Abstract
Purpose
Factors captured in a geriatric assessment can predict morbidity and mortality in older adults, but are not routinely measured in cancer clinical trials. This study evaluated the implementation of a geriatric assessment tool in the cooperative group setting.
Patients and Methods
Patients age ≥ 65 with cancer, who enrolled on cooperative group cancer trials, were eligible to enroll on Cancer and Leukemia Group B (CALGB) 360401. They completed a geriatric assessment tool before initiation of protocol therapy, consisting of valid and reliable geriatric assessment measures which are primarily self-administered and require minimal resources and time by healthcare providers. The assessment measures functional status, comorbidity, cognitive function, psychological state, social support, and nutritional status. The protocol specified criteria for incorporation of the tool in future cooperative group trials was based on the time to completion and percent of patients who could complete their portion without assistance. Patient satisfaction with the tool was captured.
Results
Of the 93 patients who enrolled in this study, five (5%) met criteria for cognitive impairment and three did not complete the cognitive screen, leaving 85 assessable patients (median age, 72 years). The median time to complete the geriatric assessment tool was 22 minutes, 87% of patients (n = 74) completed their portion without assistance, 92% (n = 78) were satisfied with the questionnaire length, 95% (n = 81) reported no difficult questions, and 96% (n = 82) reported no upsetting questions. One hundred percent of health care professionals completed their portion.
Conclusion
This brief, primarily self-administered geriatric assessment tool met the protocol specified criteria for inclusion in future cooperative group clinical trials.
INTRODUCTION
The majority of cancer incidence and mortality occurs in older adults; however, clinical trials, which set the standard of care, usually accrue younger participants with a good performance status.1–3 Since the world population is aging, and given the known association between cancer and aging,4,5 there is a critical need to improve our evidence-based knowledge regarding the care of older adults with cancer. Several studies have demonstrated that although older adults derive similar benefit from cancer therapy as do younger patients,6,7 they are at a greater risk for treatment toxicity.8–11 However, aging is a heterogeneous process that is not captured by chronologic age. The domains in a geriatric assessment are designed to capture the functional age of an older adult. This identifies those older adults who have a diminished life expectancy and/or are at risk for hospitalization and functional decline.12,13
Emerging data support the predictive and prognostic value of a geriatric assessment in weighing the risks and benefits of cancer treatment in an older adult.14–18 However, a traditional geriatric assessment is time consuming and has not been routinely incorporated into oncology practice or cooperative group clinical trials because of the time, resources, and expertise required to capture the information. To overcome this barrier, a brief geriatric assessment tool was designed, utilizing valid and reliable geriatric assessment measures which are primarily self-administered and require minimal resources and time by health care providers. The geriatric assessment tool included several validated measures of functional status, comorbidity, cognitive function, psychological state, social support, and nutritional status. This geriatric assessment tool, devised in collaboration with members from the Cancer and Leukemia Group B (CALGB) Cancer in the Elderly Committee, garnered expertise from specialists in geriatrics, oncology, psychology, quality of life, health outcomes research, and biostatistics. A comprehensive review of possible tools to measure each domain was performed. The final measures included in this brief geriatric assessment were chosen for their reliability, validity, brevity, and prognostic ability to determine risk for morbidity or mortality in an older patient. The geriatric assessment tool primarily consisted of self-reported measures which were completed by the patient. Three items were completed by the health care professional. This geriatric assessment tool was developed in two stages.
The goal of the first stage was to evaluate the feasibility of the geriatric assessment tool among older patients with a cancer diagnosis of breast, lung, colorectal cancer, or lymphoma who were receiving treatment with standard of care chemotherapy. These patients were accrued from two participating sites (Memorial Sloan-Kettering Cancer Center and the University of Chicago). The mean time to completion of the geriatric assessment tool was fewer than 30 minutes. In addition, the majority of patients were able to complete the self-administered questionnaire without assistance (78%), and were satisfied with the questionnaire length (90%). We therefore concluded that the geriatric assessment was feasible in the stated setting.19
The goal of the second stage was to determine whether this geriatric assessment tool could be successfully implemented in the cooperative group setting and to identify any barriers to implementing the tool in the cooperative group setting. Results of this study will be used to refine the geriatric assessment tool in order to achieve a final tool that will then be incorporated within cooperative group clinical trials. This report documents the findings from the second stage of development.
PATIENTS AND METHODS
CALGB 360401 was a limited-access study opened at 15 participating CALGB institutions. The study was approved by the National Cancer Institute central institutional review board and by the institutional review board at each participating institution.
Eligibility Criteria
The eligibility criteria included age at study enrollment ≥ 65 years, diagnosis of malignancy, any performance status level, and enrollment in a cooperative group treatment trial but treatment not yet started. Because several measures used in the assessment tool were not validated in other languages, eligibility was restricted to patients with the ability to follow directions in English.
Geriatric Assessment Tool
The geriatric assessment tool included validated measures of geriatric assessment across the domains of functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognitive function, and medications (Table 1).20–31 A full description of the measures included in this tool has been previously reported.19 The geriatric assessment tool was composed of a patient portion and a health care provider portion. The patient portion was composed of self-reported measures of functional status, comorbidity, psychological state, social support, nutritional status, and medications. The patient portion was designed to be completed by the patient; however, a member of the health care team assisted those who needed help. The health care provider portion consisted of three measures: rating the patient's Karnofsky performance status,22 the Timed Up and Go24 (a performance-based measure of the patient's functional status), and the Blessed Orientation-Memory-Concentration test30 (a screening measure of the patient's cognitive function).
Table 1.
Domains and Measures Captured by Geriatric Assessment Tool
| Domain With Measure | No. of Items | Description |
|---|---|---|
| Functional status | ||
| MOS physical health20 | 10 | Measures limitations in a wide range of physical functions (from bathing/dressing to vigorous activities such as running) |
| Instrumental Activities of Daily Living [subscale of the OARS]21 | 7 | Measures ability to complete activities required to maintain independence in the community (ie, meal preparation, shopping, making telephone calls, money management) |
| Karnofsky performance status (rated by the health care professional)22* | 1 | Global indicator of patient function determined by the health care professional on a scale of 0 to 100 |
| Karnofsky self-reported performance rating scale23 | 1 | Global indicator of patient function determined by patient self-report ranging from normal to severely disabled on a scale of 40 to 100 |
| No. of falls in last 6 months | 1 | No. of times patient has fallen in last 6 months |
| Timed Up and Go24* | 1 | Performance-based measure of functional status: amount of time it takes for seated patient to rise from a chair, walk 10 feet, walk back, and sit down |
| MOS social activities20 | 4 | Measures ability to participate in social activities and degree to which health status limits normal social activities |
| Comorbid medical conditions | ||
| Physical health section (subscale of the OARS)21 | 15 | List of comorbid illnesses and the degree to which they impair daily activities; patient can add additional comorbid illnesses not listed; rating of eyesight and hearing |
| Psychological state | ||
| Hospital Anxiety and Depression Scale25 | 14 | Measures of anxiety and depression |
| Social support | ||
| MOS social support survey: emotional/ information and tangible subscales26 | 12 | Perceived availability of social support |
| Nutritional status | ||
| Body mass index27 | 1 | Weight/height2 |
| Percent unintentional weight loss in past 6 months28,29 | 1 | Unintentional weight loss in last 6 months/baseline body weight × 100 |
| Cognition | ||
| Blessed Orientation-Memory-Concentration test30,31* | 6 | Gross measure of cognitive function |
| Medications | ||
| Comprehensive list of medications | 1 | List of medications including prescribed, herbal, and over-the-counter medications |
Abbreviations: MOS, Medical Outcomes Study; OARS, Older American Resources and Services.
Items completed by the healthcare professional (Karnofsky performance status, Timed Up and Go, and Blessed Orientation-Memory-Concentration test).
Patients reported their degree of satisfaction with the geriatric assessment tool. They were asked to comment on the length of the tool and to identify difficult or distressing items. The time to complete the entire geriatric assessment tool as well as the health care provider and patient portions were captured. The percent of patients who required assistance and the reasons for requiring assistance to complete the tool were recorded.
End Points
The study end points were: percentage of patients able to complete the patient portion of the assessment tool without assistance; length of time needed to complete the entire geriatric assessment tool; percent of patients missing at least one item on a scale; patient satisfaction with the patient portion, including identifying items that were distressing or difficult to comprehend and satisfaction with the length of the questionnaire; and percentage of health care professionals who completed their portion of the geriatric assessment tool. The end points were formulated by the CALGB Cancer in the Elderly Committee and Quality of Life. They were also reviewed by the CALGB executive committee. There was consensus among the members of these committees with regard to these end points.
Per protocol, successful implementation would be declared if: more than 70% of patients completed the self-report patient questionnaire without assistance, and the median time to complete the entire geriatric assessment tool was fewer than 40 minutes. With the aim of refining the geriatric assessment tool, a measure might be removed if: more than 25% of patients failed to answer at least one item on a geriatric assessment measure included within the tool, or more than 20% of patients reported that the measure was upsetting or difficult to understand. Also, if fewer than 80% of health care professionals completed the health care professional portion, this portion might be modified or removed from the geriatric assessment tool.
Study Implementation
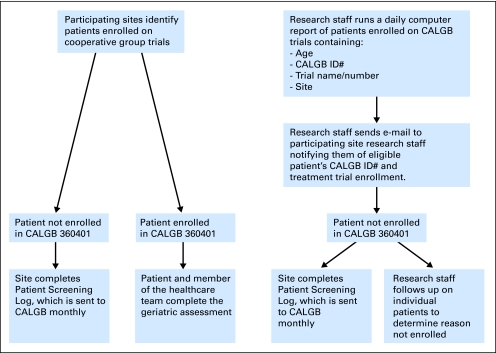
The geriatric assessment tool was completed by patients before initiation of cancer treatment. The study implementation process is summarized in Figure 1. To identify potentially eligible patients at participating institutions, CALGB information systems generated a daily report of patients age ≥ 65 registered in a CALGB trial at each participating institution. The study principal investigator or a member of the research team reviewed this report daily and notified researchers at the participating institution of potentially eligible patients. A member of the institution's research team explained the study to the patient, and informed consent was obtained from eligible patients who agreed to participate. The study team at each institution was trained by the study principal investigator via phone on protocol procedures and delivery of the geriatric assessment. A flow chart for accrual is summarized in Appendix Figure A1 (online only). Patient registration and data collection were managed by the CALGB statistical center.
Fig 1.
Cancer and Leukemia Group B (CALGB) 360401 flow chart of procedures.
Statistical Considerations
Statistical analyses were performed by CALGB statisticians. A target sample size of 80 patients was selected so that the length of a 95% CI would be no larger than 0.20 when estimating proportions higher than 0.70. Patients were categorized into two cohorts according to age, namely, 65 to 69 and ≥ 70 years. Enrollment to the 65 to 69 age cohort was capped at 25% of the study cohort in order to ensure that the median age of the study cohort would be older than 70 years. Descriptive statistics, including 95% CIs, were used to summarize data from this study.
A patient's refusal to complete the Blessed Orientation-Memory-Concentration Test30 or a score of 11 or higher was considered an indication of questionable cognitive capacity to provide accurate and reliable self-reported information. These patients were therefore excluded from all study analyses.
RESULTS
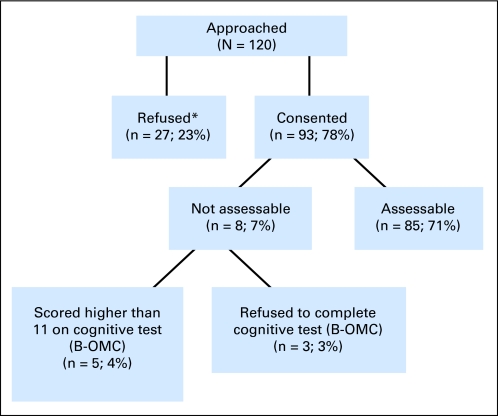
CALGB 360401 was activated in December 2006. The protocol was subsequently approved by the institutional review board at the participating sites (institutional review board approval ranging from February 2007 to April 2008). The time to obtain institutional review board approval at the individual sites contributed to the initial lag in accrual. In June 2008, the age 65 to 69 years cohort closed to accrual with 24 patients. Accrual continued to the age 70+ years cohort until January 2009, when accrual to that cohort closed with 69 patients. The final total accrual was 93 patients. Two recruitment rates were calculated: patients successfully recruited to the study of all patients screened for eligibility: 93 of 191 (49%); and patients successfully recruited to the study out of all patients approached for consent: 93 of 120 ( 78%).
Of the 93 enrolled patients, three patients refused to take the Blessed Orientation-Memory-Concentration test30 and five patients scored 11 or greater (the cutoff score for cognitive impairment). This left 85 patients assessable for analyses. The remainder of this report is based on the 85 assessable patients.
Patient demographics are summarized in Table 2. The median age of all enrolled and assessable patients was 72 (range, 65 to 90 years). Three fourths of these patients were at least 70 years of age. More than half of the patients (59%) were male; only three were nonwhite. Most (57%) patients were married. Slightly more than half (56%) of patients had at least some college background.
Table 2.
Patient Characteristics
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Assessable patients | 85 | 100 |
| Age, years | ||
| 65-69 | 21 | 25 |
| 70-74 | 34 | 40 |
| ≥ 75 | 30 | 35 |
| Sex | ||
| Female | 35 | 41 |
| Male | 50 | 59 |
| Cancer type | ||
| Breast | 12 | 14 |
| Prostate | 22 | 26 |
| Lymphoma | 11 | 13 |
| Lung | 12 | 14 |
| GI | 13 | 15 |
| Leukemia/myeloma | 8 | 9 |
| Melanoma | 2 | 2 |
| Endometrium | 2 | 2 |
| Other | 3 | 4 |
| Cancer stage | ||
| I | 6 | 7 |
| II | 17 | 20 |
| III | 15 | 18 |
| IV | 43 | 51 |
| Other* | 4 | 5 |
| Educational level | ||
| Less than high school | 9 | 11 |
| High school graduate | 27 | 32 |
| Any college | 28 | 33 |
| Any post-college | 20 | 24 |
| Missing | 1 | 1 |
| Marital status | ||
| Married | 48 | 57 |
| Widowed | 21 | 25 |
| Single | 7 | 8 |
| Separated, divorced, other | 9 | 11 |
| Employment status | ||
| Full or part-time | 14 | 16 |
| Retired, homemaker, unemployed | 70 | 82 |
| Other | 1 | 1 |
| Household composition | ||
| Lives alone | 23 | 27 |
| Lives with spouse, partner, or child | 62 | 73 |
| Race | ||
| White | 82 | 96 |
| Black | 0 | 0 |
| Hispanic | 1 | 1 |
| Asian | 1 | 1 |
| Multiracial | 1 | 1 |
Other includes three patients with leukemia and one patient with limited-stage small-cell lung cancer.
The median time to complete the geriatric assessment tool (patient and health care professional portion) was 22 minutes, with a minimum of 6 and a maximum of 60 minutes (Table 3). Patients took a median of 15 minutes (range, 3 to 45 minutes) to complete their portion and health care professionals took a median of 5 minutes (range, 1 to 30 minutes) to complete their portion of the geriatric assessment tool. Of the 85 assessable patients, 100% (n = 85) of the health care professionals completed their portion. The health care professional portion could be completed by the nurse, research assistant, or physician. Only 2% of physicians completed the health care professional questionnaire and the remainder was completed by the nurse and/or research assistant. Of the 85 assessable patients, 87% (n = 74; 95% CI, 78% to 93%) of patients completed their portion of the geriatric assessment tool without assistance (Table 4). The reasons cited for the 11 patients requiring assistance included visual problems (n = 3), fatigue (n = 1), and other reasons (n = 7), including general health, assistance with completing the medication list, frustration, non-English primary language, protective/controlling daughter, and request to have questionnaire read. Illiteracy and item difficulty were not mentioned as a reason for requiring assistance. These results meet the protocol-specified criteria to declare feasibility.
Table 3.
Time to Complete the Geriatric Assessment
| Statistic (minutes) | Instrument |
||
|---|---|---|---|
| Health Care Professional Questionnaire | Patient Questionnaire | Composite Assessment Tool | |
| Mean | 7 | 17 | 24 |
| Standard deviation | 5 | 7 | 10 |
| Median | 5 | 15 | 22* |
| Range | 1-30 | 3-45 | 6-60 |
NOTE. N = 85.
Median time to complete both the health care professional questionnaire and patient questionnaire for a given subject, and not the summation of median times for completing each questionnaire separately.
Table 4.
Study End Points
| End Point | No. of Patients | % |
|---|---|---|
| Assessable patients | 85 | 100 |
| Patient completes the patient portion of the geriatric assessment tool without assistance | 74 | 87 |
| Health care provider completes the health care provider portion of the geriatric assessment tool | 85 | 100 |
| Patient report questionnaire length satisfactory | 78 | 92 |
| Patient reports no questions too difficult to understand | 81 | 95 |
| Patient reports no questions upsetting | 82 | 96 |
Table 4 shows the degree of patient satisfaction with the self-administered questionnaire. Seventy-eight patients (92%) were satisfied with the questionnaire length, five patients (6%) felt it was long, and two patients (2%) did not respond. Eighty-one patients (95%) said there were no difficult questions; four patients (5%) reported difficult questions, citing the social support items specifically. Eighty-two patients (96%) were not upset by any questions, two patients (2%) reported the mood and social support questions upsetting, and one patient (1%) did not reply to this question.
The number of missing items for each measure in the geriatric assessment was calculated. More than 90% of patients completed all items on their questionnaires (Table 5). Results of the geriatric assessment are summarized in Table 5. Fifteen patients (18%) required assistance with instrumental activities of daily living, 17 patients (19%) reported at least one fall in the previous 6 months; 25 patients (29%) reported ≥ three comorbid illnesses; 20 patients (24%) reported fair or poor hearing, and 47 patients (55%) reported taking five or more medications. Only three patients (3%) scored above the threshold for anxiety/depression on the Hospital Anxiety and Depression Scale.25 Fifteen patients (18%) had greater than 5% unintentional weight loss, and 15 patients (18%) had a body mass index lower than 22 kg/m2.
Table 5.
Geriatric Assessment Results
| Domain With Measure | Mean | SD | Median | Range | Patients With Incomplete Data |
|
|---|---|---|---|---|---|---|
| No. | % | |||||
| Functional status | ||||||
| MOS physical health (scale 0 to 100) | 82 | 16.8 | 85 | 15-100 | 4 | 5 |
| Instrumental activities of daily living (scale 0 to 14) | 13.8 | 0.7 | 14 | 9-14 | 0 | 0 |
| Physician-rated Karnofsky performance status (scale 0 to 100) | 94.8 | 8.2 | 100 | 60-100 | 2 | 2 |
| Self-rated Karnofsky performance status (scale 40 to 100) | 89.5 | 12.8 | 90 | 40-100 | 2 | 2 |
| No. of falls in last 6 months | 0.3 | 0.7 | 0 | 0-3 | 3 | 4 |
| Timed Up and Go, seconds | 12 | 6.6 | 10 | 6-56 | 1 | 1 |
| MOS social activities (scale 0 to 100) | 66 | 18.3 | 75 | 0-94 | 1 | 1 |
| Comorbid medical conditions | ||||||
| No. of comorbid medical conditions (physical health section [subscale of the OARS]) | 2.0 | 1.6 | 2 | 0-5 | 2 | 2 |
| Psychological state | ||||||
| Hospital Anxiety and Depression Scale (scale 0 to 42) | 5.8 | 4.5 | 5 | 0-22 | 1 | 1 |
| Social support | ||||||
| MOS social support survey: emotional/information and tangible subscales | 86 | 21.6 | 98 | 15-100 | 3 | 4 |
| Nutritional status | ||||||
| Body mass index | 26.8 | 5.8 | 26 | 11-47 | 0 | 0 |
| Percent weight loss in last 6 months | 2.2 | 9.2 | 0 | 66% loss to 9% gain | 7 | 8 |
| Cognition | ||||||
| Blessed Orientation-Memory-Concentration test (scale 0 to 28) | 2.6 | 2.8 | 2 | 0-10 | 0 | 0 |
| Medications | ||||||
| No. of medications | 5.6 | 3.4 | 5 | 0-20 | 0 | 0 |
NOTE. Report is based on 85 assessable patients.
Abbreviations: SD, standard deviation; MOS, Medical Outcomes Study; OARS, Older American Resources and Services.
DISCUSSION
Approximately 60% of cancer diagnoses and 70% of cancer mortality occur in patients age ≥ 65.32 Studies have demonstrated that older adults have been under-represented in cancer clinical trials, although more recent data suggest that these statistics are starting to improve.33,34 Because characteristics other than age of older adults enrolled in these trials are not routinely captured, there is a dearth of knowledge regarding the factors other than age that identify vulnerable older adults at risk for treatment toxicity. We studied the feasibility of implementing a geriatric assessment in the cooperative group setting. Previously described barriers to incorporating a geriatric assessment in oncology care included the required time and resources. Therefore, we developed a geriatric assessment tool that could be largely self-administered with minimal provider time involved.
The rationale for the inclusion of a geriatric assessment in cooperative group clinical trials is several fold. First, since aging is a heterogeneous process, factors covered by a geriatric assessment, other than chronological age, can provide researchers with information on the overall baseline status of older individuals enrolled in their clinical trials.27,28,35–43 This information gives investigators an opportunity to account for factors other than cancer that put the older patient at risk for morbidity and mortality. Second, inclusion of a geriatric assessment provides a descriptor of the individuals enrolled on the clinical trial. Therefore, physicians in practice can have a better understanding of whether the patients included on the clinical trial have similar characteristics to the patients who they are treating in daily clinical practice. Most importantly, the geriatric assessment provided clinical information that might otherwise go unrecognized. For example, 5% of the patients enrolled on this study scored above threshold for cognitive impairment on the memory test, and these patients had signed consent to participate in a cooperative group treatment trial. This information was reported to the treating physicians so that they could determine whether any further neurologic work-up was needed. Finally, inclusion of a geriatric assessment in clinical trials could potentially identify the factors which predispose older patients to treatment toxicity. This information would be used as the basis for developing the next generation of clinical trials for vulnerable older adults that would incorporate interventions or novel treatment approaches to decrease the risk of treatment toxicity.
Several geriatric assessments have been proposed in the literature.17,44–46 Most include the domains described in this geriatric assessment, and the authors acknowledge that any of these approaches would be reasonable. However, inclusion of uniform measures across studies would increase the ease and applicability of cross-study comparison, and validate the assessment's predictive capabilities. The geriatric assessment tool described in this article includes validated and reliable measures, is primarily self-administered, requires little health care provider time and resources for completion, and was acceptable in length and in content to most patients. Nurses and research assistants primarily completed the health care provider portion. The assessment includes measures that capture a broad range of physical function as individuals who are seeking cancer treatment or treatment on clinical trial may be healthier than the general geriatric population. Furthermore, this assessment was easily incorporated into a cooperative group setting.
There are limitations to this geriatric assessment tool. It is brief and therefore may miss subtle findings that a more comprehensive assessment might detect. In addition, some items require a health care provider's attention; however, the time required to complete these items is brief. The time intervals to complete the assessment were self-reported, and the validity needs to be considered in that context; however, the average times to completion are reported as medians so that the degree of under- or over-reporting by individuals would have lesser impact. Furthermore, although patient satisfaction with the geriatric assessment tool was captured, the health care provider's satisfaction was not captured. Although most of the measures are self-explanatory, the principal investigator trained those who administered the assessment in order to increase the reliability of the data. The training was quick, however, and was completed by telephone. This study was performed at 15 CALGB sites (ie, limited access study). This limited the accrual rate. In addition, the study population consisted of older adults who enrolled on cooperative group studies which could potentially limit the generalizability of the results; however, other studies utilizing this assessment tool in a broader population of older adults not enrolled on a clinical trial have demonstrated feasibility.19,47 Lastly, few minority patients were included in this trial and black patients were more likely to decline participation. The under-representation of minority populations among older adults enrolled on National Cancer Institute sponsored trials has been previously described.48 Additional studies are needed to understand the rationale for this finding. In addition, further studies are needed to assess the feasibility of this geriatric assessment in minority populations.
Plans to further develop the geriatric assessment tool are under way. The Cancer and Aging Research Group49 has accrued more than 600 older adults with cancer to a study evaluating the geriatric assessment tool's ability to predict the risk of toxicity to chemotherapy. The assessment has also been incorporated into a cooperative group study that evaluates hormone therapy with or without bevacizumab in postmenopausal patients with metastatic cancer. The assessment is captured at baseline and in longitudinal follow-up. Several other CALGB treatment studies under development are also incorporating this geriatric assessment. The feasibility of obtaining geriatric assessment information via touch-screen computer methodology is also under study. The next generation of studies will profit from results of this research to help guide interventions or to modify treatment plans in order to decrease the risk of toxicity while maintaining therapeutic efficacy in a growing population of older adults with cancer.
Acknowledgment
The following institutions participated in this study: Dana-Farber Cancer Institute, Boston, MA–Harold J Burstein, MD, PhD, supported by CA32291; Duke University Medical Center, Durham, NC–Jeffrey Crawford, MD, supported by CA47577; Hematology-Oncology Associates of Central New York Community Clinical Oncology Program, Syracuse, NY–Jeffrey Kirshner, MD, supported by CA45389; Memorial Sloan-Kettering Cancer Center, New York, NY–Clifford A. Hudis, MD, supported by CA77651; Nevada Cancer Research Foundation Community Clinical Oncology Program, Las Vegas, NV–John A. Ellerton, MD, supported by CA35421; New Hampshire Oncology-Hematology PA, Concord, NH – Douglas J. Weckstein; Northern Indiana Cancer Research Consortium Community Clinical Oncology Program, South Bend, IN–Rafat Ansari, MD, supported by CA86726; Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, MD, supported by CA59518; Southeast Cancer Control Consortium Community Clinical Oncology Program, Goldsboro, NC–James N. Atkins, MD, supported by CA45808; The Ohio State University Medical Center, Columbus, OH–Clara D. Bloomfield, MD, supported by CA77658; University of Chicago, Chicago, IL–Hedy L. Kindler, MD, supported by CA41287; University of Minnesota, Minneapolis, MN–Bruce A. Peterson, MD, supported by CA16450, University of Vermont, Burlington, VT–Steven M. Grunberg, MD, supported by CA77406; Washington University School of Medicine, St Louis, MO–Nancy Bartlett, MD, supported by CA77440.
The authors would like to express their sincere thanks to the following investigators who assisted in patient accrual and the successful execution of the study: Gretchen Kimmick, Bercedis Peterson, Charles Shapiro, John Ellerton, Nancy Bartlett, Amy Abernethy, Vicki Morrison, and James Atkins.
Appendix
Fig A1.
Cancer and Leukemia Group B 360401: flow chart of accrual. B-OMC, Blessed Orientation-Memory-Concentration Test.*Reasons for refusal include not interested (n = 20; 74.1%), too sick (n = 1; 3.7%), too busy (n = 1; 3.7%), confidentiality concerns (n = 1; 3.7%), other (n = 4; 14.8%).
Footnotes
Written on behalf of the Cancer and Leukemia Group B.
Supported in part by Grant No. CA31946 from the National Cancer Institute to the Cancer and Leukemia Group B and by Grant No. CA33601 to the CALGB Statistical Center; by Grants No. CA33601, CA77406, CA32291, CA41287, CA59518, CA86726, CA77651, CA45389, CA47577 from the National Cancer Institute; and by K23 AG026749-01 (Paul Beeson Career Development Award in Aging Research), and American Society of Clinical Oncology–Association of Specialty Professors–Junior Development Award in Geriatric Oncology (A.H.). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00416481.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Arti Hurria, Amgen (C), Genentech (C); Richard Stone, Genzyme (C), Novartis (C), Celgene (C) Stock Ownership: None Honoraria: Richard Stone, Celgene; Ilene Galinsky, Celgene Research Funding: Arti Hurria, Abraxis BioScience, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Arti Hurria, Hyman B. Muss, Alice B. Kornblith, Richard Stone, Harvey Jay Cohen
Financial support: Hyman B. Muss
Administrative support: Arti Hurria, Hyman B. Muss
Provision of study materials or patients: Hyman B. Muss, Andrew S. Artz, Linda Schmieder, Rafat Ansari, William P. Tew, Douglas Weckstein, Jeffrey Kirshner, Richard Stone, Ilene Galinsky
Collection and assembly of data: Arti Hurria, Linda Schmieder, Jeffrey Kirshner, Kayo Togawa, Kurt Hansen, Vani Katheria, John Postiglione
Data analysis and interpretation: Arti Hurria, Constance T. Cirrincione, Hyman B. Muss, William Barry, Andrew S. Artz, Kayo Togawa, Harvey Jay Cohen
Manuscript writing: Arti Hurria, Constance T. Cirrincione, Hyman B. Muss, Alice B. Kornblith, William Barry, Andrew S. Artz, Linda Schmieder, Rafat Ansari, William P. Tew, Douglas Weckstein, Jeffrey Kirshner, Kayo Togawa, Kurt Hansen, Vani Katheria, Richard Stone, Ilene Galinsky, John Postiglione, Harvey Jay Cohen
Final approval of manuscript: Arti Hurria, Constance T. Cirrincione, Hyman B. Muss, Alice B. Kornblith, William Barry, Andrew S. Artz, Linda Schmieder, Rafat Ansari, William P. Tew, Douglas Weckstein, Jeffrey Kirshner, Kayo Togawa, Kurt Hansen, Vani Katheria, Richard Stone, Ilene Galinsky, John Postiglione, Harvey Jay Cohen
REFERENCES
- 1.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 2.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 3.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 5.Yancik R, Ries LA. Aging and cancer in America: Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. 2000;14:17–23. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 6.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 7.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 8.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 9.ten Tije AJ, Verweij J, Carducci MA, et al. Prospective evaluation of the pharmacokinetics and toxicity profile of docetaxel in the elderly. J Clin Oncol. 2005;23:1070–1077. doi: 10.1200/JCO.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 10.Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol. 2005;23:8671–8678. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 11.Crivellari D, Bonetti M, Castiglione-Gertsch M, et al. Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: The International Breast Cancer Study Group Trial VII. J Clin Oncol. 2000;18:1412–1422. doi: 10.1200/JCO.2000.18.7.1412. [DOI] [PubMed] [Google Scholar]
- 12.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 13.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann Oncol. 2005;16:1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 15.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865–6872. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 16.Tucci A, Ferrari S, Bottelli C, et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer. 2009;115:4547–4553. doi: 10.1002/cncr.24490. [DOI] [PubMed] [Google Scholar]
- 17.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 18.Rao AV, Hsieh F, Feussner JR, et al. Geriatric evaluation and management units in the care of the frail elderly cancer patient. J Gerontol A Biol Sci Med Sci. 2005;60:798–803. doi: 10.1093/gerona/60.6.798. [DOI] [PubMed] [Google Scholar]
- 19.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: A feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AL, Ware JE. Durham, NC: Duke University Press; 1992. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. [Google Scholar]
- 21.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 22.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1948. pp. 191–205. [Google Scholar]
- 23.Loprinzi CL, Laurie JA, Wieand HS, et al. Prospective evaluation of prognostic variables from patient-completed questionnaires: North Central Cancer Treatment Group. J Clin Oncol. 1994;12:601–607. doi: 10.1200/JCO.1994.12.3.601. [DOI] [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 27.Landi F, Onder G, Gambassi G, et al. Body mass index and mortality among hospitalized patients. Arch Intern Med. 2000;160:2641–2644. doi: 10.1001/archinte.160.17.2641. [DOI] [PubMed] [Google Scholar]
- 28.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients: Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 29.Newman AB, Yanez D, Harris T, et al. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 30.Kawas C, Karagiozis H, Resau L, et al. Reliability of the Blessed Telephone Information-Memory Concentration Test. J Geriatr Psychiatry Neurol. 1995;8:238–242. doi: 10.1177/089198879500800408. [DOI] [PubMed] [Google Scholar]
- 31.Katzman R, Brown T, Fuld P, et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute. National Cancer Institute; 2008. SEER cancer statistics review, 1975-2005. http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 33.Unger JM, Coltman CA, Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24:141–144. doi: 10.1200/JCO.2005.02.8928. [DOI] [PubMed] [Google Scholar]
- 34.Kimmick GG, Peterson BL, Kornblith AB, et al. Improving accrual of older persons to cancer treatment trials: A randomized trial comparing an educational intervention with standard information: CALGB 360001. J Clin Oncol. 2005;23:2201–2207. doi: 10.1200/JCO.2005.01.222. [DOI] [PubMed] [Google Scholar]
- 35.Narain P, Rubenstein LZ, Wieland GD, et al. Predictors of immediate and 6-month outcomes in hospitalized elderly patients: The importance of functional status. J Am Geriatr Soc. 1988;36:775–783. doi: 10.1111/j.1532-5415.1988.tb04259.x. [DOI] [PubMed] [Google Scholar]
- 36.Reuben DB, Rubenstein LV, Hirsch SH, et al. Value of functional status as a predictor of mortality: Results of a prospective study. Am J Med. 1992;93:663–669. doi: 10.1016/0002-9343(92)90200-u. [DOI] [PubMed] [Google Scholar]
- 37.Barberger-Gateau P, Fabrigoule C, Helmer C, et al. Functional impairment in instrumental activities of daily living: An early clinical sign of dementia? J Am Geriatr Soc. 1999;47:456–462. doi: 10.1111/j.1532-5415.1999.tb07239.x. [DOI] [PubMed] [Google Scholar]
- 38.Seeman TE, Berkman LF, Kohout F, et al. Intercommunity variations in the association between social ties and mortality in the elderly: A comparative analysis of three communities. Ann Epidemiol. 1993;3:325–335. doi: 10.1016/1047-2797(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 39.Kornblith AB, Herndon JE, Jr, Zuckerman E, et al. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer. 2001;91:443–454. doi: 10.1002/1097-0142(20010115)91:2<443::aid-cncr1020>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 40.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 41.Eagles JM, Beattie JA, Restall DB, et al. Relation between cognitive impairment and early death in the elderly. BMJ. 1990;300:239–240. doi: 10.1136/bmj.300.6719.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfson C, Wolfson DB, Asgharian M, et al. A re-evaluation of the duration of survival after the onset of dementia. N Engl J Med. 2001;344:1111–1116. doi: 10.1056/NEJM200104123441501. [DOI] [PubMed] [Google Scholar]
- 43.Landi F, Zuccala G, Gambassi G, et al. Body mass index and mortality among older people living in the community. J Am Geriatr Soc. 1999;47:1072–1076. doi: 10.1111/j.1532-5415.1999.tb05229.x. [DOI] [PubMed] [Google Scholar]
- 44.Ingram SS, Seo PH, Martell RE, et al. Comprehensive assessment of the elderly cancer patient: The feasibility of self-report methodology. J Clin Oncol. 2002;20:770–775. doi: 10.1200/JCO.2002.20.3.770. [DOI] [PubMed] [Google Scholar]
- 45.Monfardini S, Ferrucci L, Fratino L, et al. Validation of a multidimensional evaluation scale for use in elderly cancer patients. Cancer. 1996;77:395–401. doi: 10.1002/(SICI)1097-0142(19960115)77:2<395::AID-CNCR24>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 46.Molina-Garrido MJ, Guillen-Ponce C. Development of a cancer-specific Comprehensive Geriatric Assessment in a University Hospital in Spain. Crit Rev Oncol Hematol. 2011;77:148–161. doi: 10.1016/j.critrevonc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Hurria A, Mohile S, Lichtman S, et al. Geriatric assessment of older adults with cancer: Baseline data from a 500-patient multicenter study. J Clin Oncol. 2009;27(suppl):494s. abstr 9546. [Google Scholar]
- 48.Gross CP, Herrin J, Wong N, et al. Enrolling older persons in cancer trials: The effect of sociodemographic, protocol, and recruitment center characteristics. J Clin Oncol. 2005;23:4755–4763. doi: 10.1200/JCO.2005.14.365. [DOI] [PubMed] [Google Scholar]
- 49.Hurria A, Balducci L, Naeim A, et al. Mentoring junior faculty in geriatric oncology: Report from the Cancer and Aging Research Group. J Clin Oncol. 2008;26:3125–3127. doi: 10.1200/JCO.2008.16.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]