Abstract
The dynamic response of neutrophils to interleukin-8 (IL-8) is of central interest in inflammation. Chemokine –induced β2 integrin dependent adhesion can take several minutes after initial contact with IL-8 as evidenced by increased cell adhesion to intracellular adhesion molecule 1 (ICAM-1). The goal of this study is to identify signaling events that are critical for this response. We demonstrate that neither the PI3K inhibitor wortmannin, nor the PKC inhibitor bisindolymaleimide had any effect on IL-8 induced adhesion to ICAM-1. However, inhibition of PLC with U73122 or stopping the release of intracellular calcium by its downstream effector IP3 with caffeine or 2-aminoethoxydiphenyl borate completely blocked the adhesive response. Chelation of intracellular calcium with BAPTA or extracellular calcium with EGTA completely abrogated neutrophil adhesion to ICAM-1. This adhesion is mediated by LFA-1 (αLβ2) within first 300 seconds after chemokine stimulation, followed by Mac-1 (αMβ2) mediated adhesion, beginning 350 seconds after stimulus. Inhibition of p38MAP kinase results in a time course similar to Mac-1 inhibition, consistent with published evidence that Mac-1 mediated adhesion is p38MAP kinase dependent. These findings confirm a PLC dependent, PKC independent pathway from chemokine stimulus to integrin activation previously identified in other cell types, and demonstrate distinct dynamics and different requirements for LFA-1 vs. Mac-1 activation in primary human neutrophils.
Keywords: neutrophils, inflammation, integrins, chemokines, ICAM-1, adhesion
Introduction
Engineering therapeutic solutions that involve cell harvesting using microfabricated devices or the targeting of either cells or drug delivery vehicles to specific sites in the vasculature will be facilitated by a detailed understanding of the mechanisms that cells themselves use to target specific sites of injury or infection. For example, strategies for harvesting cells in microdevices may involve the use of natural signals to induce cell adhesion and arrest on natural ligands. Understanding the dynamics of increased cellular adhesion in response to specific signals should facilitate the development of such approaches. In the present report we focus on the response of neutrophils to the immobilized chemokine interleukin-8 (IL-8), identifying critical signaling intermediates in the context of dynamic changes in neutrophil adhesion to the endothelial ligand ICAM-1 (intercellular adhesion molecule-1).
The importance of neutrophil adhesion to endothelium and its regulation is evident in the extensive literature on this topic. It is well known that in the human system, the β2 integrins LFA-1 (αLβ2, aka CD11a/CD18) and Mac-1 (αMβ2, aka CD11b/CD18) are critical mediators of neutrophil arrest and migration on inflamed endothelium7, 9, 36, and furthermore that LFA-1 activity tends to precede Mac-1 mediated interactions12, 28, 29. However, to our knowledge, differences in the contributions of the different integrins have been observed either in vivo or in vitro in situations where the specific nature and timing of the stimulus, and the subsequent signaling intermediates are not well known. For the integrins to bind their counter-receptors on the endothelium they must be activated, and the mechanisms leading to this activation have also received considerable scrutiny. E-selectin mediated cell rolling has been implicated as an activator of integrins both in vitro35 and in vivo37, and activation of neutrophil integrins by chemokines, particularly IL-8, is well-documented5. Two receptors for IL-8 are expressed on neutrophils: CXCR1 and CXCR21. Both of these are G-protein coupled receptors (GPCR), and their ligation by IL-8 leads to integrin activation23, 32. Identification of principal pathways that lead from chemokine binding to integrin activation provides critical information in understanding the specific roles that different molecules play in determining neutrophil behavior.
Although chemokines can be released by endothelium into the circulation in soluble form, activation of leukocyte integrins by circulating chemokines may be unfavorable, as it would trigger integrin-mediated arrest remote from the chemokine secretion site. Early work was focused on effects of soluble chemokines5, 31, 32, 42, but new evidence demonstrates that immobilized chemokines stimulate integrin adhesiveness to endothelial ligands and promote cell motility in a much more successful manner than soluble forms8, 16, 33, 44. Thus, understanding the dynamics of the neutrophil response to immobilized chemokines is of central physiological relevance, and is also relevant to device design, where it might be advantageous to localize specific cell stimuli to surfaces.
Previously, we have shown that interaction between immobilized IL-8 and human neutrophils results in β2 integrin activation, as assessed by changes in adhesion probability to immobilized ICAM-123. Integrin activation starts several minutes after the initial contact of the cell with IL-8, and an additional 3 to 5 minutes elapses before the adhesion to ICAM-1 reaches its maximum. This long delay between IL-8 contact and integrin dependent adhesion prompted us to identify signaling pathways induced by immobilized IL-8 in human neutrophils. In these studies we employ a novel micromechanical approach that allows us to control very precisely the time of interaction as well as quantities of interacting molecules. Using different inhibitors to block specific signaling molecules, we were able to identify pathways that are and are not involved in signal transduction from IL-8 binding to its counter receptor on the surface of neutrophils to integrin-mediated adhesion. We show that this process is critically dependent phospholipase C (PLC) and subsequent on inositol 1,4,5-triphosphate (IP3) dependent calcium release, but is independent of phosphoinositide 3 kinase (PI3K) or protein kinase C (PKC) activity. We also present evidence for different temporal responses and different dependence on p38 mitogen activated protein kinase (MAP kinase) activity of LFA-1 and Mac-1 and their contributions to the adhesion.
Materials and Methods
Chemicals and Monoclonal Antibodies
The reagents used in the study: lovastatin and PI3K inhibitor wortmannin were from A. G. Scientific, Inc. (San Diego, CA), BAPTA was from Invitrogen (Grand Island, NY), PLC inhibitor U73122, its inactive analog U73413 and PKC inhibitor bisindolymaleimide I hydrochloride (BIM) were from Calbiochem (La Jolla, CA). Two inhibitors of IP3-induced calcium release, 2-aminoethoxydiphenyl borate (2APB) and caffeine, were both from Sigma.
Monoclonal antibodies against CD11b (clone ICRF44), ICAM-1 (clone 15.2) and CD45 (a protein tyrosine phosphatase, also known as the leukocyte common antigen) (clone C11) were purchased from Ancell (Bayport, MN), mAbs against IL-8 (clone 6217), human E-selectin (clone BBIG-E5) and recombinant human E-selectin were purchased from R&D Systems (Minneapolis, MN), IgG1 isotype control was obtained from Beckman Coulter Immunotech (Miami, Fl). All antibodies used for flow cytometry were FITC conjugated.
Cell Preparation
For micropipette studies neutrophils were obtained from healthy donors by diluting a drop of peripheral blood in 4% fetal bovine serum (FBS) in BSS (balanced saline solution): 5 mM KCl, 146 mM NaCl, 5.5 mM Glucose, containing 10 mM N-[2-Hydroxyethyl]piperazine-N'-[2-ethanesulfonic acid] (HEPES, Sigma, Saint Louis, MO) made with low endotoxin water obtained from Invitrogen Corp. and supplemented with 0.5 mM EGTA, 1 mM Mg2+ and 1 mM or 1 µM Ca2+, pH 7.4, 290 mOsm. The suspension was placed in a chamber on the stage of the light microscope and two micropipettes were used to manipulate a single cell into contact with the ligand-coated bead for controlled durations.
Coating Beads
Recombinant human ICAM-1 (R&D Systems, Minneapolis, MN) was coupled covalently to paramagnetic M450 Dynabeads (Dynal, Lake Success, NY) via tosyl linkage, as previously described24. Briefly, 107 beads (4.5 µm diameter) were incubated with 5 µg/ml of ligand at room temperature overnight. Then unreacted tosyl groups were blocked by incubation with 0.25 M ethanolamine. Beads were washed and stored in 0.1% BSA in PBS at 4°C. Site density was determined by flow cytometry (see below). For some experiments recombinant human E-selectin was immobilized on paramagnetic M450 beads using the same immobilization procedure.
Recombinant human IL-8 mucin-like stalk chimera (R&D Systems, Minneapolis, MN) was immobilized on the protein G coated beads (Dynal, Lake Success, NY), as previously described23. Briefly, the beads (2.8 µm diameter) were first incubated in BlockAid solution (Molecular Probes, Eugene, OR) to reduce non-specific binding. Then the antibody against His•Tag was immobilized and covalently linked through the Fc portion to those beads. The reaction was stopped by adding Tris (Sigma, St. Louis, MO) and IL-8/chimera was added to enable binding of the His•Tag sequence on the chimera to the anti-His•Tag antibody on the beads. The beads were stored at 4°C in buffer containing the chimera. For some control experiments anti-CD45 antibody was immobilized on protein G beads using the same immobilization procedure as for the His•Tag antibody immobilization.
The density of protein binding sites on the surface of the beads was determined using flow cytometry, as previously described23. Briefly, the beads were preincubated at 4°C overnight with FITC-conjugated antibody against human ICAM-1, E-selectin or IL-8, or with FITC-conjugated isotype control antibody. To correlate fluorescence intensity with the number of bound antibodies on the beads, the fluorescence signal was calibrated using Quantum Simply Cellular Beads (Flow Cytometry Standards Corp., Fishers, IN). The fluorescence intensity was converted to the number of binding sites using software provided by the manufacturer. To correct for non-specific binding, the number of non-specific "sites", detected using isotype control antibody, was subtracted from the total number of sites, detected using the specific antibody. For the IL-8 coated beads used in these studies, the site density was 900 to 1200 sites/µm2, and for ICAM-1 coated beads the site density was 130 – 440 sites/µm2.
Micropipette Measurements
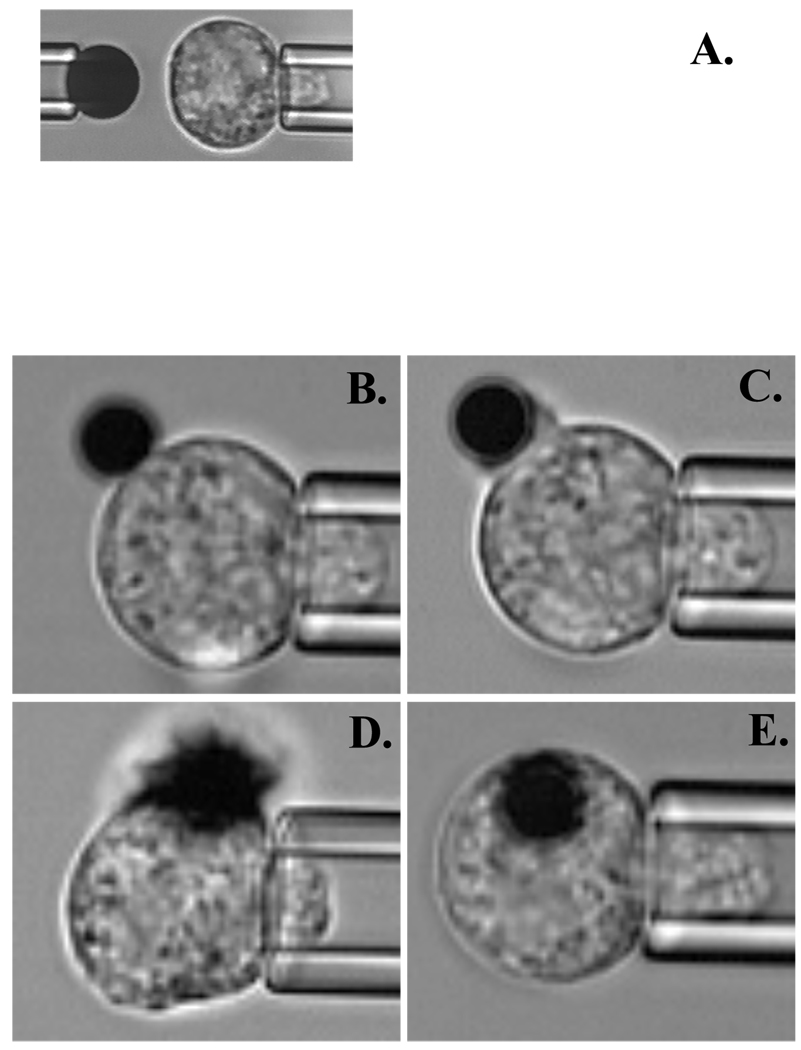
The experiments were performed on the stage of an inverted microscope. The basic procedure for determining IL-8 induced adhesion to ICAM-1 has been described previously23. Briefly, two micropipettes were positioned opposite each other in a dual entry chamber mounted on the microscope stage: one to hold the bead coated with ICAM-1, another to manipulate the cell (Fig.1A). To determine adhesion probability, the bead and the neutrophil were brought into repeated contacts of 2 seconds duration using a micromanipulator. Adhesion probability was calculated as the number of adhesive events divided by the total number of contacts. First the baseline adhesion was estimated, based on a series of contacts between an ICAM-1 coated bead and the neutrophil. Once the baseline was established, the cell was brought into contact with the bead coated with IL-8-chimera, E-selectin or anti-CD45 antibody and the adhesion test was performed continuously until maximal adhesion probability was reached or for a maximum period (800 seconds at room temperature, 600 seconds at 37° C). All IL-8 coated beads bound strongly to the neutrophil surface, and within a few tens of seconds, the cell began to engulf the bead (Fig. 1B–E). During the engulfment, the probability of adhesion to the ICAM-1-coated bead was measured continuously. To compare results across different cell bead pairs, a common set of time points was chosen, and adhesion probability for each time point was calculated for each cell based on the nearest 20 contacts, that is, the ten contacts immediately preceding that time plus the ten contacts immediately following that time.. All experiments were performed either at room temperature (22–24°C) or at 37°C as indicated. For experiments conducted at 37°C, an environmental box was used to enclose the stage and to keep humidity and temperature constant.
Figure 1.
Series of video microphotographs showing the experimental setup for the micropipette experiment. A. Initial setup. Left pipette is holding ICAM-1 coated bead (4.5µm in diameter) and right pipette is holding human neutrophil. During the experiment the adhesion probability between the neutrophil and ICAM-1 coated bead was measured. B–E. Time dependent IL-8 bead (2.8 µm in diameter) engulfment. Snapshots are taken at: B. 0 seconds (initial contact), C. 20 seconds, D. 70 seconds, E. 320 seconds – after IL-8 bead attachment to the neutrophil.
Most of the reagents used in the experiments to block specific signaling pathways, were added to the working chamber with cells and beads and kept in the chamber for the whole extent of the experiment. The only exception was BAPTA, which was loaded into the cells prior to the experiment for 15 minutes at the room temperature. Then the cells were washed three times and added to the working chamber with the beads.
Calcium Measurements
For the measurements of the intracellular calcium, granulocytes were first isolated from whole blood. Venous blood was drawn from healthy volunteers with informed consent according to protocols approved by the Institutional Human Subjects Review Board. The blood (3.5 ml) was placed over a layer of Polymorphs (Accurate Chemical & Scientific Corporation, Westbury, NY) and centrifuged for 45 minutes at 450 g. The polymorphonuclear fraction of the cells was harvested by pipette and then washed in 0.1% BSA in BSS. Traces of red blood cells were lysed by resuspending the pellets in 1:6 dilution of PBS. After 30 seconds, 4× PBS was added to adjust pH to 7.4. After one more wash in BSS, a sample of cells was counted in a hemocytometer, using trypan blue (10 µl of cells, 190 µl of BSS and 200 µl of trypan blue).
For calcium measurements cells were loaded with Fluo-4 AM (Molecular Probes) in the dark. Loading concentration was 5 µM for 107 cells per ml. Incubation was performed at 37°C for 30 minutes and then for an additional 10 minutes at room temperature. After spinning down, the supernatant was removed and cells were resuspended in BSS containing 4% FBS and left at the room temperature for de-esterification. 4×106 cells were transferred to a quartz cuvette containing a stir bar and 1ml of the same buffer. Calcium measurements were performed using a microscope photometer from Photon Technologies International (PTI, Birmingham, NJ).
Results
Pertussis Toxin
All known G-protein coupled receptors in neutrophils are pertussis toxin sensitive. We found that treatment of cells with pertussis toxin prevented the adhesion of the IL-8 coated bead to the cell, preventing any increase in integrin-mediated adhesion. Pertussis toxin prevents the interaction of G-protein with the receptor. Thus, the fact that pertussis toxin-treated cells did not adhere to IL-8 indicates that, like some but not all G-protein coupled receptors, high affinity ligand binding requires G-protein association with the receptor (data not shown).
Immobilized E-selectin is unable to induce neutrophil adhesion to ICAM-1
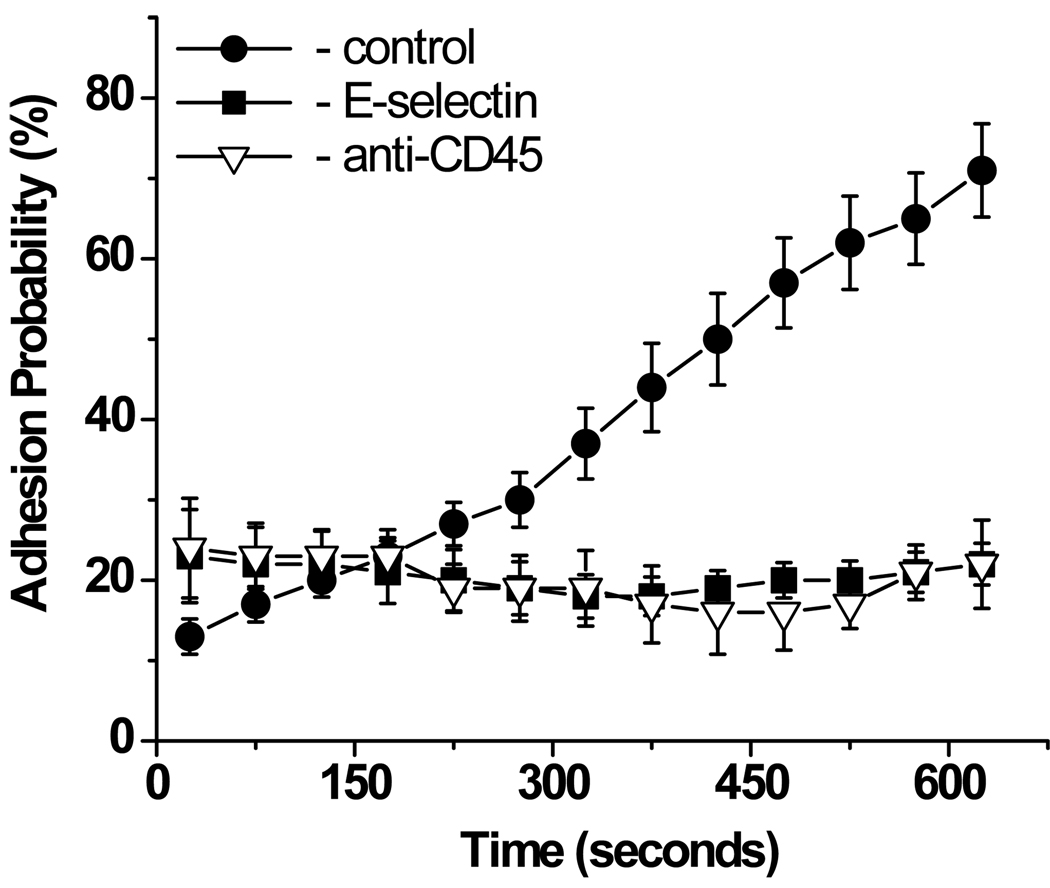
To check if integrin upregulation resulting in increased neutrophil adhesion to ICAM-1 was IL-8 specific, two control experiments were performed: IL-8 was replaced with either human E-selectin or monoclonal antibody to human CD45. Unlike IL-8 coated beads, which were engulfed by the neutrophils within minutes after the beads touched the surface of the cell, beads coated with E-selectin or CD45 stuck very strongly to neutrophils, but never were engulfed. Inasmuch as we have shown previously that engulfment is not essential for induction of signaling pathway leading from IL-8 binding to integrin activation23, experiments with E-selectin and CD45 coated beads serve as a valid controls for non-chemokine mediated adhesion to the cell, even without the bead engulfment. In both control experiments, no adhesion to ICAM-1 was observed (Fig. 2), confirming that the time dependent neutrophil adhesion through β2 integrins was induced by immobilized IL-8. (We have also shown previously that replacement of IL-8 with an identical construct containing MCP-1 (monocyte chemotactic protein-1) also elicits no response from a neutrophil23). We conclude that, in the absence of shear, E-selectin does not induce integrin activation on the neutrophil surface, as assessed by neutrophil adhesion assay to immobilized ICAM-1. However, replacement of E-selectin with a much smaller amount of IL-8 resulted in a time dependent, β2 integrin-mediated increase in ICAM-1 binding to neutrophils.
Figure 2.
Effect of immobilized IL-8 (control), E-selectin or anti-CD45 antibody on neutrophil adhesion to ICAM-1. IL-8 was immobilized on the protein G coated beads at 1,800 sites/µm2 and E-selectin was immobilized on tosyl-activated beads at 9,000 sites/µm2. anti-CD45 antibody and E-selectin coated beads stayed attached to the surface of the cell, while IL-8 coated beads got engulfed. Experiments were performed at room temperature. 43 cells from 5 donors were analyzed for this experiment. Error bars represent standard error. The measured adhesion probability for time ≥ 325 s for E-selectin is significantly different from control (IL-8) assessed by Student’s t-test (p < 0.001). The probability of adhesion for CD 45beads for times ≥ 275 s is statistically different from control (p < 0.01).
Adhesion to ICAM-1 is PLC dependent, but is independent of PI3K
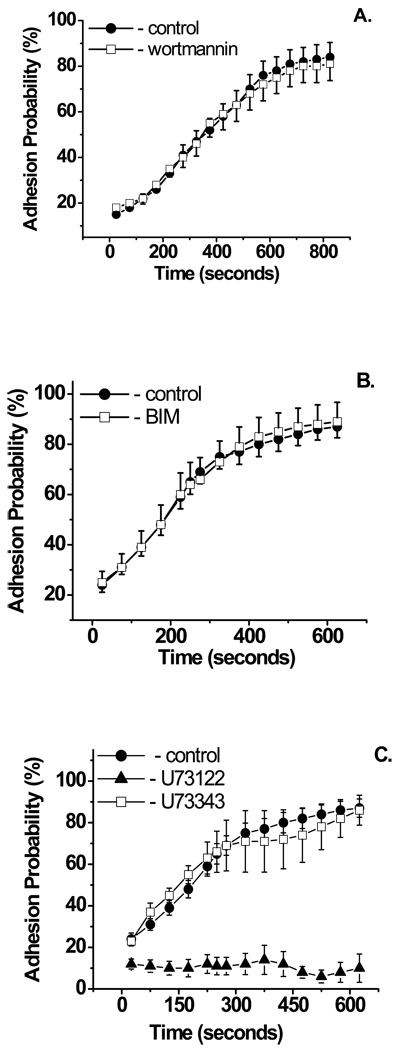
Ligation of G-protein coupled receptors sets off a host of signaling responses within cells. Binding of soluble fMLP or IL-8 to its counter receptor on the surface of a neutrophil causes PI3K and PLC activation22, 31. To check if such activation could lead to β2 integrin-mediated adhesion induced by immobilized IL-8, the PI3K inhibitor wortmannin and the PLC inhibitor U73122 were used15, 38, 42. While wortmannin (500 nM) had no effect on β2 integrin dependent adhesion (Fig. 3A), U73122 (2 µM) completely blocked neutrophil adhesion to immobilized ICAM-1, although phagocytosis of the IL-8 bead still occurred. The inactive analog U73343 (2 µM) was ineffective in inhibiting IL-8 induced integrin upregulation (Fig. 3B).
Figure 3.
Effect of signaling pathways inhibitors on IL-8 induced neutrophil adhesion to ICAM-1. A. PI3k inhibitor wortmannin (500 nM) and B. PKC inhibitor BIM (1µM) had no effect. C. PLC inhibitor U73122 (2 µM) completely blocked ICAM-1 dependent adhesion, while its inactive analog U73343 (2 µM), which was used as a control, had no effect. Experiments with BIM and PLC inhibitors were performed at 37°C, experiments with wortmannin were performed at room temperature. Ninety-five cells from six donors were analyzed for these experiments. Error bars represent standard error. Pair-wise t-tests at each time point show no statistically significant difference between wortmannin treated, BIM-treated or U73343-treated cells compared to control at the 95% confidence level, whereas the U73122-treated cells were statistically different at all time points (p < 0.01).
Calcium release from intracellular stores induces neutrophil adhesion to ICAM-1
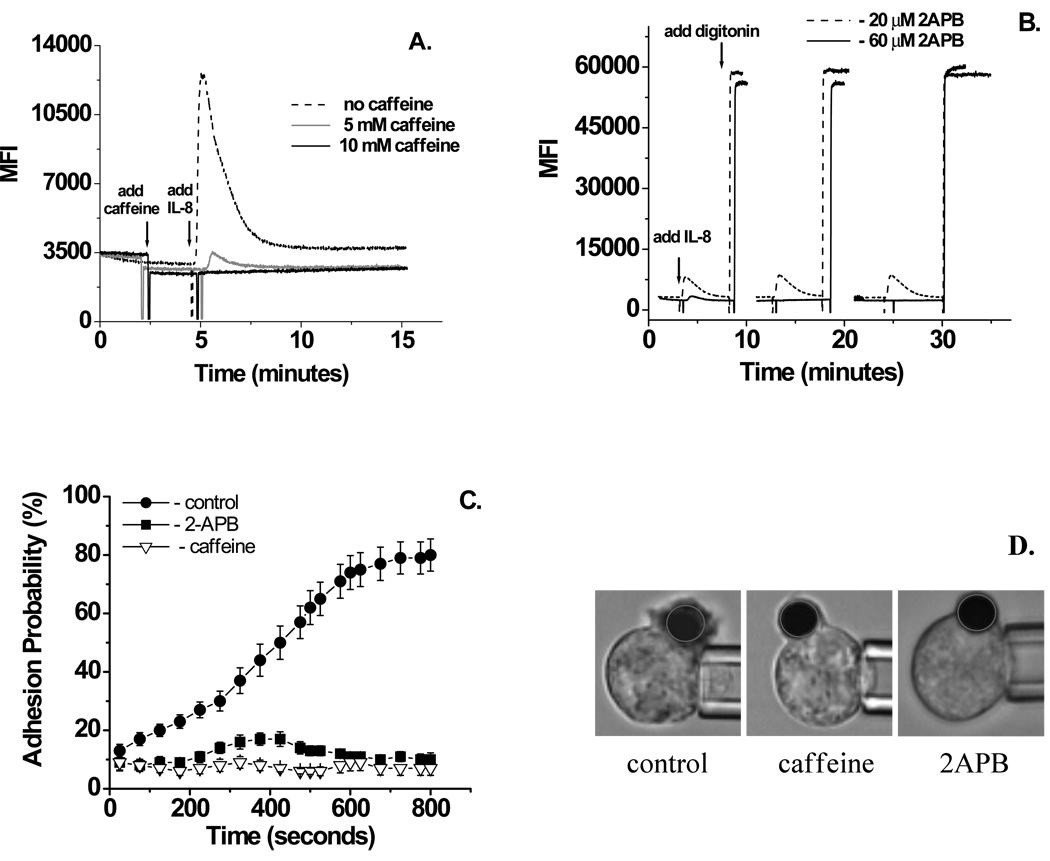
Stimulation of PLC results in DAG-mediated activation of PKC and IP3-mediated calcium release. The PKC inhibitor BIM (1µM) had no effect on neutrophil adhesion to ICAM-1 induced by immobilized IL-8 (Fig. 3C). To determine if IL-8 dependent integrin activation is IP3 dependent, two inhibitors of IP3 induced calcium release, caffeine and 2APB, were tested26, 31. As soluble IL-8 is known to induce calcium release in human neutrophils31, cells were loaded with Fluo-4 AM and then increasing concentrations of the IP3 inhibitors were applied to determine the minimum working concentration and time necessary to completely inhibit IL-8 induced calcium release. For caffeine this concentration was determined to be 10 mM (Fig. 4A) and for 2APB the effect was reached at a concentration of 60 µM after 15 minutes incubation (Fig. 4B). Inhibition of IP3-induced calcium release, using caffeine or 2APB, completely blocked IL-8 dependent neutrophil adhesion to ICAM-1 (Fig. 4C). Phagocytosis of the IL-8 bead occurred, but more slowly, and with decreased thickness of the phagocytic cup (Fig. 4D).
Figure 4.
Effect of IP3 inhibitors caffeine and 2APB on IL-8 induced calcium release and neutrophil binding to ICAM-1. A. Inhibition of calcium spark by caffeine. While cells pre-incubation with 5mM caffeine was partially effective, 10mM caffeine completely inhibited IL-8 induced calcium release. B. Timing of calcium release inhibition by 2-APB. 20 µM 2-APB had a partial effect on calcium release induced by IL-8, while pre-incubation of cells with 60 µM 2-APB for 12 minutes completely inhibited IL-8 induced calcium release. C. Inhibition of IL-8 induced neutrophils adhesion to ICAM-1 by 2-ABP (60 µM) and caffeine (10 mM). 51 cells from 5 donors were analyzed for this experiment. Error bars represent standard error. Beginning at75 s and thereafter, adhesion probability in the presence of 2APBor caffeine is significantly different from control (Student’s t-test, p < 0.001). D. Video micrograph showing phagocytic cap in the control cells and in the cells pretreated with 2-APB or caffeine 90 seconds after IL-8 bead attachment.
Calcium chelation blocks β2 integrins binding to ICAM-1
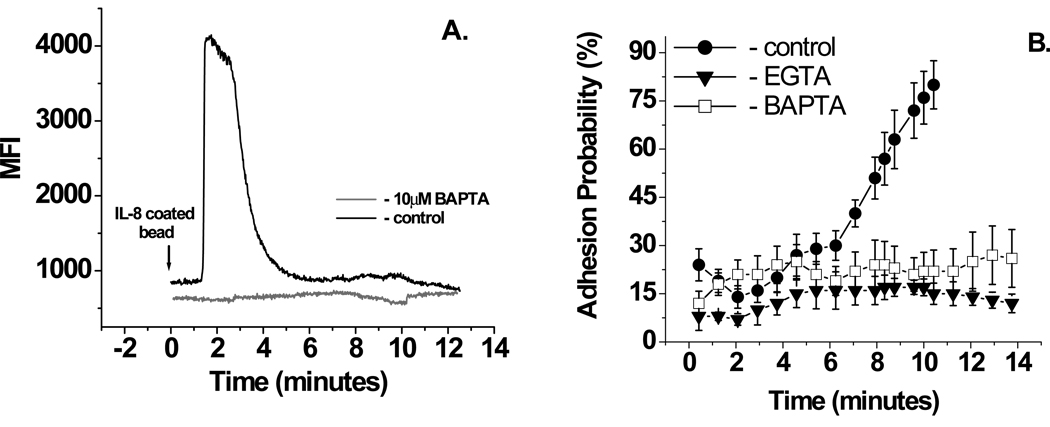
As an additional check to see if immobilized IL-8 induces integrin activation through calcium release, as soluble IL-8 or fMLP does in human neutrophils10, 16, 31, or immobilized fMLP or SDF-1 in other cell types16, the intracellular calcium chelator BAPTA was used. First the minimum amount of BAPTA needed to inhibit the IL-8 response in human neutrophils was determined to be 10 µM (Fig. 5A). It is worth noting that using our procedure, BAPTA was found to be effective at blocking IL-8 induced intracellular calcium increases only when extracellular calcium was reduced from 1mM to 1 µM. Chelation of intracellular calcium with BAPTA completely inhibited neutrophil binding to ICAM-1 induced by immobilized IL-8 (Fig. 5B).
Figure 5.
Effect on intracellular and extracellular calcium chelation on neutrophils adhesion to ICAM-1. A. Inhibition of calcium release by 10 mM BAPTA. B. Inhibition of IL-8 induced neutrophils adhesion to ICAM-1 by 10 µM BAPTA or 0.5 mM EGTA. Experiments were performed in BSS supplemented with 1µM calcium and 1mM magnesium at room temperature. 15 cells from 3 donors were analyzed for this experiment. Error bars represent standard error. Adhesion probability in the presence of EGTAis significantly different from control (Student’s t-test, p < 0.001) beginning at seven minutes and thereafter. For BAPTA, statistical significance is reached at 8 minutes and thereafter.
It is well documented that α4 integrin affinity upregulation depends on extracellular calcium16. To check if β2 integrins require external calcium for the binding to ICAM-1, cells were placed in media where all extracellular calcium was chelated by EGTA. Under this condition, adhesion to ICAM-1 was completely abolished (Fig, 5B).
LFA-1 and Mac-1 are both involved in IL-8 induced binding to ICAM-1
The fact that chemokine activation leads to integrin upregulation is well established16, 20, 33, 34, 41, 44, but the timing of this process is not easy to determine in flow channel systems. We took advantage of our system allowing us to precisely time the increase in adhesion to ICAM-1 following chemokine activation, to observe the relative kinetics of LFA-1 and Mac-1 upregulation.
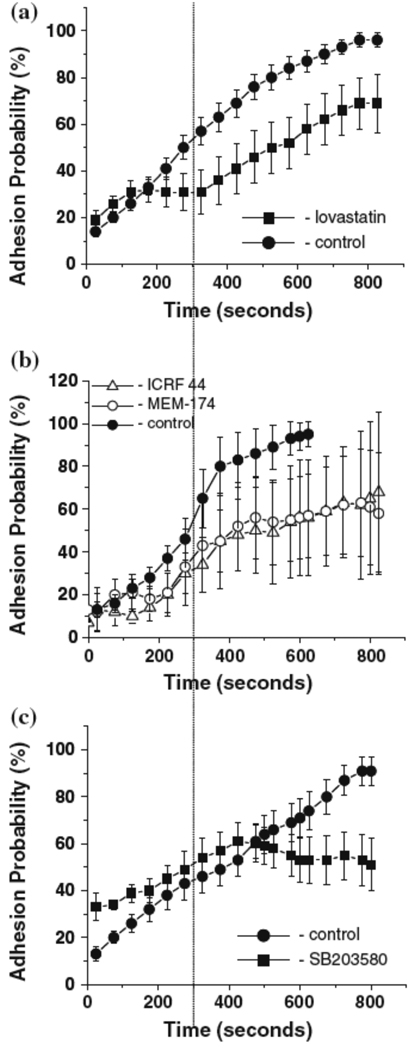
To deduce the contribution of LFA-1 to adhesion to ICAM-1, experiments were performed in the presence of lovastatin (100 µM), which stabilizes LFA-1 in a low affinity conformation and inhibits LFA-1 binding to ICAM-130, 43. In the presence of lovastatin, a significant reduction in adhesion was observed, particularly during the first 300 seconds after IL-8 activation. After 350 seconds of interaction with IL-8, adhesion to ICAM-1 increased significantly, suggesting a late onset of Mac-1 mediated adhesive response (Fig. 6A). This conclusion was further supported in experiments using blocking antibodies to Mac-1. Two different Mac-1 blocking antibodies, ICRF44 and MEM-174, had similar effects on the kinetics of adhesion. Within the first 300 seconds, blocking antibodies to Mac-1 had no effect on ICAM-1 adhesion, but after 350 seconds, adhesion was significantly inhibited (Fig. 6B). Additional experiments using the p38MAPK inhibitor SB203580 (15 µM) showed the same pattern of response as observed with Mac-1 blocking antibodies, suggesting that Mac-1 activation is p38MAPK sensitive (Fig. 6C).
Figure 6.
Time dependent IL-8 induced neutrophil adhesion to ICAM-1. Experiments were performed in the presence of A. lovastatin (100 µM); B. Mac-1 blocking antibodies MEM174 and ICRF44 (each at 15 µl/ml); C. MAPK inhibitor SB203580 (15 µM). Eighty-eight cells from 6 donors were analyzed for these experiments. Error bars represent standard error. Adhesion probability is statistically different from control (Students t-test, p < 0.05) for lovastatin at 275 s and thereafter, and for SB203580 at 600 s and thereafter. The number of cells tested with Mac-1 blocking antibodies was small (n = 4 for MEM174 and n= 3 for ICRF44). Because of the small numbers, statistical significance was not reached for either antibody individually, but combining the seven cells tested, a significant difference (Student’s t-test, p < 0.05) is obtained at 375 s and thereafter.
Discussion
Binding of ligands to chemokine receptors activates multiple signal transduction cascades and regulates diverse leukocyte functions, including adhesion, transmigration and chemotaxis. For all this to occur, integrins have to be activated. Depending on the cell type and the molecular pair under study, results on G-protein signaling can differ. For example, classical PKC (PKC-β) is involved in the chemotactic response of monocytes to the chemokine MCP-13, but only the atypical PKC (PKC-ζ) appears to be involved in downstream signaling from IL-8 receptors in neutrophils21. Thus, it is very important to delineate GPCR signaling in a defined cell type for a specific pair of interacting molecules. The neutrophil is a difficult model system because of its short life span and variability in behavior not only between donors23, but also between different cells. Nevertheless, delineating the processes that are involved in integrin upregulation in primary cells, such as neutrophils, is of major importance for understanding the physiology of inflammatory cascade in human health and disease
Upon ligation of GPCR by chemokine, free Gβγ subunits regulate multiple target proteins within the cell, including PLC and PI3K). These start two different signaling cascades, leading to distinct downstream responses22. Activation of PI3K induces cell chemotaxis and migration18, and through protein kinase B (PKB) activation, leads to well documented respiratory burst and exocytosis14. Stimulation of PLC results in generation of diacylglycerol (DAG) and IP3-mediated calcium mobilization31. While this pathway is well known for mediating activation of PKC, evidence that LFA-1 could be activated independent of either PI3K or PKC activity exists in other cell types, specifically as a result of SDF-1 activation of T-lymphocytes11, 33. Our findings that activation of both LFA-1 and Mac-1 as a result of IL-8 stimulus in neutrophils is unaffected by inhibition of PI3K or PKC indicates that a similar pathway is likely to be at work in neutrophils. Indeed, work in mice revealed that the guanine nucleotide exchange factor CalDAG-GEF-1, which is also activated by DAG and calcium, activates RAP-1 and leads to integrin-mediated adhesion of mouse neutrophils to fibronectin or fibrinogen in response to leukotriene B4 or platelet activating factor2. Neutrophils from patients with deficiencies in CalDAG GEF-1 expression also showed impairment in chemokine-induced integrin-mediated adhesion, although it was subsequently shown that the patient also lacked expression of kindlin-325, 40, a recently identified protein that plays an essential role in integrin activation27. Nevertheless, our findings are consistent with those of others, that the primary pathway from IL-8 receptor ligation to integrin activation passes through PLC and subsequent IP3-induced calcium release, ultimately involving CalDAG GEF-1 and RAP-1 mediated integrin activation.
Calcium influx and integrin adhesion
In addition to the IP3-induced intracellular calcium release, a role for extracellular calcium influx in inducing integrin mediated adhesion is suggested by the inhibitory effects of EGTA. It is well established that activation of neutrophils by IL-8 results not only in release of calcium from intracellular stores, but also activation of calcium influx through store-operated plasma membrane channels17, 31. This extracellular calcium influx has been implicated in integrin activation in U937 cells stimulated with SDF-1 or fMLP. The expected increase in VLA-4 (α4β1) integrin activation was blocked by the calcium influx inhibitor SKF9636516. The elimination of integrin mediated adhesion in our studies in the presence of EGTA suggests that calcium influx may also be necessary for β2 integrin activation in human neutrophils.
Role of E-selectin
While evidence exists that ligation of E-selectin can induce β2 integrin activation, simple ligation of E-selectin even for times up to ten minutes was not by itself sufficient to increase integrin mediated adhesion to ICAM-1 in the pure system used here. This contrasts with results obtained using cultured cells as the adhesive substrate35 or in knock-out mice37 where a role for E-selectin in β2 integrin activation has been identified. The reason for this discrepancy remains unclear, but two possibilities come to mind. First, in the more complex systems, it is possible that the E-selectin may potentiate interaction with another molecule or stimulus that leads to integrin activation. Second, it is possible that the application of force to the E-selectin bond may be required to generate activation signals. Whatever the source of the discrepancy, it is clear that E-selectin binding alone is not sufficient to activate β2 integrin-mediated adhesion to ICAM-1 over the 10 minute duration of the present experiments.
p38MAPK sensitivity of Mac-1 adhesion
The similar dynamics we observe for neutrophils responding to IL-8 in the presence of Mac-1 blocking antibodies and p38 MAP kinase inhibitor is consistent with the possibility that Mac-1 upregulation may be p38 MAP-kinase-dependent. Several other studies have linked Mac-1 activation to p38 MAP kinase activity. Heit and colleagues demonstrated that Mac-1 activation in response to fMLP is blocked by p38 MAP kinase inhibitors13, and p38 MAP kinase has also been implicated in selectin-mediated activation of Mac-139. Mac-1 mediated adhesion of human neutrophils to fibrinogen was also shown to be p38MAPK sensitive6, 41. Thus, our findings are consistent with mounting evidence indicating that Mac-1 activation is induced through p38MAPK. It also indicates an important distinction between LFA-1 and Mac-1 activation. Not only do they occur over different time scales, but LFA-1 appears to be activated independently of p38MAPK activity.
Timing of the response
One of the more surprising results of the present study is the length of time between the presentation of stimulus, and the full upregulation of integrin mediated adhesion. The result contrasts with results where soluble, rather than immobilized, IL-8 has been used as stimulus.8 We believe that the more rapid response to the soluble form is due to a much higher level of receptor occupancy than can occur for immobilized chemokine, and experiments are ongoing to test this possibility. Another possibility is that it is the localized nature of the stimulus that accounts for the long delay in the response, as time may be needed to propagate the signal from one region of the cell to another. Long delays between stimulus and integrin activation are not without precedent. Chigaev4 observed conformation changes when VLA-4 was activated using the calcium ionophore A23187and found a slow conformational unbending over a period of 8–10 minutes. These long times are also consistent with observations of extended rolling times observed for leukocytes in vivo, where cells have been shown to roll for 86 seconds or more prior to arrest19.
Phagocytosis – a red herring?
Phagocytosis of the IL-8 coated bead was first observed in a prior study23. Whether or not this has physiological significance seems questionable. In that earlier report, we demonstrated that the phagocytosis was not necessary to induce integrin-mediated adhesion to ICAM-1. In recent unpublished experiments, we observed that neutrophils spontaneously spread onto IL-8 coated surfaces. It seems likely that the spreading reaction is the natural response of the cell to contacting IL-8, and in the case of the bead, this results in phagocytosis, and in the case of a flat surface, it results in cell spreading. In the present context, this may lead to increased occupancy of IL-8 receptors and promote cell activation and integrin mediated adhesion, but as our prior results show, it is not a necessary step for inducing increased adhesion to ICAM-1.
Conclusion
Contact of neutrophils to IL-8 coated substrates leads to integrin activation through a signaling cascade that passes through PLC and the subsequent IP3-induced release of intracellular calcium. A role for calcium influx in integrin activation is also indicated. Activation of LFA-1 precedes activation of Mac-1, and inhibition of p38 MAP kinase produces a temporal response consistent with a dependence of Mac-1 (but not LFA-1) activation on the kinase.
Acknowledgements
The authors thank Foon-Yee Law for performing the experiments with soluble IL-8 and Richard Bauserman for technical support. This work was supported by the U.S. Public Health Service under NIH Grant No. PO1 HL 018208.
References
- 1.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 2.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, Housman DE, Graybiel AM, Wagner DD. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnevale KA, Cathcart MK. Protein kinase C beta is required for human monocyte chemotaxis to MCP-1. J Biol Chem. 2003;278:25317–25322. doi: 10.1074/jbc.M304182200. [DOI] [PubMed] [Google Scholar]
- 4.Chigaev A, Waller A, Zwartz GJ, Buranda T, Sklar LA. Regulation of cell adhesion by affinity and conformational unbending of alpha4beta1 integrin. J Immunol. 2007;178:6828–6839. doi: 10.4049/jimmunol.178.11.6828. [DOI] [PubMed] [Google Scholar]
- 5.Detmers PA, Powell DE, Walz A, Clark-Lewis I, Baggiolini M, Cohn ZA. Differential effects of neutrophil-activating peptide 1/IL-8 and its homologues on leukocyte adhesion and phagocytosis. J Immunol. 1991;147:4211–4217. [PubMed] [Google Scholar]
- 6.Detmers PA, Zhou D, Polizzi E, Thieringer R, Hanlon WA, Vaidya S, Bansal V. Role of stress-activated mitogen-activated protein kinase (p38) in beta 2-integrin-dependent neutrophil adhesion and the adhesion-dependent oxidative burst. J Immunol. 1998;161:1921–1929. [PubMed] [Google Scholar]
- 7.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 8.DiVietro JA, Smith MJ, Smith BR, Petruzzelli L, Larson RS, Lawrence MB. Immobilized IL-8 triggers progressive activation of neutrophils rolling in vitro on P-selectin and intercellular adhesion molecule-1. J Immunol. 2001;167:4017–4025. doi: 10.4049/jimmunol.167.7.4017. [DOI] [PubMed] [Google Scholar]
- 9.Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99:336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- 10.Elzi DJ, Bjornsen AJ, MacKenzie T, Wyman TH, Silliman CC. Ionomycin causes activation of p38 and p42/44 mitogen-activated protein kinases in human neutrophils. Am J Physiol Cell Physiol. 2001;281:C350–C360. doi: 10.1152/ajpcell.2001.281.1.C350. [DOI] [PubMed] [Google Scholar]
- 11.Ghandour H, Cullere X, Alvarez A, Luscinskas FW, Mayadas TN. Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood. 2007;110:3682–3690. doi: 10.1182/blood-2007-03-077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green CE, Schaff UY, Sarantos MR, Lum AF, Staunton DE, Simon SI. Dynamic shifts in LFA-1 affinity regulate neutrophil rolling, arrest, and transmigration on inflamed endothelium. Blood. 2006;107:2101–2111. doi: 10.1182/blood-2005-06-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heit B, Colarusso P, Kubes P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J Cell Sci. 2005;118:5205–5220. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 15.Hou C, Kirchner T, Singer M, Matheis M, Argentieri D, Cavender D. In vivo activity of a phospholipase C inhibitor, 1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole -2,5-dione (U73122), in acute and chronic inflammatory reactions. J Pharmacol Exp Ther. 2004;309:697–704. doi: 10.1124/jpet.103.060574. [DOI] [PubMed] [Google Scholar]
- 16.Hyduk SJ, Chan JR, Duffy ST, Chen M, Peterson MD, Waddell TK, Digby GC, Szaszi K, Kapus A, Cybulsky MI. Phospholipase C, calcium, and calmodulin are critical for alpha4beta1 integrin affinity up-regulation and monocyte arrest triggered by chemoattractants. Blood. 2007;109:176–184. doi: 10.1182/blood-2006-01-029199. [DOI] [PubMed] [Google Scholar]
- 17.Itagaki K, Kannan KB, Livingston DH, Deitch EA, Fekete Z, Hauser CJ. Store-operated calcium entry in human neutrophils reflects multiple contributions from independently regulated pathways. J Immunol. 2002;168:4063–4069. doi: 10.4049/jimmunol.168.8.4063. [DOI] [PubMed] [Google Scholar]
- 18.Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1997;94:3052–3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunkel EJ, Dunne JL, Ley K. Leukocyte arrest during cytokine-dependent inflammation in vivo. J Immunol. 2000;164:3301–3308. doi: 10.4049/jimmunol.164.6.3301. [DOI] [PubMed] [Google Scholar]
- 20.Laudanna C, Campbell JJ, Butcher EC. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- 21.Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998;273:30306–30315. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 23.Lomakina EB, Waugh RE. Dynamics of increased neutrophil adhesion to ICAM-1 after contacting immobilized IL-8. Ann Biomed Eng. 2006;34:1553–1563. doi: 10.1007/s10439-006-9172-y. [DOI] [PubMed] [Google Scholar]
- 24.Lomakina EB, Waugh RE. Micromechanical tests of adhesion dynamics between neutrophils and immobilized ICAM-1. Biophys J. 2004;86:1223–1233. doi: 10.1016/S0006-3495(04)74196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manevich-Mendelson E, Feigelson SW, Pasvolsky R, Aker M, Grabovsky V, Shulman Z, Kilic SS, Rosenthal-Allieri MA, Ben-Dor S, Mory A, Bernard A, Moser M, Etzioni A, Alon R. Loss of Kindlin-3 in LAD-III eliminates LFA-1 but not VLA-4 adhesiveness developed under shear flow conditions. Blood. 2009;114:2344–2353. doi: 10.1182/blood-2009-04-218636. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 27.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 28.Neelamegham S, Taylor AD, Shankaran H, Smith CW, Simon SI. Shear and time-dependent changes in Mac-1, LFA-1, and ICAM-3 binding regulate neutrophil homotypic adhesion. J Immunol. 2000;164:3798–3805. doi: 10.4049/jimmunol.164.7.3798. [DOI] [PubMed] [Google Scholar]
- 29.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarantos MR, Raychaudhuri S, Lum AF, Staunton DE, Simon SI. Leukocyte function-associated antigen 1-mediated adhesion stability is dynamically regulated through affinity and valency during bond formation with intercellular adhesion molecule-1. J Biol Chem. 2005;280:28290–28298. doi: 10.1074/jbc.M501662200. [DOI] [PubMed] [Google Scholar]
- 31.Schorr W, Swandulla D, Zeilhofer HU. Mechanisms of IL-8-induced Ca2+ signaling in human neutrophil granulocytes. Eur J Immunol. 1999;29:897–904. doi: 10.1002/(SICI)1521-4141(199903)29:03<897::AID-IMMU897>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Seo SM, McIntire LV, Smith CW. Effects of IL-8, Gro-alpha, and LTB(4) on the adhesive kinetics of LFA-1 and Mac-1 on human neutrophils. Am J Physiol Cell Physiol. 2001;281:C1568–C1578. doi: 10.1152/ajpcell.2001.281.5.C1568. [DOI] [PubMed] [Google Scholar]
- 33.Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 34.Shimaoka M, Kim M, Cohen EH, Yang W, Astrof N, Peer D, Salas A, Ferrand A, Springer TA. AL-57, a ligand-mimetic antibody to integrin LFA-1, reveals chemokine-induced affinity up-regulation in lymphocytes. Proc Natl Acad Sci U S A. 2006;103:13991–13996. doi: 10.1073/pnas.0605716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 36.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J Exp Med. 2004;200:935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 39.Smolen JE, Petersen TK, Koch C, O'Keefe SJ, Hanlon WA, Seo S, Pearson D, Fossett MC, Simon SI. L-selectin signaling of neutrophil adhesion and degranulation involves p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:15876–15884. doi: 10.1074/jbc.M906232199. [DOI] [PubMed] [Google Scholar]
- 40.Svensson L, Howarth K, McDowall A, Patzak I, Evans R, Ussar S, Moser M, Metin A, Fried M, Tomlinson I, Hogg N. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15:306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tandon R, Sha'afi RI, Thrall RS. Neutrophil β2-integrin upregulation is blocked by a p38 MAP kinase inhibitor. Biochem Biophys Res Commun. 2000;270:858–862. doi: 10.1006/bbrc.2000.2540. [DOI] [PubMed] [Google Scholar]
- 42.Tilton B, Andjelkovic M, Didichenko SA, Hemmings BA, Thelen M. G-Protein-coupled receptors and Fcgamma-receptors mediate activation of Akt/protein kinase B in human phagocytes. J Biol Chem. 1997;272:28096–28101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 43.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Medicine. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 44.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]