Abstract
Purpose
The addition of rituximab to fludarabine-based regimens in chronic lymphocytic leukemia (CLL) has been shown to produce high response rates with extended remissions. The long-term follow-up of these regimens with respect to progression, survival, risk of secondary leukemia, and impact of genomic risk factors has been limited.
Methods
We report the long-term follow-up of the chemoimmunotherapy trial CALGB 9712 from the Cancer and Leukemia Group B, for which treatment regimen was previously reported, to examine end points of progression-free survival (PFS), overall survival (OS), impact of genomic features, and risk of therapy-related myeloid neoplasm (t-MN).
Results
A total of 104 patients were enrolled on this study and now have a median follow-up of 117 months (range, 66 to 131 months). The median OS was 85 months, and 71% of patients were alive at 5 years. The median PFS was 42 months, and 27% were progression free at 5 years. An estimated 13% remained free of progression at almost 10 years of follow-up. Multivariable models of PFS and OS showed that immunoglobulin heavy chain variable region mutational status was significant for both, whereas cytogenetic abnormalities were significant only for OS. No patient developed t-MN before relapse.
Conclusion
Long-term follow-up of CALGB 9712 demonstrates extended OS and PFS with fludarabine plus rituximab. Patients treated with fludarabine plus rituximab administered concurrently or sequentially have a low risk of t-MN. These long-term data support fludarabine plus rituximab as one acceptable first-line treatment for symptomatic patients with CLL.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most prevalent type of adult leukemia for which there is no curative treatment outside of stem-cell transplantation. The lack of a curative therapy for CLL and lack of benefit to early treatment have led investigators to initiate therapy only in symptomatic disease. For many years, treatment consisted of alkylator-based therapies. Subsequent randomized, phase III trials have demonstrated both improved response and progression-free survival (PFS) with single-agent fludarabine1–5 in younger patients with CLL, whereas those older than 65 years do not appear to benefit from this.4 In addition, a recent, long-term follow-up of Cancer and Leukemia Group B study CALGB 9011 demonstrated extended overall survival (OS) in previously untreated patients with symptomatic CLL receiving fludarabine compared with chlorambucil.5 After the introduction of single-agent fludarabine, multiple studies that used fludarabine and cyclophosphamide in combination have shown improved response and PFS compared with treatment with fludarabine alone.6–8 Long-term follow-up for survival or complications from these fludarabine and cyclophosphamide combination regimens has not been reported. In particular, the risk of therapy-related myeloid neoplasm (t-MN) has not been examined.
Concurrent with the investigation of combination chemotherapy, rituximab, a chimeric monoclonal antibody targeting CD20, was introduced in the treatment of CLL. Initial studies with rituximab used a lymphoma-derived weekly administration schedule that showed modest activity in relapsed CLL.9–11 Later studies with rituximab have shown more activity when used with a more frequent administration schedule,12 at higher doses,13 or in combination with other active agents. Notably, combinations of rituximab with fludarabine (FR)14,15 or with fludarabine plus cyclophosphamide (FCR)16,17 have shown high response rates and extended PFS. A randomized, phase III trial demonstrated that FCR chemotherapy prolongs survival compared with fludarabine plus cyclophosphamide alone. Data from this study showed an overall response rate (ORR) of 95% with a complete response (CR) rate of 44%. With a follow-up of 37.7 months, median PFS was 51.8 months, and survival rate at this time point was 84.1%.18 Despite this survival advantage, it is notable that long-term follow-up of another FCR study (median follow-up, 6 years) showed that eight of 300 patients developed t-MN.19 In this report, we will describe the long-term follow-up of patients treated with FR on CALGB study 9712. The purpose of this analysis is to examine long-term PFS and OS with FR chemoimmunotherapy, examine characteristics associated with extended remission and PFS, and assess the frequency of developing t-MN.
METHODS
Patients and Assessment of Prognostic Factors
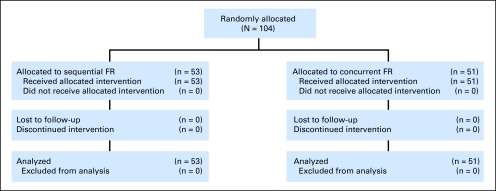
Patients were enrolled on CALGB 9712 and the corresponding tissue bank study CALGB 9665 after written informed consent was provided. The eligibility criteria for CALGB 9712 are described elsewhere14 and included untreated, symptomatic CLL as defined by the National Cancer Institute (NCI) 1996 guidelines (CONSORT, Fig 1).20 This study randomly assigned 104 patients to receive FR either sequentially (n = 53) or concurrently (n = 51). Patients were enrolled between May 1998 and January 2000; the initial data were reported in 2003.14 CR and partial response (PR) were defined by NCI 1996 criteria. ORR included CR + PR. Genomic risk factors, including interphase cytogenetics and immunoglobulin heavy chain variable region (IgVH) mutational analysis, were also analyzed as previously described.21 Long-term follow-up data for this report were obtained from the CALGB database and from patient flow sheets from individual institutions. The data used for this analysis were current as of October 2009.
Fig 1.
CONSORT diagram. FR, rituximab with fludarabine.
Statistical Considerations
OS was defined as the time from enrollment to the date of death, censoring those alive at last follow-up. PFS was defined as the time from enrollment to the date of disease progression or death, censoring patients alive and progression free at last follow-up. Two patients who withdrew from study to receive an alternative regimen before experiencing progression were censored at that time. Estimates of PFS and OS were obtained by the Kaplan-Meier method, and the log-rank test was used to compare differences between survival curves according to molecular marker status. To additionally test the association of molecular markers with outcome, proportional hazards models were used to evaluate the impact of IgVH mutational status and the presence or absence of poor risk cytogenetic markers del(17p13.1)/del(11q22.3) on outcome, adjusting for age, sex, Rai stage (high/intermediate), WBC count, lactate dehydrogenase (LDH) and splenomegaly (yes/no). Hazard ratios and 95% CIs were estimated from the models. Statistical significance was declared at P < .05. All analyses were performed by the CALGB Statistical Center.
RESULTS
Patients and Long-Term Outcome
All 104 untreated symptomatic patients enrolled on CALGB 9712 were included in this analysis. Pertinent demographic data and response data have been reported previously.14 Briefly, 77% of the patients were men, the median age was 63 years (range, 36 to 86 years), and 40% had high-risk Rai stage disease. The total ORR was 84% (95% CI, 77% to 91%). The ORR in the sequential arm (n = 53) was 77% (95% CI, 66% to 89%), and the CR rate was 28%. The ORR in the concurrent arm (n = 51) was 90% (95% CI, 82% to 98%), and the CR rate was 47%.
After a median follow-up of 117 months (range, 66 to 131 months), the median PFS and OS times for the treatment groups were similar, with an overall estimated median PFS of 42 months (95% CI, 31 to 46 months) and median OS of 85 months (95% CI, 71 to 95 months; Table 1; Fig 2). Disease progression occurred in 73 of the patients, and 15 died without documented progression. Only two patients were censored before 5 years, and both had withdrawn from study before progression or death. At 5 years, the estimated PFS rate was 28% (95% CI, 19% to 37%). Deaths occurred in 67 patients, including the two who enrolled on another study before an event on this trial. One patient died after 106 months on study, after disease progression occurred at 75 months. With respect to OS, none of the patients were censored before 5 years; 71% were still alive (95% CI, 61% to 79%) at that time.
Table 1.
Clinical Outcome for 104 Patients Enrolled on CALGB 9712 by IgVH Mutational Status and Cytogenetic Group
| Group | No. of Patients | Progression-Free Survival (months) |
Overall Survival (months) |
||||
|---|---|---|---|---|---|---|---|
| Median | 95% CI | P | Median | 95% CI | P | ||
| All Patients | 104 | 42 | 31 to 46 | NA | 85 | 71 to 95 | NA |
| IgVH status | .01 | .01 | |||||
| Unmutated | 47 | 33 | 25 to 44 | 78 | 57 to 105 | ||
| Mutated | 36 | 52 | 35 to 88 | NR | 85 to NR | ||
| Cytogenetics | .046 | .02 | |||||
| del(11q)/del(17p) | 18 | 25 | 16 to 44 | 63 | 44 to 79 | ||
| Other | 70 | 45 | 25 to 55 | 105 | 84 to NR | ||
Abbreviations: CALGB, Cancer and Leukemia Group B; IgVH, immunoglobulin heay chain variable region; NA, not applicable; NR, not reached.
Fig 2.
Kaplan-Meier estimates of progression-free survival (PFS) and overall survival (OS) for all 104 patients on Cancer and Leukemia Group B study CALGB 9712.
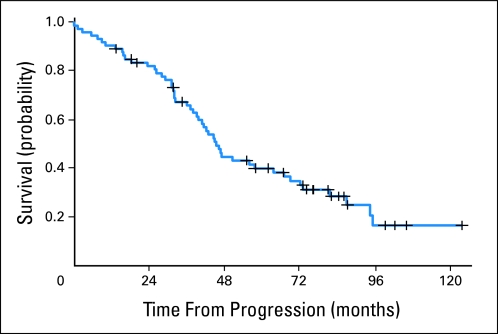
After disease progression, the survival from progression until death was analyzed to determine the utility of salvage therapies in these patients. The median time from progression until death was 45 months (95% CI, 39 to 67 months). Approximately 40% of patients (95% CI, 29% to 52%) were still alive 60 months after experiencing progression (Fig 3).
Fig 3.
Kaplan-Meier estimates of overall survival after progression.
IgVH Mutational Status and Interphase Cytogenetics
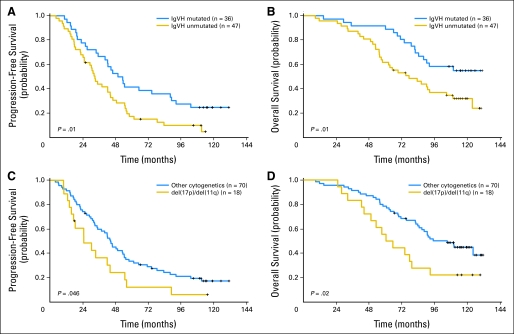
IgVH mutational analysis was performed on 83 pretreatment patient samples; 43% demonstrated mutated IgVH, and 57% were unmutated. Those patients with unmutated IgVH had a significantly poorer PFS compared with those who had mutated IgVH (median, 33 v 52 months; P = .01; Table 1; Fig 4A). Patients with unmutated IgVH also had a significantly worse OS compared with those who had mutated IgVH (median, 78 months v not reached; P = .01; Fig 4B). In multivariable models for PFS and OS, IgVH mutational status remained statistically significant (P = .02 and P = .004, respectively; Table 2).
Fig 4.
(A) Kaplan-Meier estimates of progression-free survival (PFS) in patients with mutated immunoglobulin heavy chain variable region (IgVH) versus unmutated IgVH. (B) Kaplan-Meier estimates of overall survival (OS) in patients with mutated IgVH versus unmutated IgVH. (C) Kaplan-Meier estimates of PFS in patients with del(17p13.1)/del(11q22.3) versus those without these poor-risk abnormalities. (D) Kaplan-Meier estimates of OS in patients with del(17p13.1)/del(11q22.3) versus those without these poor-risk abnormalities.
Table 2.
Multivariable Models for Progression-Free Survival and Overall Survival
| End Point | No. of Patients | Hazard Ratio | 95% CI | P |
|---|---|---|---|---|
| PFS | ||||
| Model I: IgVH status, unmutated v mutated | 78 | 1.9 | 1.1 to 3.2 | .02 |
| Model II: cytogenetics, del(17p)/ del(11q) v other | 83 | 1.9 | 1.0 to 3.6 | .06 |
| OS | ||||
| Model I: IgVH status, unmutated v mutated | 78 | 2.6 | 1.4 to 5.1 | .004 |
| Model II: cytogenetics, del(17p)/del(11q) v other | 83 | 2.3 | 1.2 to 4.4 | .01 |
NOTE. Each of the four models was adjusted for age, sex, Rai stage (high/intermediate), log-transformed white blood cell count, log-transformed lactate dehydrogenase, and splenomegaly (yes/no). Hazard ratios greater than or less than 1 correspond to an increased or decreased risk, respectively, of an event for the first category listed for dichotomous variables.
Abbreviations: IgVH, immunoglobulin heavy chain variable region; PFS, progression-free survival; OS, overall survival.
Fluorescence in situ hybridization was performed on 88 pretreatment patient samples. Poor risk cytogenetic abnormalities del(17p13.1) or del(11q22.3) were detected in 18 patients (20%). Three had del(17p13.1). These 3 patients had disease progression after 15, 18, and 89 months; two died at 28 and 49 months, and the third patient is still alive at 124 months. Compared with the 70 patients without poor risk cytogenetic abnormalities, those 18 with these abnormalities had significantly worse PFS (median, 25 v 45 months, P = .046) and OS (median, 63 v 105 months, P = .02) (Table 1; Figs 4C and 4D, respectively). Poor risk cytogenetic abnormalities were moderately associated with worse PFS, when controlling for other variables (P = .06) and remained a significant predictor for poor OS (P = .01) in a multivariable model (Table 2).
Characteristics of Patients in Long-Term remission
The extended follow-up of this study allowed assessment of pretreatment features associated with prolonged remission and survival. Although 14 patients enrolled on this study remain progression free and had a median follow-up of 114 months, we also found that 18 patients had a progression-free interval of longer than 84 months. At the other extreme, 34 patients experienced progression within 24 months of study enrollment. We analyzed pretreatment factors of these two groups in an attempt to elucidate factors predictive of extended remission duration. The characteristics of patients with greater than 84 months and less than 24 month of PFS are listed in Table 3 . Notably, these groups did not differ on the basis of age, WBC count, Rai stage, time from diagnosis to treatment, or cytogenetic group. Patients with long-term remission were more likely to be IgVH mutated, and patients with short remission were more likely to be IgVH unmutated. Response to therapy was also predictive of long-term remission. Importantly, though, of the 18 patients with long-term remission, only 50% achieved a CR by NCI 1996 criteria. One patient with long-term remission did not have a post-therapy bone marrow evaluation, so the patient was classified as a PR for this purpose. This patient had normalization of WBC and underwent splenectomy during therapy with no evidence of CLL within the spleen. Baseline and response characteristics were evaluated additionally for those eight patients who had long-term remission after achieving PR with FR chemoimmunotherapy. When using Dohner prioritization, one patient had del(17p13.1), four had trisomy 12, 2 had del(13q14.3), and one had normal cytogenetics. All but one of these patients had mutated IgVH. Six patients had palpable adenopathy before therapy, and none had adenopathy after treatment. Four patients had pretreatment splenomegaly. After therapy, one patient had no bone marrow involvement but had prolonged thrombocytopenia and would be classified as CR with incomplete marrow recovery by International Workshop on Chronic Lymphocytic Leukemia 2008 criteria.22 One patient had no morphologic evidence of CLL but did have minimal residual disease (MRD) by flow cytometry. Two patients had nodular PR: one of these had one to three lymphoid aggregates by morphology and negative flow cytometry evaluation; the other patient had one lymphoid aggregate and 25% lymphocytes in the marrow. Two patients had 10% to 15% marrow involvement, one had 30% to 40% marrow involvement, and one had 51% involvement after therapy.
Table 3.
Characteristics of Patients With Remission Duration < 24 Months or > 84 Months
| Clinical Feature | Patients With Remission Duration < 24 Months(n = 34) | Patients With Remission Duration > 84 Months(n = 18) | P* |
|---|---|---|---|
| Age, years | .66 | ||
| Median | 63 | 61 | |
| Range | 36-86 | 40-71 | |
| Male | 1.00 | ||
| No. | 25 | 13 | |
| % | 74 | 72 | |
| Leukocyte count | .06 | ||
| Median | 104.9 | 64.9 | |
| Range | 10.6-436.0 | 8.8-259.5 | |
| Hemoglobin, g/dL | .95 | ||
| Median | 12.6 | 12.5 | |
| Range | 6.3-15.1 | 8.3-15.5 | |
| Lactate dehydrogenase | .91 | ||
| Median | 212 | 213 | |
| Range | 107-950 | 102-838 | |
| β2-microglobulin | .98 | ||
| Median | 3.5 | 3.5 | |
| Range | 1.7-8.2 | 0.5-24.3 | |
| Rai stage | |||
| I/II | .78 | ||
| No. | 17 | 10 | |
| % | 50 | 56 | |
| III/IV | |||
| No. | 17 | 8 | |
| % | 50 | 44 | |
| IgVH | |||
| Mutated | .01 | ||
| No. | 8 | 13 | |
| % | 35 | 76 | |
| Unmutated | |||
| No. | 15 | 4 | |
| % | 65 | 24 | |
| Cytogenetics | |||
| del(17p) or del(11q) | .26 | ||
| No. | 7 | 2 | |
| % | 29 | 12 | |
| Other | |||
| No. | 17 | 15 | |
| % | 71 | 88 | |
| Months from diagnosis to treatment | .14 | ||
| Median | 4.1 | 16.3 | |
| Range | 0.1-113.1 | 0.3-156.1 | |
| Overall response | |||
| Complete | .002 | ||
| No. | 6 | 9 | |
| % | 18 | 50 | |
| Partial | |||
| No. | 15 | 9 | |
| % | 44 | 50 | |
| None | |||
| No. | 13 | 0 | |
| % | 38 | 0 |
The association between group and clinical feature was tested by using Fisher's exact or Wilcoxon rank sum tests for categoric and continuous variables, respectively.
Abbreviation: IgVH, immunoglobulin heavy chain variable region.
Therapy-Related Myeloid Neoplasm and Richter's Transformation
Prior small studies have suggested that there is a higher than baseline incidence of both myelodysplastic syndrome (MDS) and acute myeloid leukemia as well as Richter's transformation after receipt of fludarabine and/or fludarabine and cyclophosphamide–based therapy for CLL.23–25 As part of this long-term follow-up study, we examined the frequency of such events. No patient developed t-MN during remission or experienced early relapse after FR. One patient did develop therapy-related MDS; however, this occurred 45 months after FR treatment and 9 months after salvage therapy with FCR. Until the MDS was diagnosed, no bone marrow biopsies showed any evidence of myelodysplasia. No patient has developed acute myeloid leukemia. Four patients underwent Richter's transformation, three to large B-cell lymphomas and one to Hodgkin's lymphoma. Of the patients who experienced transformation, one had no response to FR and experienced transformation less than 3 months after study entry. One patient achieved a PR with FR and experienced transformation as the first evidence of disease progression 6 years after study entry. One patient achieved a PR with FR but experienced relapse 10 months after study entry. He was treated with four cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone before transformation at 7 months after progression. These three died within 1 year of transformation. The last patient achieved a PR with FR and experienced relapse 50 months after study entry. He experienced transformation to large B-cell lymphoma 10 months after relapse and died 30 months after transformation.
DISCUSSION
Here we have described the long-term follow-up of patients enrolled on CALGB 9712, one of the first chemoimmunotherapy protocols initiated in which fludarabine was combined with rituximab, administered either sequentially or concurrently, in previously untreated, patients with symptomatic CLL. An earlier report by our group26 showed improved overall response, CR, PFS, and OS compared with those previously reported with fludarabine alone. This report extends follow-up an additional 5 years for these patients and demonstrates continued durable remissions in a subset of these patients, with an estimated 13% of patients remaining free of disease progression at almost 10 years of follow-up. We have also found that the median time from progression until death was 45 months, which shows the efficacy of salvage therapies in these patients. As this study was not designed to capture information related to salvage therapies, additional studies will be needed to determine the efficacy of specific salvage regimens. As identified previously,21 patients with CLL treated with FR who have IgVH-mutated disease or who lack del(11q22.3) or del(17p13.1) abnormalities have better outcomes. In separate multivariable analyses, unmutated IgVH was significantly associated with inferior PFS and OS compared with mutated IgVH, whereas the presence of poor-risk cytogenetic abnormalities del(17p13.1) and del(11q22.3) tended to be associated with poor PFS and significantly associated with poor OS compared with those not harboring these cytogenetic abnormalities. In the models for OS, age was the only other variable significant with P < .05, and, in the models for PFS, no other variable was significant. Because poor-risk cytogenetics tend to be associated with unmutated IgVH, a prospective study with larger numbers of patients would be required to determine the relative contributions of these two variables.
The application of more aggressive therapy in all types of cancer, including CLL, can have implications for long-term survivorship including development of secondary leukemia. In a long-term follow-up study of first-line FCR therapy, with a median follow-up of 6 years, there were eight occurrences of MDS (2.8%) that occurred during first remission.19 Studies examining fludarabine and chlorambucil therapies or consolidative autologous stem-cell transplantation in CLL have also reported on the risk of t-MN.23,27,28 t-MN is a devastating complication of therapy, is not easily treated, and most often leads to death. One possible advantage to initial chemoimmunotherapy without the use of alkylating agents may be the lower risk of this complication. Consideration of t-MN is particularly relevant to low-risk genomic groups that may achieve significant benefit from less aggressive regimens. Notably, no patient receiving FR chemoimmunotherapy on this trial developed t-MN during their progression-free interval.
We examined the pretreatment characteristics of patients who experienced extended remission duration after treatment with FR and compared these patients with those who had short remission duration. The only discriminating pretreatment feature was IgVH mutational status; those patients with extended remissions were more likely to have IgVH-mutated disease, and those with short remission duration were likely to have IgVH-unmutated disease. Notably, 50% of these patients did not attain a CR as defined by the NCI 1996 criteria20 and, yet, still experienced extended remission duration. Previous studies have examined the impact of MRD on PFS and OS. In a retrospective study of patients with relapsed disease who were receiving alemtuzumab, eradication of MRD was associated with improved OS at a median follow-up of 36 months.29 Another study of 137 patients who had been treated with a variety of regimens showed that, with a median follow-up of 3.1 years, patients without MRD by flow cytometry had improved OS and that this distinction was clearer for patients undergoing first treatment.30 Similarly, at 6 years follow-up after FCR therapy, patients without MRD had prolonged time to progression and OS.19 These data are slightly incongruent with what we have observed in this study, in which 39% of patients with long-term remissions had residual disease on post-therapy bone marrow evaluation. Much of the published MRD data comes from patients who have experienced relapsed disease, and follow-up on these studies is often significantly shorter than this report. Patients experiencing relapse within a short time of initial treatment and also included in relapsed studies are often enriched with IgVH-unmutated disease. Our findings in this study with extended follow-up suggest that a subset of patients with IgVH-mutated disease do not require disease eradication for long-term disease control, and the identification of these patients pretreatment would be clinically important. Additional study is ongoing into molecular characterization and more detailed analysis of these patients.
How do the long-term follow-up data from this study factor into our current treatment approach of symptomatic, previously untreated patients with CLL today? A randomized, phase III study comparing FCR with FC demonstrated improved CR, ORR, PFS, and OS with combination chemoimmunotherapy.18 Benefit in that study was observed for most genetic groups except for patients with del(17p13.1) or normal karyotypes. Other studies examining either FCR- or FC-based therapy have demonstrated that patients with del(11q22.3) gain benefit from the fractioned cyclophosphamide, changing this genomic factor to a neutral predictive prognostic factor.31 In contrast, in this study, for which no alkylating agent was included, patients with del(11q22.3) did not do as well as those without this cytogenetic marker. This is similar to other fludarabine-based studies that did not include an alkylating agent and substantiates the claim that patients with del(11q22.3) should be treated with FCR as a first-line regimen. Given the greater acute toxicity and the long-term risks of t-MN after FCR-based therapy, a reasonable approach might be to consider FR for low-risk genomic patients, whereas patients with CLL and with del(11q22.3) should receive FCR therapy. High ORR and CR rates as well as extended PFS have been seen with both FCR and FR, and it is as yet unclear which regimen is superior for individual patients in intermediate risk groups. Differences in follow-up make direct comparison of published studies difficult. However, a randomized, US intergroup study (CALGB 10404) is examining this important scientific question.
In summary, the long-term data from CALGB 9712 demonstrate that chemoimmunotherapy with FR is associated with extended PFS and OS in a subset of patients and is not associated with t-MN. These data support FR chemoimmunotherapy as one appropriate first-line therapy for patients with low-risk CLL.
Footnotes
Written on behalf of the Cancer and Leukemia Group B (Chicago, IL).
Supported in part by Grant No. CA31946 from the National Cancer Institute to the Cancer and Leukemia Group B (R.L.S., Chairman) P50 CA140158-02 (J.C.B.), and from the Leukemia and Lymphoma Society (J.C.B.), D. Warren Brown Foundation (J.C.B.), Karches Family Foundation (K.R.R.), Nash Family Foundation (K.R.R.), Peter J. Sharp Foundation (K.R.R.), and Prince Family Foundation (K.R.R.).
Presented in part at the 51st American Society of Hematology Annual Meeting, December 5-8, 2009, New Orleans, LA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: John G. Gribben, Roche/Genentech, Celgene, Mundipharma; John C. Byrd, Gentech Research Funding: John C. Byrd, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Kanti R. Rai, Richard A. Larson, John C. Byrd
Financial support: Richard A. Larson, John C. Byrd
Administrative support: Vicki A. Morrison, Richard A. Larson,John C. Byrd
Provision of study materials or patients: Nyla A. Heerema, John G. Gribben, Vicki A. Morrison, Kanti R. Rai, Richard A. Larson,John C. Byrd
Collection and assembly of data: Jennifer A. Woyach, Amy S. Ruppert, Nyla A. Heerema, Vicki A. Morrison, Kanti R. Rai, Richard A. Larson, John C. Byrd
Data analysis and interpretation: Jennifer A. Woyach, Amy S. Ruppert, Nyla A. Heerema, Bercedis L. Peterson, Vicki A. Morrison, Kanti R. Rai, Richard A. Larson, John C. Byrd
Manuscript writing: Jennifer A. Woyach, Amy S. Ruppert, Nyla A. Heerema, Bercedis L. Peterson, John G. Gribben, Vicki A. Morrison, Kanti R. Rai, Richard A. Larson, John C. Byrd
Final approval of manuscript: Jennifer A. Woyach, Amy S. Ruppert, Nyla A. Heerema, Bercedis L. Peterson, John G. Gribben, Vicki A. Morrison, Kanti R. Rai, Richard A. Larson, John C. Byrd
REFERENCES
- 1.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, Smith AG, Löffler H, et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia: The French Cooperative Group on CLL. Lancet. 1996;347:1432–1438. doi: 10.1016/s0140-6736(96)91681-5. [DOI] [PubMed] [Google Scholar]
- 3.Leporrier M, Chevret S, Cazin B, et al. Randomized comparison of fludarabine, CAP, and ChOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001;98:2319–2325. doi: 10.1182/blood.v98.8.2319. [DOI] [PubMed] [Google Scholar]
- 4.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 5.Rai K, Peterson BL, Appelbaum FR, et al. Long-term survival analysis of the north american intergroup study c9011 comparing fludarabine and chlorambucil in previously untreated patients with chronic lymphocytic leukemia. Blood. 2009;114:536. abstr 536. [Google Scholar]
- 6.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 7.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 8.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 9.Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: A phase II trial of the Minnie Pearl Cancer Res Network. J Clin Oncol. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Itälä M, Geisler CH, Kimby E, et al. Standard-dose anti-CD20 antibody rituximab has efficacy in chronic lymphocytic leukaemia: Results from a Nordic multicentre study. Eur J Haematol. 2002;69:129–134. doi: 10.1034/j.1600-0609.2002.02786.x. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin P, Grillo- López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 14.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: Results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 15.Schulz H, Klein SK, Rehwald U, et al. Phase 2 study of a combined immunochemotherapy using rituximab and fludarabine in patients with chronic lymphocytic leukemia. Blood. 2002;100:3115–3120. doi: 10.1182/blood-2002-03-0972. [DOI] [PubMed] [Google Scholar]
- 16.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 17.Stilgenbauer S, Zenz T, Winkler D, et al. Genomic aberrations, VH mutation status and outcome after fludarabine and cyclophosphamide (FC) or FC plus rituximab (FCR) in the CLL8 trial. Blood. 2008;112:781. abstr 781. [Google Scholar]
- 18.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 19.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 21.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: Justification for risk-adapted therapy. J Clin Oncol. 2006;24:437–443. doi: 10.1200/JCO.2005.03.1021. [DOI] [PubMed] [Google Scholar]
- 22.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: Results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 24.Morrison VA. In reply to: Fludarabine-related myeloid leukemia. J Clin Oncol. 2003;21:3709–3710. doi: 10.1200/JCO.2003.99.090. [DOI] [PubMed] [Google Scholar]
- 25.Lam CC, Ma ES, Kwong YL. Therapy-related acute myeloid leukemia after single-agent treatment with fludarabine for chronic lymphocytic leukemia. Am J Hematol. 2005;79:288–290. doi: 10.1002/ajh.20340. [DOI] [PubMed] [Google Scholar]
- 26.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: An updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 27.Sutton L, Chevret S, Maloum K, et al. Autologous stem cell transplantation (ASCT) in CLL. results of a phase III randomized multicenter trial. Blood. 2009:878. abstr 878. [Google Scholar]
- 28.Gribben JG, Zahrieh D, Stephans K, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005;106:4389–4396. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreton P, Kennedy B, Lucas G, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23:2971–2979. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Kwok MRA, Varghese A, Hillmen P. Minimal residual disease is a predictor for progression-free and overall survival in chronic lymphocytic leukemia (CLL) that is independent of the type or line of therapy. Blood. 2009;114:540. abstr 540. [Google Scholar]
- 31.Tsimberidou AM, Tam C, Abruzzo LV, et al. Chemoimmunotherapy may overcome the adverse prognostic significance of 11q deletion in previously untreated patients with chronic lymphocytic leukemia. Cancer. 2009;115:373–380. doi: 10.1002/cncr.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]