis and chronic urticaria. The primary elimination pathway of fexofenadine in humans is biliary excretion of the unchanged species (1). Tahara et al. demonstrated that biliary clearance accounts for ~56–72% and 40% of total body clearance in mice and rats, respectively (2). Fexofenadine has a low passive membrane permeability with reported Papp values in the absorptive direction of 0.17 × 106 – 0.27 × 106 cm/s determined in the Caco-2 model (3). Furthermore, the effective jejunal permeability in healthy human volunteers also was very low: 0.06 × 10−4 ± 0.07 × 10−4 cm/s and 0.04 × 10−4 ± 0.07 × 10−4 cm/s when fexofenadine was administered alone and with verapamil using jejunal single-pass perfusion (4, 5). Therefore, fexofenadine relies on active hepatic transport as one of the determinants for systemic clearance.
Identifying the degree and importance of drug-drug interactions in the human liver is very challenging because the bile and liver compartments are not readily accessible due to the anatomy of the human hepatobiliary tract. The absolute oral bioavailability of fexofenadine was reported to be 33% (product information, Hoechst Marion, Roussel, Laval, Quebec, Canada), with 12% of the total administered dose recovered in urine (1). Therefore, two-thirds of the bioavailable dose of fexofenadine is presumed to be excreted in bile, underscoring the importance of this excretory route to overall systemic clearance. Fexofenadine has been used extensively as an in vivo probe substrate to assess P-glycoprotein/multidrug resistance protein 1 (P-gp/MDR1) activity; altered systemic concentrations and overall exposure have been reported due to perturbations in P-gp efflux in the intestine (6–9). The contribution of P-gp to fexofenadine hepatobiliary transport, or the degree of interaction with hepatic P-gp, are poorly understood in humans.
The organic anion transporting polypeptides (OATPs), specifically OATP1B1, OATP1B3 and OATP1A2 in humans, and Oatp1a1, Oatp1a4, and Oatp1b2 in rats, have been implicated in the cellular uptake of fexofenadine (8, 10–12). In vitro studies demonstrated that P-gp mediated the cellular efflux of fexofenadine (10). Thus, fexofenadine has been recommended as an in vivo probe substrate for P-gp interactions in the intestine (13). In fact many drug-drug and food-drug interaction studies in humans utilizing fexofenadine as a probe substrate for P-gp activity have been performed with various co-administered drugs, herbal supplements and food components (6–9, 14–17). Although P-gp plays a role in limiting intestinal absorption and blood-brain barrier penetration of fexofenadine (2, 10), multiple transport mechanisms play a role in the biliary excretion of fexofenadine (18, 19).
In mice fexofenadine is excreted into bile predominantly by multidrug resistance-associated protein 2 (Mrp2), and to a minor extent by P-gp and other yet unidentified mechanisms (18). The mechanisms of biliary excretion of fexofenadine in rats and humans have not been elucidated. However, the biliary excretion rate and intrinsic biliary clearance of fexofenadine in naturally occurring Mrp2-deficient TR− and Eisai hyperbilirubinemic (EHBR) rats were unchanged compared to wild-type rats (2, 18). In the present studies, the role of other canalicular transport proteins including the bile salt export pump (Bsep) and P-gp in fexofenadine biliary clearance was investigated in rat perfused livers.
Bsep is primarily involved in the biliary excretion of bile acids (20), but also is capable of transporting some xenobiotics including vinblastine, pravastatin and fexofenadine in transfected expression systems (19, 21, 22). P-gp serves an important barrier function and is responsible for biliary excretion of bulky hydrophobic and cationic substrates, including many chemotherapeutics agents, cardiac glycosides, cyclosporin A, HIV protease inhibitors, and fexofenadine, as mentioned above (23). Bcrp transports sulfated steroids and many anticancer agents in addition to some organic anions and cations (24–26). Fexofenadine does not appear to be a BCRP/Bcrp substrate based on the results of studies utilizing Bcrp knockout mice and MDCKII cells transfected with human BCRP (2, 19).
The objective of the present study was to examine the suitability of fexofenadine as a probe substrate for altered hepatobiliary transport in humans. A pharmacokinetic model was developed to describe the disposition of fexofenadine in healthy human subjects, and simulations were conducted to examine the effect of inhibition or induction of hepatic transport processes on fexofenadine disposition. The findings from compartmental modeling and simulation were tested using chemical modulators of hepatic transport proteins. The effect of GF120918, a human P-gp and BCRP inhibitor, was assessed in human sandwich-cultured hepatocytes (SCH). In addition, livers from Mrp2-deficient TR− rats were perfused in situ in a single-pass manner in the presence and absence of the Bsep inhibitor bosentan (27), and GF120918 to examine the impact of modulation of uptake and efflux proteins on fexofendadine hepatobiliary disposition. Furthermore, the taurocholate infusion in perfused livers was withheld to perturb trafficking of ATP-binding cassette (ABC) transport proteins to the canalicular membrane (28, 29) in order to assess the contribution of impaired biliary excretion to fexofenadine disposition.
Material and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), insulin, MEM non-essential amino acids solution (100x), L-glutamine, insulin and penicillin G-streptomycin solution were purchased from Invitrogen (Carlsbad, CA). Collagenase (type 4), fetal bovine serum (FBS), fexofenadine, cetirizine, sodium taurocholate, digoxin, Triton X-100, dexamethasone, methanol, Krebs-Henseleit buffer packs (K-3753) and Hanks’ balanced salt solution (HBSS) modified with (H-1387) or without (H-4891) calcium chloride were obtained from Sigma-Aldrich (St. Louis, MO). BioCoat™ collagen I plates, Matrigel™ basement membrane matrix, and ITS+™ (insulin/transferrin/selenium) culture supplement were purchased from BD Biosciences Discovery Labware (Bedford, MA). [3H]-taurocholate (5 Ci/mmol, >97% purity) and [3H]-digoxin (40 Ci/mmol, >97% purity) were obtained from PerkinElmer Life and Analytical Sciences (Boston, MA). Bio-Safe II™ liquid scintillation cocktail was obtained from Research Products International (Mt. Prospect, IL). Centrifree® micropartition devices were obtained from Millipore (Billerica, MA). Bicinchoninic acid (BCA) protein assay reagents and BSA for the protein assay standard were purchased from Pierce Chemical Co. (Rockford, IL). N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide (GF120918) was a gift from GlaxoSmithKline (Research Triangle Park, NC). All other chemicals and reagents were of analytical grade and available from commercial sources.
Pharmacokinetic Modeling
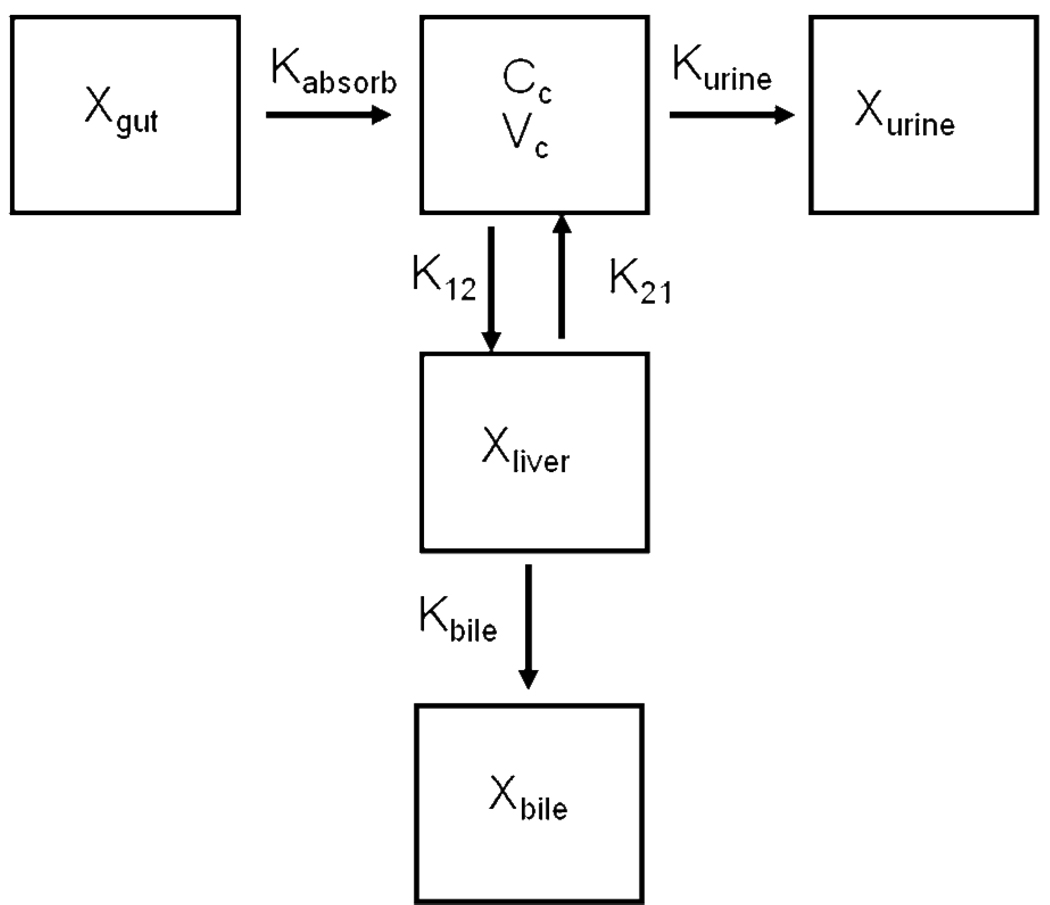
Mean fexofenadine plasma concentrations and urinary excretion rate data were estimated from previously published data in healthy human subjects after a single oral dose of 120 mg (9). Cumulative mass in bile after 48 hours was estimated based on the assumption that bioavailability of oral fexofenadine was 33% (product information, Hoechst Marion, Roussel, Laval, Quebec, Canada), of which approximately one-third of the 120 mg dose was excreted into urine (30), and two-thirds was excreted into bile. To achieve model parsimony, bioavailability of fexofendine was fixed at the aforementioned literature value (33%). The adoption of fixed bioavailability accounted for the first pass extraction of fexofenadine and the remaining fixed fraction of dose was absorbed directly into the systemic compartment. This is supported by the fact that fexofenadine has a low hepatic uptake clearance. A number of different compartmental models were developed (including a peripheral compartment in addition to the liver compartment, with elimination from the peripheral, central and/or liver compartments) using the concentration- and mass-time profiles. Differential equations describing the disposition of fexofenadine in the compartments were fit simultaneously to plasma, urine and bile profiles using nonlinear least-squares regression (WinNonlin v. 4.1 Pharsight Corporation, Mountain View, CA). Different initial parameter values were used in the fitting process along with broad lower and upper bounds to have confidence in the final parameter estimates and avoid convergence at a local minimum. The goodness of fit was based on Akaike’s Information Criterion, the residual sum of squares, coefficients of variation on the parameter estimates, the condition number, and visual inspection of the residual plots and of model fitting to the concentration- and mass-time profiles. Due to the disparity in the number of data points for the mass in bile and the urinary excretion rate data compared with the plasma concentration data, a weighting scheme was used to account for the relative contribution of each data set to the overall number of data points. Each data point was assigned a weight of, where n is the fraction of the total number of data points that support a particular sample matrix. The optimal model that best described the data is shown in Figure I. This model included a compartment depicting the systemic circulation described by the plasma concentration-time profile after absorption, represented by a first-order rate constant Kabsorb, from the gut compartment. A first-order rate constant, K12, governed fexofenadine uptake from the central compartment into a peripheral compartment, representing the liver. K21 represented a first-order rate constant for hepatic basolateral efflux; Kbile and Kurine represented biliary and urinary excretion rate constants, respectively. The following differential equations described fexofenadine disposition in healthy human subjects:
Figure I.
Model scheme depicting fexofenadine disposition in healthy humans. Xgut represents the amount of fexofenadine in the gut after an oral dose; Kabsorb represents the first–order absorption rate constant; CC represents the concentration of fexofenadine in the central compartment (systemic circulation) with volume VC; K12 represents the first–order rate constant for hepatic uptake; K21 represents the first-order rate constant for hepatic basolateral efflux; Xliver represents the amount of fexofenadine in the liver; Kbile represents the first–order rate constant for biliary excretion; Xbile represents the amount of fexofenadine in bile; Kurine represents the first–order rate constant for urinary excretion; Xurine represents the amount of fexofenadine in urine.
Pharmacokinetic Simulations
The contribution of each parameter in this compartment model to the overall disposition of fexofenadine was assessed through a series of simulation experiments. Each parameter was varied sequentially from the baseline value by either increasing (i.e. induction) or decreasing (i.e. inhibition) the parameter estimate by 50%, 100% or 300%. The concentration-, mass- and excretion rate-time profiles were simulated using WinNonlin v.4.1, and the area under the curve (AUC) for the plasma concentration-time and hepatic mass-time profiles were calculated utilizing a standard noncompartmental approach.
Isolation and In Vitro Culture of Primary Human Hepatocytes
Human liver tissue was obtained by qualified medical staff from the University of North Carolina, School of Medicine, as waste from surgical resection. Written informed consent was obtained from patients before undergoing hepatic surgery. IRB approval was obtained for the collection of human liver tissue. Hepatocytes were isolated by a modification of the two-step collagenase perfusion, as described previously (31). Cell viability, determined by trypan blue exclusion, was >88%. Hepatocytes were seeded (~1.5 × 106 cells/well) in 6-well BioCoat™ plates in DMEM without phenol red supplemented with 2 mM L-glutamine, 1% (v/v) MEM non-essential amino acids, 100 units penicillin G sodium, 100 µg streptomycin sulfate, 1 µM dexamethasone, 5% (v/v) FBS, and 10 µM insulin (day 0 of culture), and allowed to attach for 2–6 h in a humidified incubator (95% O2, 5% CO2) at 37°C. After cell attachment, culture plates were swirled gently and the culture medium was replaced with the same medium. Cells were overlaid 16–24 h (day 1 of culture) after seeding with ice-cold Matrigel™ basement membrane matrix (0.25 mg/mL) in 2 mL/well cold serum-free DMEM containing 2 mM L-glutamine, 1% (v/v) MEM non-essential amino acids, 100 units penicillin G sodium, 100 µg streptomycin sulfate, 0.1 µM dexamethasone, and 1% (v/v) ITS+™. The culture medium was changed every 24 h until experiments were performed on day 9–10 of culture.
Accumulation Experiments
The method to determine substrate accumulation in SCH has been described previously (32). Briefly, hepatocytes were rinsed twice with 2 mL warm HBSS containing Ca2+ (standard buffer) or Ca2+-free HBSS and incubated with 2 mL of the same buffer for 10 min at 37°C (to maintain tight junction integrity and bile canalicular networks, or disrupt tight junctions and open bile canalicular networks, respectively). The buffer was removed, and the cells were incubated for 10 min at 37°C with 1.5 mL of [3H]-taurocholate (1 µM), [3H]-digoxin (1 µM) in the presence and absence of 2 µM GF120918, or for 30 min with fexofenadine (5 µM) in the presence and absence of 2 µM GF120918 in standard buffer. Hepatocytes were rinsed vigorously three times with 2 mL ice-cold standard buffer following the incubation. Taurocholate- and digoxin-treated hepatocytes were lysed with 1 mL 0.5% (v/v) Triton X-100 in phosphate-buffered saline by placing plates on an orbital shaker for a minimum of 20 min at room temperature. Substrate uptake was corrected for nonspecific binding by subtracting uptake on blank six-well Biocoat™ plates overlaid with Matrigel™. Data were normalized to protein concentration in each well, determined in duplicate aliquots using BCA protein assay reagent kit (Pierce) as instructed by the manufacturer. BSA, as supplied by the manufacturer, was used as a standard (0.2 – 1 mg/mL). Due to incompatability of the protein assay with methanol, the average protein concentration for standard HBSS or Ca2+-free HBSS incubations in the same liver preparation was used to normalize fexofenadine content. The [3H]-taurocholate and [3H]-digoxin samples were analyzed by liquid scintillation spectroscopy in a Packard Tri-Carb scintillation counter (PerkinElmer Life and Analytical Sciences). Fexofenadine-treated hepatocytes were lysed with 1 mL 70% (v/v) ice-cold methanol/water and stored at −80°C until analysis. The cells were scraped off the plates and centrifuged at 10,000 g for 5 min before analysis by LC/MS/MS.
Animals
Male Mrp2-deficient TR− rats bred at the University of North Carolina (212–341 g; breeding stock obtained from Dr. Mary Vore, University of Kentucky, Lexington, KY) were used for perfused liver studies. Rats had free access to water and food prior to surgery. All animal procedures complied with the guidelines of the Institutional Animal Care and Use Committee (University of North Carolina, Chapel Hill, NC).
In Situ Liver Perfusion
All experimental procedures were performed under full anesthesia induced with ketamine/xylazine (140/8 mg/kg i.p.). The liver perfusion procedure was modified slightly from the previous report of Tian et al (2008). Briefly, the common bile duct was cannulated with polyethylene PE-10 tubing (Becton Dickinson, Parsippany, NJ). The portal vein was cannulated with a 16-gauge catheter (B. Braun Medical Inc., Bethlehem, PA), the abdominal vena cava below the liver was severed immediately by incision, and the inferior vena cava above the liver was cannulated with a 14-gauge catheter. Subsequently, the inferior vena cava was ligated between the liver and kidney to direct all perfusate outflow through the cannulated inferior vena cava above the liver. The liver was perfused in a single-pass manner (30 ml/min with fexofenadine-free, continually oxygenated, Krebs-Henseleit buffer in the presence of 5 µM taurocholate, unless otherwise indicated) for an equilibration period of ~15 min. Following the pre-perfusion period to allow for equilibration of temperature (37°C) and bile flow, the liver was perfused for 60 min with buffer containing 5 µM taurocholate, unless otherwise indicated, and 0.5 µM fexofenadine alone, or with 2 µM GF120918 or 5 µM bosentan. Bile and outflow perfusate were collected in 10-min intervals in toto, and livers were isolated at the end of perfusion and stored at −80°C until analysis. Perfusion pressure and bile flow were used to assess liver viability (33).
Determination of Protein Binding
Livers were thawed and homogenenized in three volumes (v/w) of 0.1 M phosphate buffer (pH 7.4). Liver homogenate samples (500 µL) were placed in Centrifree® micropartition devices and centrifuged at 1000 g at 4°C for 10 min to pass ~10% of the original volume through the filter. The absence-of-protein condition was used to assess non-specific binding. Samples were obtained from above (total concentration in the presence of protein) and below (unbound concentration) the filter, and analyzed by liquid chromatography with detection by tandem mass spectrometry (LC/MS/MS).
Analytical Methods
Bile, outflow perfusate, liver homogenate, ultracentrifugation filtrate and human SCH lysates were analyzed by LC/MS/MS (Applied Biosystems API 4000 triple quadrupole with TurboIonSpray interface, MDS Sciex, Concord, ON, Canada). Fexofenadine and cetirizine (internal standard) were eluted from an Aquasil C18 column (5µm, 50mm × 2.1mm, Thermo-Electron, Waltham, MA) using a mobile phase gradient at a flow rate of 0.75 mL/min (A: 0.1% formic acid in water, B: 0.1% formic acid in methanol); 0–0.8 min hold at 10% B, 0.8–3.5 min linear increase to 85% B, 3.5–4.0 min hold at 85% B, 4.0–4.5 min return to 10% B, 4.5–5 min hold at 10% B (Shimazdu solvent delivery system, Columbia, MD). Fexofenadine and the internal standard, cetirizine, were detected in positive ion mode using multiple reaction monitoring: fexofenadine, 502.3 → 466.4 m/z; cetirizine, 389.0 → 201.0 m/z. Fexofenadine was quantified with seven point standard curves prepared in the appropriate matrix, and coefficients of variation were <14%.
Data Analysis
For SCH studies, the biliary excretion index (BEI, %) and in vitro biliary clearance (Clbiliary, mL/min/kg) were calculated using B-CLEAR® technology [Qualyst, Inc., Raleigh, NC; (32)]:
| (1) |
where substrate accumulation in the cell + bile compartments was determined in hepatocytes preincubated in standard buffer; cellular accumulation of substrate was determined in hepatocytes preincubated with Ca2+-free HBSS.
| (2) |
where AUC0-T represents the product of the incubation time (T) and the initial concentration in the medium. In vitro Clbiliary values were scaled per kilogram body weight using 1 mg protein/1.5 × 106 cells (the typical value obtained in all preparations), 107 × 106 hepatocytes/g of human liver tissue (34), and 25.7 g of liver tissue per kg of body weight (35).
Steady-state fexofenadine concentrations were defined as the average perfusate concentration during the 50- to 60-min interval based on experiments performed by (18) in which outflow fexofenadine concentrations reached a plateau after 50 min of infusion in in situ single-pass perfused rat liver studies. The hepatic extraction ratio was calculated as the ratio of the difference between steady-state inflow and outflow fexofenadine concentrations and the steady-state inflow concentration. Unbound intrinsic biliary clearance (Cl’biliary) was calculated as the ratio of the fexofenadine biliary excretion rate and the unbound liver concentration (Cliver,unbound).
All data were reported as mean ± SD (n = 3–4 per group). Statistical significance was assessed by one-way analysis of variance (ANOVA) with Dunnett’s post-hoc test. In all cases, p < 0.05 was considered to be statistically significant.
Results
Pharmacokinetic Modeling and Simulation of Data from Healthy Human Subjects
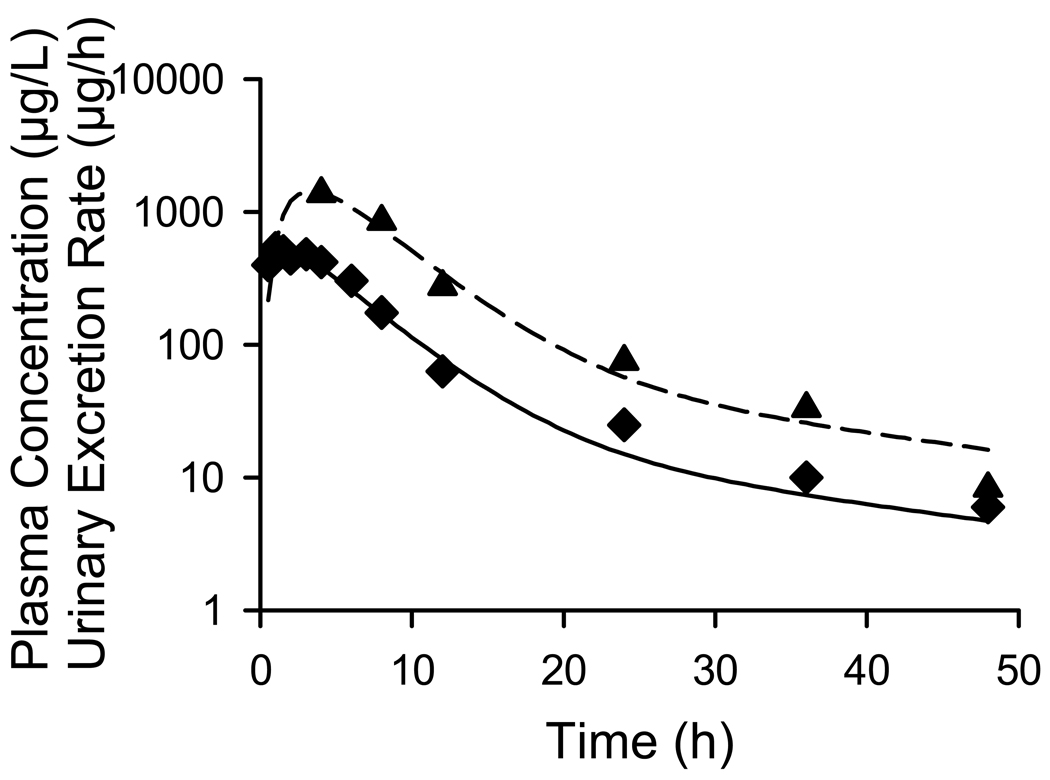
The model scheme depicted in Fig. I best described the fexofenadine plasma concentration vs. time and urinary excretion rate vs. time obtained from previously published data (9), as shown in Fig. II. Parameter estimates for fexofenadine disposition based on the human data are reported in Table I. The ratio of the biliary (Kbile) and basolateral (K21) efflux rate constants was 5.6, suggesting that once in the liver, the predominant process that determines fexofenadine disposition is excretion into bile.
Figure II.
Mean fexofenadine disposition in healthy humans. The curves represent the best fit of the pharmacokinetic model based on the scheme depicted in Fig. I to the data obtained from Shimizu et al. (9). The plasma concentration-time profile (♦ solid line) and urinary excretion rate-time profile (▲ dashed line) are plotted on log-linear scale. Symbols represent actual data points while lines represent the best fit of the model to the data.
Table I.
Pharmacokinetic parameters governing fexofenadine disposition in healthy humans estimated using nonlinear regression analysis based on the compartmental model scheme depicted in Fig. I. Parameter estimates and coefficients of variation were generated based on mean data (n=8 healthy subjects) from a previously published report (6).
| Parameters | Estimate | CV (%) |
|---|---|---|
| Kabsorb (h−1) | 1.67 | 23.0 |
| Vc (L) | 55.8 | 7.71 |
| K12 (h−1) | 0.124 | 11.0 |
| K21 (h−1) | 0.00788 | 49.7 |
| Kbile (h−1) | 0.0438 | 11.1 |
| Kurine (h−1) | 0.0689 | 10.1 |
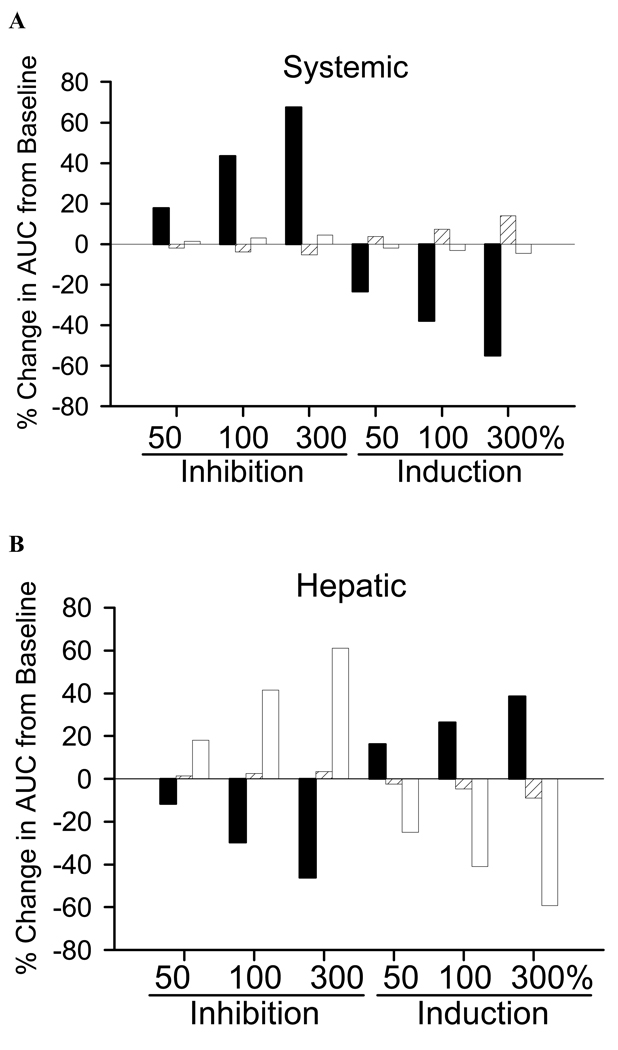
To assess the influence of alterations in hepatic uptake (K12) or excretion (K21, Kbile) on fexofenadine systemic and hepatic exposure, simulations were performed using the parameter estimates in Table I. Based on these simulations, a 50%, 100% and 300% change in the hepatic uptake rate constant (K12) resulted in a 18% or greater difference in the systemic exposure of fexofenadine, determined as the AUC of the plasma concentration-time profile (Fig. III.A). Similar changes in the basolateral efflux rate constant (K21), and biliary excretion rate constant (Kbile), resulted in less than a 7% change in the systemic AUC, except for the 300% increase in basolateral efflux, which increased the systemic AUC ~14%. Importantly, hepatic exposure, determined as the AUC of the hepatic mass-time profile, was sensitive to changes in the hepatic uptake rate constant (K12) as well as the biliary excretion rate constant (Kbile). A 50%, 100% and 300% change in K12 resulted in a 12–16%, 26–30% and 39–46% change, respectively in hepatic exposure. In contrast to systemic exposure, perturbations in Kbile produced at least a 18% difference in hepatic exposure for all simulations. Alterations in the basolateral efflux rate constant (K21) resulted in negligible changes in hepatic exposure of fexofenadine.
Figure III.
Simulated percentage change from baseline in the AUC of the (A) plasma concentration-time profile, predictive of systemic exposure, and (B) hepatic mass-time profile, predictive of hepatic exposure, associated with perturbations in the hepatic uptake rate constant (K12; solid bars), basolateral efflux rate constant (K21; hatched bars) and biliary excretion rate constant (Kbile; open bars).
Biliary Excretion of Fexofenadine in Human Sandwich-Cultured Hepatocytes
The in vitro biliary excretion of [3H]taurocholate, [3H]digoxin and fexofenadine was measured in human SCH from two unrelated donors. Cellular accumulation of the model bile acid [3H]taurocholate (73.8 and 57.8 pmol/mg protein) and BEI [58.1 and 52.4 %, (Table II)] were consistent with previous data generated in this model system. As a positive control, the P-gp substrate [3H]digoxin was evaluated in the presence and absence of GF120918. Cellular accumulation of [3H]digoxin was increased slightly by GF120918 for one liver (21.0 vs. 29.9 pmol/mg protein); the BEI and in vitro biliary clearance of [3H]digoxin were abolished completely by GF120918 in both livers (Table II). Fexofenadine cellular accumulation increased slightly in the presence of GF120918 (66.2 vs. 93.2 and 62.5 vs. 65.1 pmol/mg protein), but fexofenadine BEI and in vitro biliary clearance were not affected by GF120918 in human SCH. Both, BEI and in vitro biliary clearance values were decreased in the second liver donor compared to the first for all three compounds, suggesting decreased development of the bile canalicular networks and/or canalicular protein function in hepatocytes from this liver donor.
Table II.
Accumulation, BEI and in vitro Clbiliary of [3H]taurocholate, [3H]digoxin and fexofenadine in human SCH. SCH were incubated with 1 µM [3H]taurocholate (10 min), 1 µM [3H]digoxin (10 min) or 5 µM fexofenadine (30 min) in the presence and absence of 2 µM GF120918. Results are presented as representative data from triplicate experiments from two livers. The incubation time (t) is indicated in parentheses.
| Substrate | Accumulation Cells + Bile (pmol/mg) |
Accumulation Cells (pmol/mg) |
BEI % |
in vitro Clbiliary (mL/min/kg) |
||||
|---|---|---|---|---|---|---|---|---|
| Liver 1 | Liver 2 | Liver 1 | Liver 2 | Liver 1 | Liver 2 | Liver 1 | Liver 2 | |
| [3H]Taurocholate (t=10 min) |
176 ± 5 | 121 ± 11 | 73.8 ± 2.3 | 57.8 ± 5.5 | 58.1 | 52.4 | 18.7 | 11.6 |
| [3H]Digoxin (t=10 min) |
28.1 ± 1.6 | 26.9 ± 1.5 | 21.0 ± 1.3 | 25.1 ± 2.4 | 25.0 | 6.76 | 1.29 | 0.33 |
| [3H]Digoxin + 2 µM GF120918 (t=10 min) |
26.2 ± 0.9 | 23.4 ± 2.3 | 29.9 ± 1.6 | 25.2 ± 1.6 | 0 | 0 | 0 | 0 |
| Fexofenadine (t=30 min) |
78.8 ± 8.2 | 66.3 ± 19.1 | 66.2 ± 3.7 | 62.5 ± 4.3 | 16.0 | 5.79 | 0.15 | 0.05 |
| Fexofenadine + 2 µM GF120918 (t=30 min) |
112 ± 6 | 73.2 ± 3.8 | 93.2 ± 9.7 | 65.1 ± 2.3 | 16.8 | 11.0 | 0.23 | 0.10 |
Fexofenadine Recovery in Perfused Livers from TR− Rats
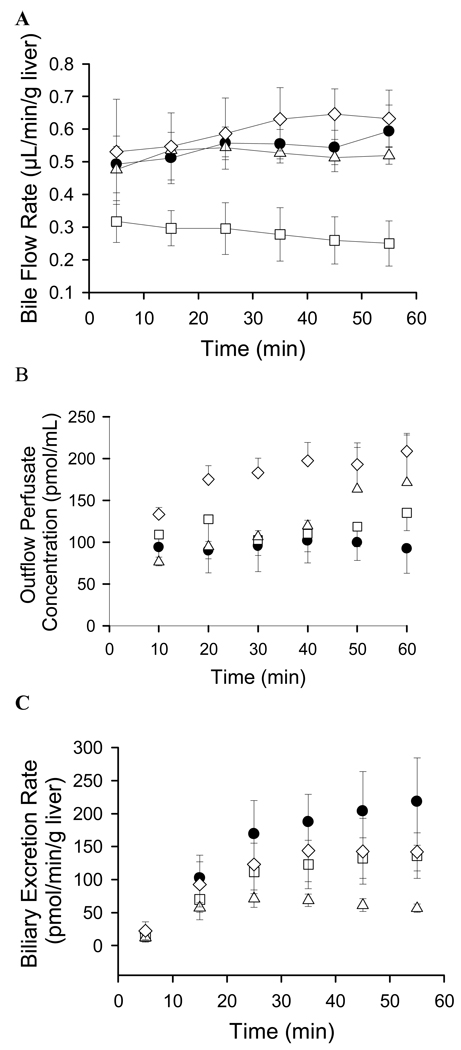
Bile flow rates (mean ± SD) in TR− rat livers perfused with 5 µM taurocholate and 0.5 µM fexofenadine (control) or in combination with either 2 µM GF120918 or 5 µM bosentan, were 0.54 ± 0.04, 0.52 ± 0.02 and 0.60 ± 0.05 µL/min/g liver, respectively. Compared to control values, bile flow rates in livers from TR− rats perfused without 5 µM taurocholate were decreased ~50% to 0.28 ± 0.03 µL/min/g liver. Bile flow in each group was stable during the 60-min perfusion period (Fig. IV.A). The total recovery of fexofenadine at the end of the perfusion period, as a percentage of the total infused dose, was 86 ± 16% in control, 77 ± 9% without taurocholate, 75 ± 4% with GF120918, and 81 ± 9% with bosentan.
Figure IV.
Data generated in perfused livers from Mrp2-deficient TR− rats. (A) Bile flow rates, (B) Fexofenadine concentrations in outflow perfusate, and (C) Biliary excretion rates of fexofenadine in livers perfused with 0.5 µM fexofenadine (●), 0.5 µM fexofenadine without 5 µM taurocholate (□), 0.5 µM fexofenadine + GF120918 (△), and 0.5 µM fexofenadine + bosentan (◊). Perfusate buffer contained 5 µM taurocholate, unless otherwise noted. Data represent mean ± SD (n-3–4 per group).
Fexofenadine concentrations in outflow perfusate are presented in Fig. IV.B (mean ± SD). Outflow perfusate concentrations at the end of the infusion were significantly increased in the presence of GF120918 (171.5 ± 58.8 pmol/mL) and bosentan (209.0 ± 19.4 pmol/mL), compared to control (92.3 ± 29.4 pmol/mL); GF120918 and bosentan significantly decreased the extraction ratio of fexofenadine (Fig. IV.B; Table III)]. The biliary excretion rate of fexofenadine was decreased ~38% when taurocholate was withheld from the inflow perfusate; coadministration of GF120918 and bosentan decreased the fexofenadine biliary excretion rate ~75% and ~35%, respectively (Fig. IV.C; Table III). Unbound fexofenadine concentrations in livers were not significantly different among the treatment groups, although unbound liver concentrations tended to be higher in the presence of GF120918. Bosentan significantly decreased the ratio of fexofenadine unbound liver concentrations to outflow perfusate concentrations, consistent with inhibition of fexofenadine partitioning from perfusate to liver. Fexofenadine Clbiliary was only significantly decreased in the presence of GF120918.
Table III.
Fexofenadine disposition in single-pass perfused TR− rat livers. Mean ± SD (n = 3–4 per group).
| 0.5 µM Fexofenadine |
0.5 µM Fexofenadine without TC |
0.5 µM Fexofenadine + 2 µM GF120918 |
0.5 µM Fexofenadine + 5 µM Bosentan |
|
|---|---|---|---|---|
| Css,out (pmol/mL) # | 92.3 ± 29.4 | 135 ± 21 | 172 ± 59* | 209 ± 19* |
| Cliver (pmol/mL) | 7295 ± 786 | 7156 ± 1529 | 8159 ± 720 | 5561 ± 873 |
| Cliver, unbound (pmol/mL) | 1389 ± 196 | 1282 ± 142 | 1631 ± 620 | 1235 ± 369 |
| Cliver, unbound / Css,out | 16.2 ± 5.4 | 9.53± 0.46 | 10.8 ± 6.0 | 6.05 ± 2.34* |
| Extraction Ratio | 0.82 ± 0.06 | 0.73 ± 0.04 | 0.66 ± 0.12* | 0.58± 0.04* |
| Biliary Excretion Rate (pmol/min/g liver) |
218 ± 66 | 136 ± 34* | 55.6 ± 6.4* | 142 ± 29 |
| Cl’biliary (mL/h/g liver) | 8.57 ± 2.00 | 6.56 ± 2.27 | 2.36 ± 1.12* | 7.52 ± 2.78 |
p< 0.05 fexofenadine ± modulator vs. fexofenadine alone.
Total concentration determined based on 50–60-min collection interval. Perfusate buffer contained 5 µM taurocholate (TC), unless otherwise noted.
Discussion
To evaluate the utility of fexofenadine as a probe substrate for hepatobiliary transport function,, pharmacokinetic modeling and simulation studies based on previously published data were utilized to examine the impact of hepatic transport modulation on the systemic and hepatic exposure of fexofenadine in humans. Furthermore, modeling and simulation of fexofenadine data in humans was supported by in vitro studies in human SCH and perfused rat livers using various chemical modulators of hepatic transport proteins.
The pharmacokinetic model adequately described the mean fexofenadine plasma concentrations, urinary excretion rate and estimated cumulative mass in bile data after a 120 mg oral dose (Fig II); low variability was associated with the parameter estimates (Table I). The predominant process determining the systemic clearance of fexofenadine in humans appears to be hepatic uptake when comparing the rate constants (Table I) governing disposition of fexofenadine in humans. Although, this is a very low clearance process when comparing the product of K12 (hepatic uptake) and Vc which is 6.92 L/h with hepatic blood flow in humans ~87 L/h (35).
To examine the sensitivity of systemic and hepatic exposure to changes in hepatic uptake and efflux processes in humans, a simulation approach was utilized, and the rate constants representing the active transport processes governing hepatic uptake and efflux were modulated in a systematic manner. The results indicated that the central compartment, representing the systemic circulation, is most sensitive to changes in the hepatic uptake rate constant, rather than the basolateral efflux rate constant, or the biliary excretion rate constant (Fig. III). This finding is supported by the absence of drug-drug interactions (defined as a significant change in fexofenadine pharmacokinetics) when fexofenadine is administered with known P-gp modulators such as diltiazem (9) or verapamil (5), or in individuals with P-gp polymorphisms (C3435T and G2677T) (14, 36, 37). Furthermore, simulations are consistent with drug-drug interaction studies with known OATP modulators such as fruit juices (30), and in individuals with polymorphisms of the SLCO1B1 gene (38), where significant changes in fexofenadine AUC have been reported. In contrast, simulations revealed that hepatic fexofenadine exposure in humans was sensitive to alterations in either the hepatic uptake rate constant or the biliary excretion rate constant. Most importantly, the marked increase in fexofenadine hepatic exposure due to modulation of Kbile was not reflected by changes in the systemic concentration-time profile in humans (Fig. III). This demonstrates the difficulty in assessing hepatic exposure by only measuring systemic concentrations of a substrate like fexofenadine. In humans, multiple transport proteins appear to be involved in fexofenadine biliary excretion, thus, the suitability of this substrate as a probe to assess altered hepatic excretion in humans is limited. However, based on perfused rat liver data, the GF120918-sensitive component of biliary excretion plays a predominant role in canalicular transport in rats; inhibition of fexofenadine biliary excretion resulted in increased perfusate concentrations. These data underscore the importance of species differences in hepatobiliary drug transport, and the important role that compensatory transport proteins may play in determining the systemic and/or hepatic exposure of a substrate when hepatic transport processes are modulated. Furthermore, as depicted in Figure III, significant interactions in hepatic excretion of a drug that displays hepatobiliary disposition characteristics similar to fexofenadine may not be reflected by changes in the plasma concentration-time profile.
To investigate the changes in systemic and hepatic exposure after inhibition of fexofenadine biliary excretion, and examine the involvement of P-gp in the biliary excretion of fexofenadine in humans, studies were performed in human SCH using the inhibitor GF120918. The possible involvement of BCRP was ruled out based on previously published data generated in MDCKII cells transfected with human BCRP demonstrating that BCRP does not transport fexofenadine (19). OATP and P-gp function were assessed by the probe substrate [3H]digoxin as a positive control. Intracellular accumulation and BEI values of [3H]digoxin were comparable to previously reported data (39, 40). GF120918 (2 µM), used previously to inhibit P-gp in rat SCH (39), completely abolished the BEI and in vitro Clbiliary of [3H]digoxin in human SCH (Table II). Fexofenadine exhibited a low BEI and in vitro Clbiliary which was marginally affected by GF120918. The trend towards increased hepatocellular accumulation of fexofenadine in the presence of GF120918 (Table II) is in agreement with the pharmacokinetic simulations which predicted that hepatic accumulation would increase when the biliary excretion rate constant was decreased (Fig III.B). However the possibility that GF120918-mediated inhibition of basolateral efflux results in increased hepatocellular accumulation of fexofenadine cannot be ruled out. These data suggest that GF120918-sensitive protein(s) (presumably P-gp) play a minor role in fexofenadine biliary excretion in human hepatocytes. Quite possibly, other canalicular transport proteins fully compensate when GF120918-sensitive protein(s) is/are inhibited. Fexofenadine is a known substrate for human MRP2 and BSEP based on previously published studies using MDCKII and HEK293 transfected cells (11, 19). These data imply that fexofenadine would not be a suitable probe substrate in humans to assess altered biliary excretion associated with a specific canalicular transport process.
Due to the limited availability of human hepatocytes, the lack of specific MRP2 inhibitors that would not affect OATP-mediated fexofenadine uptake, and the multiplicity of efflux transport proteins involved in fexofenadine hepatobiliary disposition in humans (19, 41), further investigations were conducted in rat perfused livers. Previous studies demonstrated that the biliary excretion and intrinsic biliary clearance of fexofenadine were not significantly different in wild-type and Mrp2-deficient rats (2, 18). Therefore, TR− rats were employed in the present studies to rule out any compensatory role of Mrp2 in the biliary excretion of fexofenadine. Co-infusion of fexofenadine and GF120918 resulted in a significant decrease in both the biliary excretion rate and the intrinsic biliary clearance of fexofenadine (Fig. IV.C; Table III). Fexofenadine is a substrate of the basolateral efflux protein Mrp3 (19, 41), which is upregulated 7-fold in TR− rats (42) and may explain the significant increase in outflow perfusate concentrations of fexofenadine in the presence of GF120918. This observation is supported by simulations examining the impact of induction of the basolateral efflux rate constant (K21) on fexofenadine disposition, which resulted in noticeable changes in the plasma AUC (Fig III.A). Although fexofenadine liver concentrations tended to be higher when biliary excretion was inhibited by GF120918 in perfused TR− rat livers, as predicted in humans based on the pharmacokinetic simulations, differences failed to reach statistical significance. This apparent discrepancy could be due to species differences in the role of basolateral efflux proteins as a determinant of fexofenadine hepatobiliary disposition. In humans perturbations in biliary excretion only affected hepatic exposure; basolateral efflux was such a minor pathway that systemic exposure was not influenced by such perturbations. Basolateral efflux may play a more important role in systemic exposure in the rat based on this data, and also the mouse as reported previously using Mrp2 knock-out mice (19, 41).
Fexofenadine is not a substrate of human or mouse BCRP/Bcrp based on previously published data generated in MDCKII cells transfected with human BCRP, or in Bcrp knockout mice (2, 19). Therefore, the predominant pathway for excretion of fexofenadine into rat bile is likely P-gp, in contrast to the mouse in which Mrp2 plays the greatest role (18). This is supported by Milne et al., who demonstrated that the P-gp inhibitor erythromycin decreased cumulative recovery of fexofenadine in bile, and slightly increased hepatic accumulation and the perfusate AUC of fexofenadine in rat isolated perfused livers (43). Interestingly, mass balance data reported by these investigators indicated that 50% of the fexofenadine dose remained in the liver at the end of perfusion. Our studies revealed that ~40% of the fexofenadine dose was retained within the rat liver at the end of perfusion. As previously suggested, the liver may act as a reversible binding or sequestration site as well as an elimination organ for fexofenadine (5).
Withholding taurocholate from the perfusion buffer resulted in a significant decrease in the biliary excretion rate of fexofenadine (Fig. IV.C; Table III). Hepatic fexofenadine concentrations were similar to control livers perfused with fexofenadine and taurocholate. The significant decrease in the biliary excretion of fexofenadine may be due to reduced canalicular localization of Bsep and other ATP binding cassette (ABC) transport proteins that mediate fexofenadine biliary excretion, causing a modest shift in the route of fexofenadine excretion to perfusate. Treatment of rats in vivo with taurocholate has been shown to modestly increase canalicular ABC transport protein expression on the hepatocyte canalicular membrane within minutes (28, 29).
Fexofenadine has been shown to be a substrate of human and rat BSEP/Bsep using in vitro transfected systems (19). Therefore, bosentan, a known inhibitor of Bsep (27), was concurrently infused into the perfusate. Although bosentan was expected to decrease fexofenadine biliary excretion, only a 35% decrease in the biliary excretion rate was noted (Table III). Interestingly, bosentan significantly decreased the extraction ratio and partitioning of fexofenadine from perfusate to liver (Table III). These results may be due to bosentan-mediated inhibition of fexofenadine hepatic uptake, and/or to increased basolateral efflux as a result of inhibition of fexofenadine biliary excretion; either scenario would result in significantly increased fexofenadine perfusate concentrations (Fig. IV.B; Table III). Bosentan is a substrate of all three Oatp isoforms in the rat liver [Oatp1a1, 1a4 and 1b2; (44)] which are responsible for fexofenadine hepatic uptake (8). Furthermore, bosentan (100 µM) competitively inhibited (by ~40%) the transport of model probes of the human OATP1B1, 1B3 and 2B1 isoforms in transfected CHO cell lines (45). Interestingly, the simulations of impaired hepatic uptake of fexofenadine in humans agreed with the results from perfused rat liver studies: perfusate (a surrogate for plasma) concentrations significantly increased in the presence of bosentan (Fig. IV.B).
Conclusion
In summary, pharmacokinetic modeling and simulation studies revealed that fexofenadine in humans is not an optimal probe for any specific hepatic efflux transport protein due to compensatory hepatic transport mechanisms. Systemic fexofenadine concentrations are most sensitive to changes in fexofenadine hepatic uptake. Increased or decreased hepatocyte concentrations as a result of impaired or induced biliary or hepatic basolateral efflux, respectively, associated with drug-drug interactions or genetic mutations, has minimal impact on systemic exposure. Furthermore, pharmacokinetic modeling and simulation studies were in good agreement with preclinical data that predicted changes in systemic and liver exposure due to inhibition of hepatic uptake. Studies in perfused livers from TR− rats demonstrated that fexofenadine biliary excretion is mediated primarily by a GF120918-sensitive (presumably P-gp) mechanism. In contrast, either P-gp plays a minor role in fexofenadine biliary excretion in human hepatocytes, or other canalicular transport proteins fully compensate when P-gp is inhibited.
Acknowledgment
The authors would like to thank Arlene S. Bridges, Ph. D. for her analytical support, and Yiwei Rong, for her technical expertise in the isolation of human hepatocytes. This research was supported by a grant from the National Institutes of Health (RO1 GM41935). Brandon Swift is supported by an Eli Lilly predoctoral fellowship.
List of Nonstandard Abbreviations
- SCH
sandwich-cultured hepatocytes
- OATP
organic anion transporting polypeptide
- MDR1
multidrug resistance protein 1
- P-gp
P-glycoprotein
- Mrp
multidrug resistance-associated protein
- TR−
Mrp2-deficient Wistar rat
- EHBR
Eisai hyperbilirubinemic rats
- Bsep
bile salt export pump
- Bcrp
breast cancer resistance protein
- WT
wild-type Wistar rat
- GF120918
N-(4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl]-phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide
- ABC
ATP-binding cassette
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
Fetal bovine serum
- HBSS
Hanks’ balanced salt solution
- BCA
Bicinchoninic acid
- LC/MS/MS
liquid chromatography with detection by tandem mass spectrometry
- AUC
area under the curve
- ABC
ATP binding cassette
References
- 1.Lippert C, Ling J, Brown P, Burmaster S, Eller M, Cheng L, Thompson R, Weir S. Mass Balance and Pharmacokinetics of MDL 16,455A in Healthy, Male Volunteers. Pharmaceutical Research. 1995;12:S-390. [Google Scholar]
- 2.Tahara H, Kusuhara H, Fuse E, Sugiyama Y. P-glycoprotein plays a major role in the efflux of fexofenadine in the small intestine and blood-brain barrier, but only a limited role in its biliary excretion. Drug Metab Dispos. 2005;33:963–968. doi: 10.1124/dmd.105.004192. [DOI] [PubMed] [Google Scholar]
- 3.Petri N, Tannergren C, Rungstad D, Lennernas H. Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm Res. 2004;21:1398–1404. doi: 10.1023/b:pham.0000036913.90332.b1. [DOI] [PubMed] [Google Scholar]
- 4.Tannergren C, Knutson T, Knutson L, Lennernas H. The effect of ketoconazole on the in vivo intestinal permeability of fexofenadine using a regional perfusion technique. Br J Clin Pharmacol. 2003;55:182–190. doi: 10.1046/j.1365-2125.2003.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tannergren C, Petri N, Knutson L, Hedeland M, Bondesson U, Lennernas H. Multiple transport mechanisms involved in the intestinal absorption and first-pass extraction of fexofenadine. Clin Pharmacol Ther. 2003;74:423–436. doi: 10.1016/S0009-9236(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 6.Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69:114–121. doi: 10.1067/mcp.2001.113697. [DOI] [PubMed] [Google Scholar]
- 7.Banfield C, Gupta S, Marino M, Lim J, Affrime M. Grapefruit juice reduces the oral bioavailability of fexofenadine but not desloratadine. Clin Pharmacokinet. 2002;41:311–318. doi: 10.2165/00003088-200241040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–370. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Uno T, Sugawara K, Tateishi T. Effects of itraconazole and diltiazem on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein. Br J Clin Pharmacol. 2006;61:538–544. doi: 10.1111/j.1365-2125.2006.02613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- 11.Matsushima S, Maeda K, Ishiguro N, Igarashi T, Sugiyama Y. Investigation of the inhibitory effects of various drugs on the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2008 doi: 10.1124/dmd.107.017814. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, Sugiyama Y. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33:1477–1481. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- 13.Tucker GT, Houston JB, Huang SM. Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential--towards a consensus. Br J Clin Pharmacol. 2001;52:107–117. doi: 10.1046/j.0306-5251.2001.temp.1441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shon JH, Yoon YR, Hong WS, Nguyen PM, Lee SS, Choi YG, Cha IJ, Shin JG. Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharmacol Ther. 2005;78:191–201. doi: 10.1016/j.clpt.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Chan E, Pan SQ, Huang M, Lee EJ. Pharmacokinetic interactions of drugs with St John's wort. J Psychopharmacol. 2004;18:262–276. doi: 10.1177/0269881104042632. [DOI] [PubMed] [Google Scholar]
- 16.Lemma GL, Wang Z, Hamman MA, Zaheer NA, Gorski JC, Hall SD. The effect of short- and long-term administration of verapamil on the disposition of cytochrome P450 3A and P-glycoprotein substrates. Clin Pharmacol Ther. 2006;79:218–230. doi: 10.1016/j.clpt.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Yasui-Furukori N, Uno T, Sugawara K, Tateishi T. Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. Clin Pharmacol Ther. 2005;77:17–23. doi: 10.1016/j.clpt.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Tian X, Zamek-Gliszczynski MJ, Li J, Bridges AS, Nezasa K, Patel NJ, Raub TJ, Brouwer KL. Multidrug resistance-associated protein 2 is primarily responsible for the biliary excretion of fexofenadine in mice. Drug Metab Dispos. 2008;36:61–64. doi: 10.1124/dmd.107.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushima S, Maeda K, Hayashi H, Debori Y, Schinkel AH, Schuetz JD, Kusuhara H, Sugiyama Y. Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol. 2008 doi: 10.1124/mol.107.041459. [DOI] [PubMed] [Google Scholar]
- 20.Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123:1649–1658. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- 21.Hirano M, Maeda K, Hayashi H, Kusuhara H, Sugiyama Y. Bile salt export pump (BSEP/ABCB11) can transport a nonbile acid substrate, pravastatin. J Pharmacol Exp Ther. 2005;314:876–882. doi: 10.1124/jpet.105.084830. [DOI] [PubMed] [Google Scholar]
- 22.Lecureur V, Sun D, Hargrove P, Schuetz EG, Kim RB, Lan LB, Schuetz JD. Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol Pharmacol. 2000;57:24–35. [PubMed] [Google Scholar]
- 23.Hoffmannand HK, Kroemer U. The ABC transporters MDR1 and MRP2: multiple functions in disposition of xenobiotics and drug resistance. Drug Metab Rev. 2004;36:669–701. doi: 10.1081/dmr-200033473. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata S, Oka M, Shiozawa K, Tsukamoto K, Nakatomi K, Soda H, Fukuda M, Ikegami Y, Sugahara K, Yamada Y, Kamihira S, Doyle LA, Ross DD, Kohno S. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280:1216–1223. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278:22644–22649. doi: 10.1074/jbc.M212399200. [DOI] [PubMed] [Google Scholar]
- 26.Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol. 2005;67:1758–1764. doi: 10.1124/mol.104.010439. [DOI] [PubMed] [Google Scholar]
- 27.Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69:223–231. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- 28.Gatmaitan ZC, Nies AT, Arias IM. Regulation and translocation of ATP-dependent apical membrane proteins in rat liver. Am J Physiol. 1997;272:G1041–G1049. doi: 10.1152/ajpgi.1997.272.5.G1041. [DOI] [PubMed] [Google Scholar]
- 29.Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 30.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 31.LeCluyse EL, Bullock PL, Parkinson A, Hochman JH. Cultured rat hepatocytes. Pharm Biotechnol. 1996;8:121–159. doi: 10.1007/978-1-4899-1863-5_9. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KL. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol. 1999;277:G12–G21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- 33.Chandra P, Johnson BM, Zhang P, Pollack GM, Brouwer KL. Modulation of hepatic canalicular or basolateral transport proteins alters hepatobiliary disposition of a model organic anion in the isolated perfused rat liver. Drug Metab Dispos. 2005;33:1238–1243. doi: 10.1124/dmd.105.003665. [DOI] [PubMed] [Google Scholar]
- 34.Wilson ZE, Rostami-Hodjegan A, Burn JL, Tooley A, Boyle J, Ellis SW, Tucker GT. Inter-individual variability in levels of human microsomal protein and hepatocellularity per gram of liver. Br J Clin Pharmacol. 2003;56:433–440. doi: 10.1046/j.1365-2125.2003.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daviesand T, Morris B. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 36.Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF. MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol. 2002;53:526–534. doi: 10.1046/j.1365-2125.2002.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–199. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 38.Niemi M, Kivisto KT, Hofmann U, Schwab M, Eichelbaum M, Fromm MF. Fexofenadine pharmacokinetics are associated with a polymorphism of the SLCO1B1 gene (encoding OATP1B1) Br J Clin Pharmacol. 2005;59:602–604. doi: 10.1111/j.1365-2125.2005.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Annaert PP, Turncliff RZ, Booth CL, Thakker DR, Brouwer KL. P-glycoprotein-mediated in vitro biliary excretion in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2001;29:1277–1283. [PubMed] [Google Scholar]
- 40.Bi YA, Kazolias D, Duignan DB. Use of cryopreserved human hepatocytes in sandwich culture to measure hepatobiliary transport. Drug Metab Dispos. 2006;34:1658–1665. doi: 10.1124/dmd.105.009118. [DOI] [PubMed] [Google Scholar]
- 41.Tian X, Swift B, Zamek-Gliszczynski MJ, Belinsky MG, Kruh GD, Brouwer KL. Impact of basolateral multidrug resistance-associated protein (Mrp) 3 and Mrp4 on the hepatobiliary disposition of fexofenadine in perfused mouse livers. Drug Metab Dispos. 2008;36:911–915. doi: 10.1124/dmd.107.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong H, Turner KC, Ward ES, Jansen PL, Brouwer KL. Altered hepatobiliary disposition of acetaminophen glucuronide in isolated perfused livers from multidrug resistance-associated protein 2-deficient TR(-) rats. J Pharmacol Exp Ther. 2000;295:512–518. [PubMed] [Google Scholar]
- 43.Milne RW, Larsen LA, Jorgensen KL, Bastlund J, Stretch GR, Evans AM. Hepatic disposition of fexofenadine: influence of the transport inhibitors erythromycin and dibromosulphothalein. Pharm Res. 2000;17:1511–1515. doi: 10.1023/a:1007609225851. [DOI] [PubMed] [Google Scholar]
- 44.Treiber A, Schneiter R, Delahaye S, Clozel M. Inhibition of organic anion transporting polypeptide-mediated hepatic uptake is the major determinant in the pharmacokinetic interaction between bosentan and cyclosporin A in the rat. J Pharmacol Exp Ther. 2004;308:1121–1129. doi: 10.1124/jpet.103.061614. [DOI] [PubMed] [Google Scholar]
- 45.Treiber A, Schneiter R, Hausler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos. 2007;35:1400–1407. doi: 10.1124/dmd.106.013615. [DOI] [PubMed] [Google Scholar]