Abstract
Epigenetic chromatin remodeling, including reversible histone methylation, regulates gene transcription in brain development and synaptic plasticity. Aberrant chromatin modifications due to mutant chromatin enzymes or chemical exposures have been associated with neurological or psychiatric disorders such as mental retardation, schizophrenia, depression, and drug addiction. Some chromatin enzymes, such as histone demethylases JARID1C and UTX, are coded by X-linked genes which are not X-inactivated in females. The higher expression of JARID1C and UTX in females could contribute to sex differences in brain development and behavior.
Keywords: Epigenetics, Hippocampus, Neuron, Synapse, GABAergic, Mental retardation, Schizophrenia, Anxiety, Depression, Addiction
Histone methylation is carried out by cell type- and gene-specific enzymes
Histone methylation, one form of epigenetic chromatin remodeling, has recently been linked to a number of neurological and psychiatric disorders, suggesting its importance in normal brain development and function (Akbarian and Huang, 2009). Both histones H3 and H4 can be methylated at specific lysine (K) or arginine (R) residues (Klose and Zhang, 2007). This review focuses on histone lysine methylation, of which more is known about its implication in the brain.
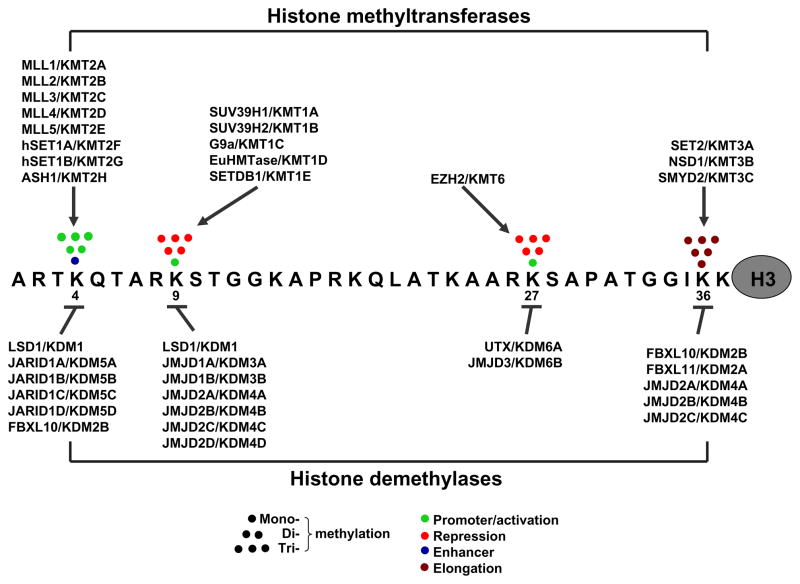
Specific lysine residues, including H3K4, H3K9, H3K27, H3K36, H3K79, and H4K20, can be mono-, di-, or tri-methylated. Some of these methylation modifications are associated with gene activation (H3K4, H3K36, and H3K79) while others are associated with gene repression (di- or tri-methylation at H3K9 and at H3K27) (Fig. 1). The addition and removal of methyl moieties at these lysine residues are carried out by histone methyltransferases and histone demethylases respectively, with each enzyme acting at histone proteins found at particular gene sequences (i.e. chromatin loci) in particular cell types (Fig. 1).
Fig. 1.
Dynamic regulation and diverse effects of histone lysine methylation on gene transcription. Histone H3 Lysine 4 (K4), -9 (K9), -27 (K27), and -36 (K36) on H3’s N-terminal tail are among primary targets of specific histone methyltransferases and demethylases. Each enzyme is enzymatically active in certain cell types and at histone substrates found at certain DNA sequences/genes. Depending on which lysine is methylated and how it is methylated (i.e. mono-, di-, or tri-methylated), gene transcription is differently affected which is indicated by the different colors. Although human enzymes are shown here, many have homologues in other species including rodents. For each enzyme two symbols are given, its previous name and the recently proposed systematic. This figure is modified from Fig. 1 of Mosammaparast and Shi, 2010 and Fig. 1 of Agger et al., 2008.
Methylation of H3K4 has been studied extensively in developmental and cancer biology. Methyl-H3K4 at a gene promoter is recognized by specific protein factors, which are recruited to the promoter and initiate the assembly of transcriptional machinery; in contrast, demethylation of methyl-H3K4 at a promoter leads to transcriptional silencing (Kouzarides, 2007; Mosammaparast and Shi, 2010). In humans, H3K4-specific methyltransferases include MLL1, -2, -3, -4, -5, hSET1a, -1b, and ASH1, all containing a SET (Suppressor of variegation, Enhancer of zeste, and Trithorax) protein domain; H3K4-specific demethylases include LSD1, JARID1A, -1B, -1C, -1D, and FBXL10. All of these demethylases, except for LSD1, contain the catalytic JmjC domain. Some H3K4 demethylases act at mono- or di-methyl-H3K4 and others act at di- or tri-methyl-H3K4 (Agger et al., 2008; Lan et al., 2008). Each of these enzymes regulates a specific set of genes and is involved in specific cellular processes, i.e. there is little functional overlap between two histone enzymes. For instance, Mll1 knockout causes embryonic lethality, a phenotype not compensated for by other H3K4 methyltransferases (Yu et al., 1995; Lubitz et al., 2007). Note: In this review gene symbols are italicized and protein designations are not italicized. For a human gene, all letters are uppercase (e.g. MLL1); and for a mouse gene, only the first letter is uppercase and the remaining letters are lowercase (e.g. Mll1).
Each histone methyltransferase or demethylase is preferentially expressed in certain cell types where it exerts its enzymatic activity at target genes. The target genes of a given enzyme can differ between cell types. In development, for instance, JARID1A specifically represses HOX genes (Christensen et al., 2007); in adults, it regulates genes involved in lymphocyte development (Su et al., 2002; Lopez-Bigas et al., 2008). When associated with the tumor-suppressor RB1, JARID1A contributes to transcriptional silencing of genes involved in cell proliferation and tumorigenesis (Fattaey et al., 1993). However, when bound to the oncoprotein MYC, it becomes enzymatically inactive and certain tumorigenesis genes are subsequently transcribed (Secombe et al., 2007). JARID1B, another H3K4-specific demethylase, is particularly abundant in gonads where it regulates genes involved in germ line development (Su et al., 2002). JARID1B has been implicated in breast cancer where it silences genes involved in cell growth and thus accelerates cell division and tumorigenesis (Lu et al., 1999). FBXL10, a newly identified H3K4-specific demethylase, specifically represses rRNA gene transcription (Frescas et al., 2007). When mutated, FBXL10 causes mental retardation, indicating that FBXL10’s repression of rRNA transcription is particularly important for brain development/function (see below). Some chromatin enzymes are expressed in males or females only. JMJD1A, for instance, is a testis-specific H3K9 demethylase which activates genes essential for spermatogenesis and male fertility (e.g. androgen receptor (AR) gene) (Okada et al., 2007).
When bound with a nuclear receptor such as AR or ER (estrogen receptor), the histone demethylase LSD1 switches from a H3K4 demethylase to a H3K9 demethylase. By demethylating at H3K9, LSD1 facilitates transcription of the genes targeted by the liganded nuclear receptor (Metzger et al., 2005; Yamane et al., 2006; Garcia-Bassets et al., 2007; Wissmann et al., 2007). In fact, demethylation of H3K9, catalyzed by LSD1 or other demethylases such as JMJD1C and JMJD2C, seems to be an essential step in nuclear receptor-induced gene expression (Lee et al., 1995; Wissmann et al., 2007). Moreover, expression of H3K9 demethylases, such as JMJD1C, is highly correlated with that of AR across a variety of brain regions (Wolf et al., 2007).
Being both a H3K4- and H3K9-demethylase, LSD1 plays a key role in gene regulation during development. In the developing pituitary gland, for instance, LSD1 is involved in stem cell lineage commitment, cell differentiation, and production of pituitary hormones (Wang et al., 2007). In these distinct processes LSD1 participates in different protein complexes, some being transcriptional activators and some being transcriptional repressors. It has been speculated that LSD1’s diverse involvements in cellular differentiation and physiology have emerged through evolution; its more conserved function is likely the regulation of germ cell development and meiosis. Mutations in LSD1 homologues result in defective sporulation in yeast and disrupted ovary development in fiies. In adult mice, Lsd1 is more highly expressed in gonads than in other tissues, consistent with LSD1’s importance in germ cell development (Di Stefano et al., 2007; Godmann et al., 2007; Lan et al., 2007a).
Nuclear processes modified by coordinated histone methylation/demethylation
Histone methylation is involved in nuclear processes such as DNA replication, recombination, repair, RNA transcription and splicing. The assembly of splicesomes, for instance, is initiated when methyl-H3K4 at specific DNA sites is recognized by the PHD (Plant Homeodomain) finger domain protein CHD1 (Sims et al., 2007). V(D)J (Variable, Diversity, and Joining) DNA fragment recombination in immune responses is facilitated by RAG2, another PHD finger domain protein, which binds at methyl-H3K4 at specific DNA sequences (Matthews et al., 2007). When the methyl-H3K4 marks are removed from these DNA sequences, or when the interaction between RAG2 and methyl-H3K4 is blocked, V(D)J recombination can no longer proceed normally (Matthews et al., 2007).
In neurons the major role of histone methylation/demethylation is likely transcriptional regulation. Histone methylation marks at promoters, gene body sequences, or 3′ downstream sequences could affect transcription initiation, elongation, or termination respectively. Trimethyl-H3K4 is often found at promoters of active genes, close to the transcription start site (TSS), while monomethyl-H3K4 is more likely found at enhancer sequences more upstream from the TSS. In addition to trimethyl-H3K4, active promoters typically contain acetyl-H3K9/K14 and trimethyl-H3K36 marks (Guenther et al., 2007; Vermeulen et al., 2007). Each of the three histone marks makes distinct contributions to transcription initiation. Acetyl groups are negatively charged and thus acetylated histones repel the DNA strand away from the histone octamer complexes. Methyl groups are neutrally charged; trimethyl-H3K4 are recognized by specific proteins with a PHD finger domain such as TAF3, a subunit of the transcription complex TFIID (Vermeulen et al., 2007). Other PHD finger domain proteins, such as ING2, ING4, ING5, RAG2, and BPTF, have also been shown to be able to bind at methyl-H3K4. Epigenetic chromatin remodeling likely contributes to the swift response and high efficiency of transcriptional regulation. The structural conformation of a combination of specific histone marks is likely recognized by the transcriptional machinery more readily than the promoter DNA sequence. When functionally related genes contain similar histone marks at their promoters, they are likely transcribed in sync, which results in optimized pathway activity, i.e. no gene is over- or under-transcribed.
Once methyl-H3K4 marks are formed at a promoter, this DNA site typically becomes unavailable for repressive histone marks such as methyl-H3K27 (i.e. H3K27 residues at this promoter would remain to be unmethylated). The reverse also holds true, namely a promoter with the methyl-H3K27 mark typically contains unmethylated H3K4 residues. This mutual exclusion between these two modifications is accomplished through the joint action of histone enzymes—H3K4 methyltransferases such as MLL1 are often found in protein complexes which contain a H3K27 demethylase such as UTX (also known as KDM6a) (Lee et al., 2007b). In development the protein complex containing both MLL1 and UTX is responsible for activating HOX genes in a cell lineage-specific fashion (Lee et al., 2007b). Conversely, H3K4 demethylases such as JARID1C (also known as SMCX and KDM5C) are often found in protein complexes which also possess H3K27 methyltransferase activities. Polycomb Repressive Complex 1 (PRC1), for instance, carry out simultaneous H3K4 demethylation and H3K27 methylation. In development PRC1 is responsible for keeping hundreds of genes, known as PcG target genes, in transcriptional silence (Simon and Kingston, 2009). In the PRC1 complex, JARID1C is found to be in contact with the ubiquitin ligases RING1a and -1b (Lee et al., 2007a; Tahiliani et al., 2007). Ubiquitination of histone H2a at lysine119 (H2aK119) by these two RING proteins is suggested to be a prerequisite for JARID1C-mediated demethylation of H3K4 (Weake and Workman, 2008).
Pluripotent stem cells are the exception as they have both methyl-H3K4 and methyl-H3K27 marks at a large number of promoters (Mikkelsen et al., 2007). As stem cells are induced to differentiate along a particular cell lineage, some of these binary promoters retain the methyl-H3K4 mark and these genes are activated; other binary promoters retain the methyl-H3K27 mark and these genes stay silenced.
In order to switch off transcription, active histone marks such as trimethyl-H3K4 and acetyl-H3K9/K14 have to be removed from the promoter. It has been shown that at some promoters the disassembly starts with the HDAC1/2-mediated histone deacetylation. CoREST, a transcription co-repressor, is then recruited to the deacetylated H3. The H3K4 demethylase LSD1 is then called to action which demethylates at trimethylated H3K4 residues. For long-term repression, the BHC80 protein is found to be present adjacent to the H3K4 residue preventing this residue from being re-methylated (Shi et al., 2005).
Histone methylation/demethylation in brain development and brain disorders
Histone methylation is implicated in brain development and function as well as in brain disorders. It is likely that in most cases multiple modifications are involved instead of individual ones, as alteration at one modification often triggers a chain reaction affecting other modifications (see above).
Methyl-H3K4
Various H3K4-specific demethylases and methyltransferases have been implicated in brain disorders. The H3K4 demethylase JARID1C causes mental retardation when mutated, which we will return to in the next section. The H3K4 methyltransferase MLL1 has been found to be down-regulated in the prefrontal cortex of patients with schizophrenia, which is consistent with altered emotional behaviors in brain-conditioned Mll1 KO mice such as the increased propensity to display depression-like behaviors (Huang et al., 2007a).
The methylation modification at H3K4 is also involved in learning and memory formation. In adult mice, contextual fear conditioning up-regulates methyl-H3K4 at specific promoters (e.g. Zif268 and Bdnf) 1 h after the contextual exposure, which returns to baseline by 24 h (Gupta et al., 2010). Moreover, Mll1 deficient mice are defective in fear memory formation (Akbarian and Huang, 2009). Other histone modifications that have been demonstrated to be involved in learning and memory formation include histone acetylation and phosphorylation (Borrelli et al., 2008).
Methylation of H3K4 regulates brain development and neurotransmission in adult brains. In neuronal differentiation, genes such as synapsin become activated as a result of MLL1-mediated H3K4 methylation at these genes’ promoters (Wynder et al., 2005). In the adult brain, promoters of glutamate receptor genes contain high levels of methyl-H3K4, which is absent at promoters of globin genes (Stadler et al., 2005). Across brain regions, the level of methyl-H3K4 correlates positively with that of glutamate receptor mRNA (Stadler et al., 2005). Similar to what has been found in other cell types, trimethyl-H3K4 and acetyl-H3K9/K14 are co-localized at promoters of actively transcribed genes in the brain. When cultured neurons are treated with sodium butyrate, a histone deacetylase inhibitor, acetyl-H3K9/K14, as well as methyl-H3K4, becomes more enriched at promoters of genes such as Gad1 (Huang et al., 2007a). Therefore, both glutamatergic and GABAergic neurotransmission are modified by methylation of H3K4 at specific promoter sequences.
Methyl-H3K9
Methyl-H3K9 is a repressive histone modification which has been implicated in neurological and psychiatric conditions (e.g. Friedreich ataxia, Fragile X syndrome, Huntington’s disease, and addiction). Friedreich ataxia is a neurodegenerative disease due to abnormally high copy numbers of GAA repeats in the FXN gene, which result in transcriptional reduction or even silence (Campuzano et al., 1996). The transcriptional silencing of FXN involves chromatin remodeling at these GAA repeats, including increased levels of methyl-H3K9, which is confirmed in patients with Friedreich ataxia relative to controls (Al-Mahdawi et al., 2008). In addition, DNA methyltransferases (DNMTs) are recruited by the methyl-H3K9 modification and lead to cytosine hyper-methylation at the GAA repeats (Al-Mahdawi et al., 2008). In Huntington’s disease, another trinucleotide repeat expansion disorder, elevated levels of H3K9 methyltransferase ESET and methyl-H3K9 enrichment are found in postmortem striatal tissues (Ryu et al., 2006).
PHF8 is a H3K9 demethylase whose mutations result in mental retardation and facial deformity such as cleft lip and palate (Laumonnier et al., 2005; Abidi et al., 2007; Koivisto et al., 2007). PHF8 is found typically in a protein complex which also includes the H3K4 methyltransferase WDR5 and RNA polymerase and is responsible for rRNA gene transcription (Feng et al., 2010; Horton et al., 2010; Kleine-Kohlbrecher et al., 2010). It is not clear why PHF8 mutations result in specific cognitive impairment and facial deformity.
The 9q34.3 subtelomeric deletion syndrome (also known as Kleefstra syndrome) is due to haploinsufficiency of the H3K9 methyltransferase EHMT1. Symptoms include mental retardation, behavioral disorders (e.g. aggression, hypoactivity, autistic-like behavior), delayed developmental milestones, and facial dysmorphisms (Stewart and Kleefstra, 2007). Similar neurological and behavioral phenotypes are observed in mice with heterozygous Ehmt1 knockout (Balemans et al., 2010). Relative to wildtype littermates, these mutant mice are more anxious and less social—engaging less in social play and showing no social novelty preference. In another mouse model of this brain disorder, Ehmt1 is ablated in specific brain regions (e.g. forebrain, striatum, hippocampus, and hypothalamus) of neonatal mice (Schaefer et al., 2009). In these mice, a large number of genes, normally transcribed in peripheral tissues (e.g. skeletal, muscular, cardiovascular, hematological, and immunological systems), are found to be expressed in the brain. This transcriptional abnormality likely interferes with brain function leading to widespread changes in the behavior of these mice, which explore much less in a novel environment and show defects in fear memory formation.
Drug-induced long term changes in gene transcription and neuronal activity also involve methylation of H3K9. In mice, chronic cocaine treatments cause a nucleus accumbens-specific reduction of methyl-transferase G9a and methyl-H3K9 enrichment, which coincides with increased dendritic spines in this brain region and the development of a cocaine preference (Maze et al., 2010). When down-regulation of G9a is blocked, chronic cocaine treatment fails to produce these morphological and behavioral changes. The effects of cocaine appear to be highly enzyme-specific. Suv39h1, another H3K9 methyltransferase, is in fact up-regulated instead of being down-regulated; up-regulation of Suv39h1 results in increased levels of methyl-H3K9 at specific promoters such as cFos (Renthal and Nestler, 2008).
Methylation of H3K9 also plays a role in the stress response. In rats, the level of trimethyl-H3K9 in the hippocampus is increased in acute stress but decreased following chronic restraint stress for 21 days. The long-term stress-induced reduction of methyl-H3K9 can be blocked by selective serotonin reuptake inhibitor (SSRI) fluoxetine, which may act directly at H3K9-specific methyltransferases or demethylases (Hunter et al., 2009).
Methyl-H3K27
The repressive methyl-H3K27 mark is implicated in the development of depression-like behaviors in mice. Repeated social defeats in mice cause behavioral and neurochemical changes similar to those found in human patients with depression disorders. In this mouse model of depression, extensive chromatin remodeling events take place at specific DNA sequences, including increased levels of methyl-H3K27 at Bdnf promoters in the hippocampus (Tsankova et al., 2006). The high levels of methyl-H3K27 at the Bdnf promoters persist weeks after these “depressed” mice have been removed to a harassment-free environment. Treatments with the antidepressant imipramine effectively revert the depression-like behaviors, in concordance with the substitution of methyl-H3K27 by methyl-H3K4 and acetyl-H3 marks at the Bdnf promoters. This epigenetic switch is not a byproduct of the treatment or a consequence of the behavioral change; instead it contributes to imipramine’s effect on behaviors—when this chromatin remodeling process (i.e. replacement of methyl-H3K27 by methyl-H3K4 and acetyl-H3) is blocked, imipramine is no longer effective in reversing depression (Tsankova et al., 2006).
Methyl-H3K27 is also involved in the neural regulation of circadian rhythms (Etchegaray et al., 2006). EZH2, a H3K27 methyltransferase, is co-immunoprecipitated with the Clock protein; together they form methyl-H3K27 at the Period1 and Period2 promoters and result in transient transcriptional silencing of the two Period genes. In contrast, EZH2 knockdown results in irregular circadian rhythms (Etchegaray et al., 2003).
Mutations in the JARID1C demethylase result in mental retardation
JARID1C mutations cause X-linked mental retardation (XLMR) (Jensen et al., 2005; Santos et al., 2006; Tzschach et al., 2006; Abidi et al., 2008; Adegbola et al., 2008). More than 20 mutations have been identified so far through screening families with XLMR, making JARID1C one of the most frequently mutated genes among XLMR cases. Since not all neural systems are equally affected by JARID1C mutations—sensory perception and motor function, for instance, are largely intact in these patients—it is possible that JARID1C is particularly important in brain regions where it is expressed at high levels (e.g. hippocampus), and neural functions associated with these brain regions are more affected (Xu et al., 2008a). Some patients suffer from additional symptoms such as seizure, delayed language and social development, high agitation, and autism-like behavior (Jensen et al., 2005; Santos et al., 2006; Tzschach et al., 2006; Abidi et al., 2008; Adegbola et al., 2008). The variable phenotypes of JARID1C mutations might be accounted for by the amino acid locations of the mutations—JARID1C is suggested to be involved in multiple distinct protein complexes (Tahiliani et al., 2007); a particular mutation disrupts JARID1C’s protein–protein interaction in certain protein complexes and thus gene transcription normally regulated by these complexes. Peripheral functions are largely preserved in these patients, suggesting that JARID1C is particularly important for CNS function and targets neuronal genes. The brain function-specificity of the JARID1C demethylase is consistent with its expression pattern: although ubiquitously expressed in all tissues and at all ages tested, brain tissue, in particular fetal brain tissue, has higher levels of JARID1C transcripts compared to other tissues (Agulnik et al., 1994; Wu et al., 1994; Xu et al., 2002, 2008a; Jensen et al., 2005). About one third of patients show growth retardation, which might be accounted for by JARID1C’s activities in the cytoplasm, where JARID1C is associated with SMAD3, a key player in the TGFβ pathway important in bone formation (Kim et al., 2008).
The neurophenotypes of JARID1C mutations could arise due to disrupted brain development, a notion supported by several lines of evidence. In zebrafish, JARID1C KD with anti-sense oligos leads to increased neuronal cell death (Iwase et al., 2007). In cultured rat cerebellar neurons, Jarid1c KD with RNAi results in shorter dendrites (but normal axons) (Iwase et al., 2007). It is not clear which genes are regulated by JARID1C in the developing brain and become mis-regulated when JARID1C is mutated in humans or knocked down in model systems. In cultured cells, JARID1C is found to be associated with the REST silencer complex (Tzschach et al., 2006). In embryonic brain tissue, the REST complex represses a large number of neuronal genes (e.g. BDNF and Synapsin) from premature expression in stem cells and is thus crucial for brain development (Ballas and Mandel, 2005).
To determine if JARID1C plays a role in adult brains independent of its contribution to brain development, we knocked down Jarid1c with small interfering RNAs (siRNAs) in discrete brain regions of adult mice (Xu et al., unpublished). When Jarid1c was repressed in the dorsal hippocampus/CA1, mice showed deficiencies in the object recognition test, a behavioral assay in rodents assessing episodic memory (neuronal representation of physical features of an object or an event such as color, sound, and smell). In these CA1-specific Jarid1c KD mice, multiple genes involved in GABAergic neurotransmission were found to be up-regulated, and promoters of these genes contain increased levels of trimethyl-H3K4. We hypothesize that in learning and memory formation these GABAergic genes are repressed by JARID1C; in patients with JARID1C mutations and in Jarid1c KD mice a hyperactive GABA system in the hippocampus contributes to the cognitive phenotype. This hypothesis is in agreement with previous findings such as that learning and memory formation require the repression of GABAergic neurotransmission in the hippocampus (Cui et al., 2008). It is possible that other symptoms in patients with JARID1C mutations (e.g. seizure, high irritation, and autism-like behavior) could also be attributed to abnormally high activities of GABAergic neurotransmission. For instance, elevated GABAergic activity in the prefrontal cortex contributes to aggressive outbursts in humans and rodent models (Miczek et al., 2003).
The potential involvement of histone modifications in brain sexual differentiation
The X chromosome is enriched for genes important for brain function
Both JARID1C and PHF8 are histone demethylases whose mutations cause mental retardation in humans. It may not be a coincidence that both genes map on the X chromosome, where genes affecting cognitive function are represented disproportionally higher when compared to autosomes (Ropers and Hamel, 2005). A middle-sized chromosome with ~1600 genes (including non-coding genes), the X chromosome contains over 100 genes whose mutations result in mental retardation, making an X-linked gene on average three times more likely to be involved in cognition than an autosomal gene (Zechner et al., 2001). It is known that X-linked disease genes, relative to their autosomal counterparts, are easier to be identified due to the mother-to-son transmission pattern of phenotypes. However, the X-linkage bias for cognitive genes is not due to the ascertainment bias of X-linked disease genes. It is suggested that X-linked genes might have been selected for cognitive functions through female mate choices for “smart” males (Zechner et al., 2001; Skuse, 2005; Graves et al., 2006).
X-linked histone enzyme genes JARID1C and UTX are not X-inactivated in females and expressed dimorphically expressed in the brain
For each X-linked gene, female mammals possess two copies whereas males possess only one copy, except genes in the pseudoautosomal regions (PARs) which are presented equally in both sexes. In humans, both X and Y chromosomes have two PARs, PAR1 and PAR2, located at the terminal region of the short and long arm respectively. Twenty-four genes map to PAR1 and five genes map to PAR2. When examined in various human tissues on expression arrays, most PAR genes are found to be expressed similarly between the two sexes, as anticipated (Nguyen and Disteche, 2006). Mouse chromosomes are telocentric (i.e. one arm only) and thus there is only one PAR on the X and Y chromosome, located adjacent to the telomere. Sts is the single mouse PAR gene identified so far and it codes for steroid sulfatase, an enzyme involved in dendritic morphogenesis as well as behaviors such as aggression (Compagnone et al., 1997; Mortaud et al., 2010).
The majority of X-linked genes are found outside PARs in humans and mice; nevertheless, most non-PAR X-linked genes are expressed similarly between males and females despite of the 2-to-1 gene dosage difference (Arnold et al., 2008). This sex equality is probably a byproduct of genome-wide transcriptional regulation operating in all individuals of both sexes. The genome-wide regulation ensures coordinated expression of genes involved in the same biological pathway or the same multi-protein functional complex. Two mechanisms, X-upregulation and X-inactivation, have evolved in mammals to facilitate the transcriptional balance between sex-linked and autosomal genes in both males and females: (a) X-linked genes are up-regulated so that in males expression of X-linked genes and autosomal genes is balanced; (b) in females, X-upregulation results in excess of X-linked transcripts relative to autosomal transcripts; this imbalance is thought to be resolved through female-specific X-inactivation, i.e. one of the two X chromosomes in female cells becomes transcriptionally silenced. It is not clear if the transcription of Y-linked and autosomal genes is compensated. The Y chromosome contains only a handful of genes, many of which are exclusively expressed in the testes where they contribute solely to spermatogenesis (Skaletsky et al., 2003).
More is known about X inactivation than X upregulation. In both humans and mice, X inactivation is implemented in early embryonic development: in each female cell one X chromosome is chosen randomly to be inactivated, followed by a cascade of chromosome-wide epigenetic remodeling events including histone deacetylation, DNA hypermethylation, and recruitment of non-coding RNA Xist (Heard and Disteche, 2006). Consequently this X chromosome appears as a densely packed sphere, known as a Barr Body, located characteristically at the periphery of a nucleus in female human and mouse cells. Transcriptional activities seem to be shut down entirely on this chromosome. However, when allelic-specific transcription was assessed gene by gene, 15–25% of genes on the human inactive X (Xi) were found to be making transcripts, although typically at reduced levels relative to their alleles on the active X (Xa) (Carrel and Willard, 2005). In humans, these escapee genes tend to appear in clusters on the Xi, particularly on the short arm (Disteche et al., 2002). In mice, fewer X-linked genes, 13 out of 393 genes tested, are found to be transcribed bi-allelically in females (Yang et al., 2010). Although the gene content of the X chromosome is highly conserved across mammalian species, it is likely that the list of X escapees is variable between humans and mice.
We know little about the implication of these X escapees in sex differences in health and disease. In fact, when tested in human lymphocytes and other cell types, many of the X escapee genes are expressed at similar levels between the two sexes (Johnston et al., 2008). One might speculate that the Xa alleles of these genes in females might be less active compared to these genes in males, due to some sort of feedback systems which bring about balanced expression between these genes and their functionally related genes. Nevertheless, expression of these genes has not been analyzed in a variety of conditions, such as exposure to stress or pathogens, to determine if it makes a difference between having one and two transcriptionally competent alleles. Nor has it been tested whether if these genes are involved in sexual differentiation during development (Prothero et al., 2009).
JARID1C escapes X-inactivation in both human and mouse females, resulting in its dimorphic expression favoring females. The sex difference in levels of JARID1C mRNA, however, varies between ages and between tissues (Carrel et al., 1996; Sheardown et al., 1996; Xu et al., 2008a). In fact, when X inactivation is established initially on Xi, Jarid1c is turned off like its neighboring genes (Lingenfelter et al., 1998). The inactive state of the Xi allele of Jarid1c is then reverted due to, at least in part, the presence of the protein insulator CTCF. By binding at specific DNA sites upstream of the Jarid1c gene, CTCF could prevent the Jarid1c promoter from being hyper-methylated (Filippova et al., 2005). Without DNA methylation, repressive histone modifications such as hypoacetyl-H3, initially formed at the Jarid1c promoter, cannot be stabilized and thus disassembled from this sequence. As a result, the Xi allele of Jarid1c becomes reactivated (Filippova et al., 2005). It is not clear if a similar process occurs at other X escapees.
UTX, coding for a H3K27 demethylase (Agger et al., 2007; Lan et al., 2007b; Lee et al., 2007b), is another X-linked escapee gene in humans and mice (Greenfield et al., 1998). Mutations in UTX have recently been identified in a variety of cancer types including brain tumors, suggesting faulty histone methylation as a common feature in tumorigenesis (van Haaften et al., 2009). As mentioned earlier, methyl-H3K27, the repressive mark targeted by UTX, and methyl-H3K4, the active histone mark targeted by JARID1C, are mutually exclusive at gene promoters. It would be interesting to test whether the balanced doses of JARID1C and UTX, both being expressed from double copies in females and single copies in males, bear functional significance in transcriptional regulation. It is not clear if PHF8, the X-linked H3K9 demethylase, escapes X inactivation or not in the brain.
In adult mouse brains, escapee genes, including Jarid1c and Utx, are expressed more highly in females than in males (Xu et al., 2002, 2008a,b). In addition, Usp9x, which escapes X inactivation in humans but is suggested to be X-inactivated in mice (Jones et al., 1996), is also found to be expressed dimorphically in mouse brains favoring females (Xu et al., 2002, 2005). Usp9x codes for a deubiquitinating enzyme which regulates synaptic formation in fruit flies (DiAntonio et al., 2001).
When expression of these mouse escapees was quantified in brain sections, the sex differences (female>male) were confirmed in most brain regions (Xu et al., 2002, 2005, 2006, 2008b). However, in certain brain regions, such as the amygdala, expression of Utx was comparable between the two sexes (Xu et al., 2008b). The functional significance of the disappearance of a sex difference in Utx expression in particular brain regions is not clear.
Many autosomal and sex-linked genes are expressed dimorphically in the brain; in most cases these differences are attributed to sex-specific hormones and are reverted following manipulations of steroids such as androgens or estrogens in development or adulthood (Morris et al., 2004; Becker et al., 2005; McCarthy and Konkle, 2005). To determine if the higher expression of mouse escapee genes in female brains is due to sex hormones or gene dosages, these genes were analyzed in the 4-core genotype (FCG) mouse model. In this model, the gonadal sex (testes vs. ovaries) and genetic sex (XY vs. XX) are separated: a mouse with testes (and in turn the male-typical hormonal milieu) could have either an XX or an XY sex chromosome complement; similarly, a mouse with ovaries (and in turn the female-typical hormonal milieu) could have either an XX or XY sex chromosome complement (Arnold and Chen, 2009). In these FCG mice the escapee genes were found to be expressed more highly in XX mice than XY mice, irrespective of the gonadal status (Xu et al., 2005, 2006, 2008a,b). Therefore, dimorphic expression of X-linked escapee genes could contribute to sexual differentiation in brain and behavior independent of gonadal hormones.
The X-linked histone methylases could be implicated in the behavioral differences between XX and XY mice in the FCG model. Relative to XX females, XY females are more aggressive in the resident-intruder test and less maternal as measured by the latency to retrieve dislocated pups (Gatewood et al., 2006); moreover, XY mice, regardless of gonadal sex, spend more time than XX mice engaging in social play such as sniffing or grooming a stimulus mouse (McPhie-Lalmansingh et al., 2008). In addition, sex behavior was examined in male mice with sex chromosome aneuploidy (e.g. XXY and XYY males), and it was found that male mice with two copies of the X chromosome take longer to mount a receptive female than males with one copy of the X (Park et al., 2008). In these studies all mice were gonadectomized at puberty, and these behavioral differences between different sex chromosome complements are thus not due to sex steroids in adults. Since a number of neuroanatomical phenotypes known to be sensitive to perinatal testosterone are found to be similar between XX and XY mice within the same gonadal sex, the XX–XY behavioral differences may not be attributed to the developmental hormone exposure (De Vries et al., 2002; Wagner et al., 2004). Therefore, these behavioral phenotypes are possibly affected by differential expression of X escapees and/or Y-linked genes between XX and XY mice. Jarid1c, for example, could be involved in the phenotype of higher aggression in XY than XX females as aggression is often noticed in patients with JARID1C mutations (Jensen et al., 2005).
In some cases, the gonadal sex and genetic sex affect a phenotype in opposite directions within male or female sex and thus bring the two sexes closer instead of apart on this phenotype. In a mouse model of autoimmune diseases, for instance, the male genetic sex (XY sex chromosome complement) stimulates immune responses (e.g. cytokine production and lymphocyte proliferation), whereas the male gonadal sex (testes which secrete testosterone) represses these immune responses (Palaszynski et al., 2005).
Since the histone demethylase JARID1C is expressed more highly in the brain of females than males, it is plausible that males would have higher levels of methyl-H3K4 at specific promoters than females. As a result, these genes would be transcribed more abundantly in males than females. In parallel, the higher expression of UTX in females would lead to transcription of specific genes more abundantly in females than males. These potential sex differences in methyl-H3K4 and methyl-H3K27 have not been tested yet. Among various forms of histone methylation modifications, H3K9 methylation has been compared between the two sexes in mouse brain (Tsai et al., 2009). Males have higher levels of methyl-H3K9 as well as acetyl-H3 than females in the cortex and hippocampus when mice are examined at embryonic day 18, the day of birth, and postnatal day 6. Exposure to prenatal testosterone in males is likely responsible for the sex difference in acetyl-H3 but not for the sex difference in methyl-H3K9, because prenatally androgenized females have male-like levels of acetyl-H3 but female-like levels of methyl-H3K9. It is possible that methyl-H3K9 is a histone mark influenced by genetic sex at some genes and by hormonal sex at other genes. In a female cell, relative to a male cell, the higher level of the X escapee JARID1C, which often complexes with the H3K9 methyltransferase G9a, results in increased methyl-H3K9 modifications at some JARID1C-targeted promoters (and gene silencing); on the other hand, the higher level of estrogen-liganded receptors which bind at H3K9 demethylases including LSD1, results in decreased methyl-H3K9 modifications at ER responsive promoters (and gene activation). JARID1C and LSD1, whose levels are affected respectively by the genetic sex and by steroids, likely target at different gene promoters, since JARID1C and LSD1 are not functionally overlapping which is suggested by the neurological disorder due to JARID1C mutations.
Could the dimorphism of X-linked histone enzymes be compensated by their Y-linked paralogues?
The sexually dimorphic expression of X escapee genes has been suggested to be compensated for by a handful of Y-linked genes in males. The female advantage in transcription of Jarid1c and Utx, for instance, might be compensated for by male expression of Y-linked Jarid1d and Uty. However, expression and functional examinations are not always consistent with the suggested compensation between XX and XY. For several X–Y gene pairs whose expression is measured in mouse brain, the summed expression of two X-linked gene copies in females typically surpasses that of the X plus Y copies in males (Xu et al., 2002). Expression of the X and Y paralogues is not always concordant in abundance between different ages and between different tissues, implying that the two paralogues might be regulated by separate signaling pathways and thus implicated in separate cellular functions (Xu et al., 2002). In some cases, the two paralogues show different distribution across brain regions. Between Utx and Uty, for example, Utx is highly expressed in the amygdala whereas Uty is highly expressed in the paraventricular nucleus of the hypothalamus (PVN) (Xu et al., 2008b). More importantly, the X- and Y-paralogue may not be functionally interchangeable. UTX has been demonstrated in biochemical assays to be a histone demethylase; similar analysis could not determine if UTY possesses demethylase activity (Lan et al., 2007b). Even though both JARID1C and JARID1D have been confirmed to be H3K4 demethylases in in vitro assays, their biological activities may differ in aspects such as target genes. This hypothesis is supported by clinical observations of male patients with JARID1C mutations, whose normal copies of JARID1D do not spare these individuals from symptoms such as cognitive deficiencies (Jensen et al., 2005).
X escapee genes are likely implicated in the neurophenotype of 45, XO Turner syndrome
It is not clear if X-linked histone demethylases such as JARID1C and UTX play a role in the neurophenotypes of XO Turner syndrome (TS). In women with TS, one entire X chromosome or a significant portion of one X chromosome has been lost in early embryonic development. Diagnostic features of TS include short stature (due to haploinsufficiency of the SHOX gene located in the pseudoautosomal region PAR1), ovarian degeneration and in turn reduced levels of female steroids (due to chromosome aneuploid-induced oocyte atrophy), and webbed neck. A high percentage of patients also suffer from neurological impairments, mainly compromised ability in spatial reasoning and in social cognition, such as interpreting facial emotions of others (Ross et al., 2006). Neuroanatomical alterations have also been detected in individuals with TS relative to controls in brain regions such as the orbitofrontal cortex and amygdala (Good et al., 2003). Individuals with TS do not suffer from mental retardation; they tend to outperform healthy controls in verbal IQ tests but perform poorly in non-verbal tests. Since the spatial reasoning or social competence deficiency is not ameliorated by the estrogen replacement treatment typically given to girls with TS starting at puberty, the TS neurophenotypes are not likely due to the insufficient levels of sex steroids but more likely due to the insufficient dosages of X-linked genes, especially the escapee genes (Ross et al., 2006). Efforts of deletion mapping have pointed to the Xp22.3 region, which contains several candidate genes, in the TS spatial reasoning phenotype (Zinn et al., 2007). In addition, the social cognition deficiency seems to be more severe in individuals retaining a maternal X than those with a paternal X, which leads to the hypothesis of the involvement of imprinted genes on the X chromosome in social behavior (Skuse et al., 1997).
XO mice are not considered to be a faithful model of human TS because these XO mice grow normally. Relative to wild type mice, XO females are subfertile with a shorter reproductive span, possibly due to enhanced atrophy of oocytes in these mice (Burgoyne et al., 1998). Although they have no obvious peripheral defects, these mice perform differently relative to control XX mice in a number of behavioral assays: XO mice show increased anxiety in the elevated plus maze test (Isles et al., 2004); they are less adaptive in response to location changes of the rewards in the reversal learning task, likely due to deficiency in working memory and spatial memory (Davies et al., 2007). Genes responsible for these phenotypes have not been identified yet.
Histone modifications are affected by individual or parental environment
Besides genetic factors such as mutant histone enzymes, environment (e.g. diet, drugs, toxic substances) could also induce chromatin remodeling in the brain. Social stress-induced depression or cocaine exposure, as described above, brings about long lasting changes in chromatin modifications at specific genes. These environment-originated chromatin modifications may explain, at least in part, behavioral differences between identical twins (Baranzini et al., 2010). It is through chromatin remodeling that environment, or “nurture”, gets in direct contact with and exerts long term influences on “nature”, i.e. one’s DNA sequences. Even more striking, certain environment-induced epigenetic marks are transmitted to following generations and affect behaviors of offspring which have not been exposed to the initial environmental factor (Crews et al., 2007; Arai et al., 2009). It is possible that some of the environment-induced epigenetic marks at neuronal genes are preserved or re-established in germ line development, similar to what happens in imprinting (Whitelaw and Whitelaw, 2008).
Histone modifications are promising therapeutic targets in brain disorders
Since chromatin modifications are reversible, it is possible that neurological or psychiatric disorders could be treated or managed through epigenetic approaches. In fact, a number of medications, whose pharmacologic mechanisms have been elusive in the past, have now been shown to be effective through inducing chromatin remodeling (Tsankova et al., 2007; Akbarian and Huang, 2009). In addition to imipramine which is mentioned above, certain monoamine oxidase inhibitors also exert anti-depressant effects through chromatin remodeling, due to the structural similarity between monoamine oxidases and the histone demethylase LSD1. In animal models these monoamine oxidase inhibitors reactivate abnormally silenced genes by increasing levels of methyl-H3K4 at promoters of these genes (Lee et al., 2006; Huang et al., 2007b). In Fragile X mental retardation syndrome, the CGG repeat expansion at the FMR1 gene leads to hyper-DNA methylation and transcriptional repression. When FMR1-deficient cells are treated with 5-aza, a DNA methylation inhibitor, the FMR1 gene becomes reactivated in concordance with increased levels of methyl-H3K4 and acetyl-H3 at the FMR1 promoter (Tabolacci et al., 2005). Various high through-put screening projects are underway for small molecules capable of inducing chromatin remodeling, and some of these molecules might have brain-specific effects.
In sum, in both developing and adult brains, histone methylation modifications are formed at or removed from certain DNA sequences in specific cell populations by designated methyltransferases or demethylases. The local chromatin conformation, created by specific combinations of methylation and other chromatin modifications, is recognized by particular proteins which in turn carry out nuclear processes including transcriptional regulation. Consequently, developmental events such as neuronal differentiation and dendritic outgrowth as well as neurotransmission in adult brains are affected. A better understanding of these chromatin modifications in brain tissue and in brain disorders would give hope for new effective treatment methods which work through chromatin remodeling.
Acknowledgments
We thank Drs. Christine Disteche (Univ. Washington), Schahram Akbarian (Univ. Massachusetts Medical School), Lars Jensen and Andreas Kuss (Max Planck Institute of Molecular Genetics) for fruitful discussions. We acknowledge the current and previous research assistants in Xu lab for their contributions to the Jarid1c knockdown project: Marc Siegel, Kelsey Graham, Alex Newbury, Michael Campagna, Cristine S. De La Hoz Ulloa, Jordan Goldberg. We also thank Ms. Janine Stuczko for her administrative assistance. This project was supported by a Tufts start-up fund, seed grants from Tufts Cummings School of Veterinary Medicine, and a Core Award from Tufts School of Medicine Center for Neuroscience Research (JX).
References
- Abidi FE, Miano MG, Murray JC, Schwartz CE. A novel mutation in the PHF8 gene is associated with X-linked mental retardation with cleft lip/cleft palate. Clin Genet. 2007;72:19–22. doi: 10.1111/j.1399-0004.2007.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidi FE, Holloway L, Moore CA, Weaver DD, Simensen RJ, Stevenson RE, Rogers RC, Schwartz CE. Mutations in JARID1C are associated with X-linked mental retardation, short stature and hyperreflexia. J Med Genet. 2008;45:787–793. doi: 10.1136/jmg.2008.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adegbola A, Gao H, Sommer S, Browning M. A novel mutation in JARID1C/SMCX in a patient with autism spectrum disorder (ASD) Am J Med Genet A. 2008;146A:505–511. doi: 10.1002/ajmg.a.32142. [DOI] [PubMed] [Google Scholar]
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- Agger K, Christensen J, Cloos PA, Helin K. The emerging functions of histone demethylases. Curr Opin Genet Dev. 2008;18:159–168. doi: 10.1016/j.gde.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Agulnik AI, Mitchell MJ, Mattei MG, Borsani G, Avner PA, Lerner JL, Bishop CE. A novel X gene with a widely transcribed Y-linked homologue escapes X-inactivation in mouse and human. Hum Mol Genet. 1994;3:879–884. doi: 10.1093/hmg/3.6.879. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Epigenetic regulation in human brain-focus on histone lysine methylation. Biol Psychiatry. 2009;65:198–203. doi: 10.1016/j.biopsych.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mahdawi S, Pinto RM, Ismail O, Varshney D, Lymperi S, Sandi C, Trabzuni D, Pook M. The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum Mol Genet. 2008;17:735–746. doi: 10.1093/hmg/ddm346. [DOI] [PubMed] [Google Scholar]
- Arai JA, Li S, Hartley DM, Feig LA. Transgenerational rescue of a genetic defect in long-term potentiation and memory formation by juvenile enrichment. J Neurosci. 2009;29:1496–1502. doi: 10.1523/JNEUROSCI.5057-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Itoh Y, Melamed E. A bird’s-eye view of sex chromosome dosage compensation. Annu Rev Genomics Hum Genet. 2008;9:109–127. doi: 10.1146/annurev.genom.9.081307.164220. [DOI] [PubMed] [Google Scholar]
- Balemans MC, Huibers MM, Eikelenboom NW, Kuipers AJ, van Summeren RC, Pijpers MM, Tachibana M, Shinkai Y, van Bokhoven H, Van der Zee CE. Reduced exploration, increased anxiety, and altered social behavior: autistic-like features of euchromatin histone methyltransferase 1 heterozygous knockout mice. Behav Brain Res. 2010;208:47–55. doi: 10.1016/j.bbr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol. 2005;15:500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Baranzini SE, et al. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature. 2010;464:1351–1356. doi: 10.1038/nature08990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80:37–40. doi: 10.1159/000014954. [DOI] [PubMed] [Google Scholar]
- Campuzano V, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Carrel L, Hunt PA, Willard HF. Tissue and lineage-specific variation in inactive X chromosome expression of the murine Smcx gene. Hum Mol Genet. 1996;5:1361–1366. doi: 10.1093/hmg/5.9.1361. [DOI] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 belongs to a family of demethylases, specific for tri- and dimethylated lysine 4 on histone 3. Cell. 2007;128:1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Salido E, Shapiro LJ, Mellon SH. Expression of steroid sulfatase during embryogenesis. Endocrinology. 1997;138:4768–4773. doi: 10.1210/endo.138.11.5504. [DOI] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W, Humby T, Isles AR, Burgoyne PS, Wilkinson LS. X-monosomy effects on visuospatial attention in mice: a candidate gene and implications for Turner syndrome and attention deficit hyperactivity disorder. Biol Psychiatry. 2007;61:1351–1360. doi: 10.1016/j.biopsych.2006.08.011. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano L, Ji JY, Moon NS, Herr A, Dyson N. Mutation of Drosophila Lsd1 disrupts H3-K4 methylation, resulting in tissue-specific defects during development. Curr Biol. 2007;17:808–812. doi: 10.1016/j.cub.2007.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Haghighi AP, Portman SL, Lee JD, Amaranto AM, Goodman CS. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature. 2001;412:449–452. doi: 10.1038/35086595. [DOI] [PubMed] [Google Scholar]
- Disteche CM, Filippova GN, Tsuchiya KD. Escape from X inactivation. Cytogenet Genome Res. 2002;99:36–43. doi: 10.1159/000071572. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Yang X, DeBruyne JP, Peters AH, Weaver DR, Jenuwein T, Reppert SM. The polycomb group protein EZH2 is required for mammalian circadian clock function. J Biol Chem. 2006;281:21209–21215. doi: 10.1074/jbc.M603722200. [DOI] [PubMed] [Google Scholar]
- Fattaey AR, Helin K, Dembski MS, Dyson N, Harlow E, Vuocolo GA, Hanobik MG, Haskell KM, Oliff A, Defeo-Jones D, et al. Characterization of the retinoblastoma binding proteins RBP1 and RBP2. Oncogene. 1993;8:3149–3156. [PubMed] [Google Scholar]
- Feng W, Yonezawa M, Ye J, Jenuwein T, Grummt I. PHF8 activates transcription of rRNA genes through H3K4me3 binding and H3K9me1/2 demethylation. Nat Struct Mol Biol. 2010;17:445–450. doi: 10.1038/nsmb.1778. [DOI] [PubMed] [Google Scholar]
- Filippova GN, Cheng MK, Moore JM, Truong JP, Hu YJ, Nguyen DK, Tsuchiya KD, Disteche CM. Boundaries between chromosomal domains of X inactivation and escape bind CTCF and lack CpG methylation during early development. Dev Cell. 2005;8:31–42. doi: 10.1016/j.devcel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Frescas D, Guardavaccaro D, Bassermann F, Koyama-Nasu R, Pagano M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature. 2007;450:309–313. doi: 10.1038/nature06255. [DOI] [PubMed] [Google Scholar]
- Garcia-Bassets I, Kwon YS, Telese F, Prefontaine GG, Hutt KR, Cheng CS, Ju BG, Ohgi KA, Wang J, Escoubet-Lozach L, Rose DW, Glass CK, Fu XD, Rosenfeld MG. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128:505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godmann M, Auger V, Ferraroni-Aguiar V, Di Sauro A, Sette C, Behr R, Kimmins S. Dynamic regulation of histone H3 methylation at lysine 4 in mammalian spermatogenesis. Biol Reprod. 2007;77:754–764. doi: 10.1095/biolreprod.107.062265. [DOI] [PubMed] [Google Scholar]
- Good CD, Lawrence K, Thomas NS, Price CJ, Ashburner J, Friston KJ, Frackowiak RS, Oreland L, Skuse DH. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 2003;126:2431–2446. doi: 10.1093/brain/awg242. [DOI] [PubMed] [Google Scholar]
- Graves JA, Koina E, Sankovic N. How the gene content of human sex chromosomes evolved. Curr Opin Genet Dev. 2006;16:219–224. doi: 10.1016/j.gde.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Greenfield A, Carrel L, Pennisi D, Philippe C, Quaderi N, Siggers P, Steiner K, Tam PP, Monaco AP, Willard HF, Koopman P. The UTX gene escapes X inactivation in mice and humans. Hum Mol Genet. 1998;7:737–742. doi: 10.1093/hmg/7.4.737. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20:1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- Horton JR, Upadhyay AK, Qi HH, Zhang X, Shi Y, Cheng X. Enzymatic and structural insights for substrate specificity of a family of jumonji histone lysine demethylases. Nat Struct Mol Biol. 2010;17:38–43. doi: 10.1038/nsmb.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007a;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Greene E, Murray Stewart T, Goodwin AC, Baylin SB, Woster PM, Casero RA., Jr Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc Natl Acad Sci U S A. 2007b;104:8023–8028. doi: 10.1073/pnas.0700720104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci U S A. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles AR, Davies W, Burrmann D, Burgoyne PS, Wilkinson LS. Effects on fear reactivity in XO mice are due to haploinsufficiency of a non-PAR X gene: implications for emotional function in Turner’s syndrome. Hum Mol Genet. 2004;13:1849–1855. doi: 10.1093/hmg/ddh203. [DOI] [PubMed] [Google Scholar]
- Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Qi HH, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–1088. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Jensen LR, Amende M, Gurok U, Moser B, Gimmel V, Tzschach A, Janecke AR, Tariverdian G, Chelly J, Fryns JP, Van Esch H, Kleefstra T, Hamel B, Moraine C, Gecz J, Turner G, Reinhardt R, Kalscheuer VM, Ropers HH, Lenzner S. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am J Hum Genet. 2005;76:227–236. doi: 10.1086/427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CM, Lovell FL, Leongamornlert DA, Stranger BE, Dermitzakis ET, Ross MT. Large-scale population study of human cell lines indicates that dosage compensation is virtually complete. PLoS Genet. 2008;4:e9. doi: 10.1371/journal.pgen.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MH, Furlong RA, Burkin H, Chalmers IJ, Brown GM, Khwaja O, Affara NA. The Drosophila developmental gene fat facets has a human homologue in Xp11.4 which escapes X-inactivation and has related sequences on Yq11.2. Hum Mol Genet. 1996;5:1695–1701. doi: 10.1093/hmg/5.11.1695. [DOI] [PubMed] [Google Scholar]
- Kim TD, Shin S, Janknecht R. Repression of Smad3 activity by histone demethylase SMCX/JARID1C. Biochem Biophys Res Commun. 2008;366:563–567. doi: 10.1016/j.bbrc.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Kleine-Kohlbrecher D, Christensen J, Vandamme J, Abarrategui I, Bak M, Tommerup N, Shi X, Gozani O, Rappsilber J, Salcini AE, Helin K. A functional link between the histone demethylase PHF8 and the transcription factor ZNF711 in X-linked mental retardation. Mol Cell. 2010;38:165–178. doi: 10.1016/j.molcel.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307–318. doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- Koivisto AM, Ala-Mello S, Lemmela S, Komu HA, Rautio J, Jarvela I. Screening of mutations in the PHF8 gene and identification of a novel mutation in a Finnish family with XLMR and cleft lip/cleft palate. Clin Genet. 2007;72:145–149. doi: 10.1111/j.1399-0004.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lan F, Zaratiegui M, Villen J, Vaughn MW, Verdel A, Huarte M, Shi Y, Gygi SP, Moazed D, Martienssen RA. S. pombe LSD1 homologs regulate heterochromatin propagation and euchromatic gene transcription. Mol Cell. 2007a;26:89–101. doi: 10.1016/j.molcel.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, Alpatov R, Issaeva I, Canaani E, Roberts TM, Chang HY, Shi Y. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007b;449:689–694. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- Lan F, Nottke AC, Shi Y. Mechanisms involved in the regulation of histone lysine demethylases. Curr Opin Cell Biol. 2008;20:316–325. doi: 10.1016/j.ceb.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Holbert S, Ronce N, Faravelli F, Lenzner S, Schwartz CE, Lespinasse J, Van Esch H, Lacombe D, Goizet C, Phan-Dinh Tuy F, van Bokhoven H, Fryns JP, Chelly J, Ropers HH, Moraine C, Hamel BC, Briault S. Mutations in PHF8 are associated with X linked mental retardation and cleft lip/cleft palate. J Med Genet. 2005;42:780–786. doi: 10.1136/jmg.2004.029439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Choi HS, Gyuris J, Brent R, Moore DD. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995;9:243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem Biol. 2006;13:563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Lee MG, Norman J, Shilatifard A, Shiekhattar R. Physical and functional association of a trimethyl H3K4 demethylase and Ring6a/MBLR, a polycomb-like protein. Cell. 2007a;128:877–887. doi: 10.1016/j.cell.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007b;318:447–450. doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- Lingenfelter PA, Adler DA, Poslinski D, Thomas S, Elliott RW, Chapman VM, Disteche CM. Escape from X inactivation of Smcx is preceded by silencing during mouse development. Nat Genet. 1998;18:212–213. doi: 10.1038/ng0398-212. [DOI] [PubMed] [Google Scholar]
- Lopez-Bigas N, Kisiel TA, Dewaal DC, Holmes KB, Volkert TL, Gupta S, Love J, Murray HL, Young RA, Benevolenskaya EV. Genome-wide analysis of the H3K4 histone demethylase RBP2 reveals a transcriptional program controlling differentiation. Mol Cell. 2008;31:520–530. doi: 10.1016/j.molcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PJ, Sundquist K, Baeckstrom D, Poulsom R, Hanby A, Meier-Ewert S, Jones T, Mitchell M, Pitha-Rowe P, Freemont P, Taylor-Papadimitriou J. A novel gene (PLU-1) containing highly conserved putative DNA/chromatin binding motifs is specifically up-regulated in breast cancer. J Biol Chem. 1999;274:15633–15645. doi: 10.1074/jbc.274.22.15633. [DOI] [PubMed] [Google Scholar]
- Lubitz S, Glaser S, Schaft J, Stewart AF, Anastassiadis K. Increased apoptosis and skewed differentiation in mouse embryonic stem cells lacking the histone methyltransferase Mll2. Mol Biol Cell. 2007;18:2356–2366. doi: 10.1091/mbc.E06-11-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews AG, Kuo AJ, Ramon-Maiques S, Han S, Champagne KS, Ivanov D, Gallardo M, Carney D, Cheung P, Ciccone DN, Walter KL, Utz PJ, Shi Y, Kutateladze TG, Yang W, Gozani O, Oettinger MA. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450:1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE, III, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF. Sex chromosome complement affects social interactions in mice. Horm Behav. 2008;54:565–570. doi: 10.1016/j.yhbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–257. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Mortaud S, Nicolas L, Pinoteau W, Tordjman S, Carlier M, Roubertoux PL. Brain pathways mediating the pro-aggressive effect of the steroid sulfatase (Sts) gene. Behav Genet. 2010;40:211–219. doi: 10.1007/s10519-010-9340-6. [DOI] [PubMed] [Google Scholar]
- Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem. 2010;79:155–179. doi: 10.1146/annurev.biochem.78.070907.103946. [DOI] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38:47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- Okada Y, Scott G, Ray MK, Mishina Y, Zhang Y. Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature. 2007;450:119–123. doi: 10.1038/nature06236. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- Park JH, Burns-Cusato M, Dominguez-Salazar E, Riggan A, Shetty S, Arnold AP, Rissman EF. Effects of sex chromosome aneuploidy on male sexual behavior. Genes Brain Behav. 2008;7:609–617. doi: 10.1111/j.1601-183X.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prothero KE, Stahl JM, Carrel L. Dosage compensation and gene expression on the mammalian X chromosome: one plus one does not always equal two. Chromosome Res. 2009;17:637–648. doi: 10.1007/s10577-009-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropers HH, Hamel BC. X-linked mental retardation. Nat Rev Genet. 2005;6:46–57. doi: 10.1038/nrg1501. [DOI] [PubMed] [Google Scholar]
- Ross J, Roeltgen D, Zinn A. Cognition and the sex chromosomes: studies in Turner syndrome. Horm Res. 2006;65:47–56. doi: 10.1159/000090698. [DOI] [PubMed] [Google Scholar]
- Ryu H, Lee J, Hagerty SW, Soh BY, McAlpin SE, Cormier KA, Smith KM, Ferrante RJ. ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington’s disease. Proc Natl Acad Sci U S A. 2006;103:19176–19181. doi: 10.1073/pnas.0606373103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C, Rodriguez-Revenga L, Madrigal I, Badenas C, Pineda M, Mila M. A novel mutation in JARID1C gene associated with mental retardation. Eur J Hum Genet. 2006;14:583–586. doi: 10.1038/sj.ejhg.5201608. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Sampath SC, Intrator A, Min A, Gertler TS, Surmeier DJ, Tarakhovsky A, Greengard P. Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron. 2009;64:678–691. doi: 10.1016/j.neuron.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secombe J, Li L, Carlos L, Eisenman RN. The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 2007;21:537–551. doi: 10.1101/gad.1523007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown S, Norris D, Fisher A, Brockdorff N. The mouse Smcx gene exhibits developmental and tissue specific variation in degree of escape from X inactivation. Hum Mol Genet. 1996;5:1355–1360. doi: 10.1093/hmg/5.9.1355. [DOI] [PubMed] [Google Scholar]
- Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell. 2005;19:857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- Sims RJ, III, Millhouse S, Chen CF, Lewis BA, Erdjument-Bromage H, Tempst P, Manley JL, Reinberg D. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol Cell. 2007;28:665–676. doi: 10.1016/j.molcel.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaletsky H, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- Skuse DH. X-linked genes and mental functioning. Hum Mol Genet. 2005;14 (Spec1):R27–R32. doi: 10.1093/hmg/ddi112. [DOI] [PubMed] [Google Scholar]
- Skuse DH, James RS, Bishop DV, Coppin B, Dalton P, Aamodt-Leeper G, Bacarese-Hamilton M, Creswell C, McGurk R, Jacobs PA. Evidence from Turner’s syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387:705–708. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- Stadler F, Kolb G, Rubusch L, Baker SP, Jones EG, Akbarian S. Histone methylation at gene promoters is associated with developmental regulation and region-specific expression of ionotropic and metabotropic glutamate receptors in human brain. J Neurochem. 2005;94:324–336. doi: 10.1111/j.1471-4159.2005.03190.x. [DOI] [PubMed] [Google Scholar]
- Stewart DR, Kleefstra T. The chromosome 9q subtelomere deletion syndrome. Am J Med Genet C Semin Med Genet. 2007;145C:383–392. doi: 10.1002/ajmg.c.30148. [DOI] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabolacci E, Pietrobono R, Moscato U, Oostra BA, Chiurazzi P, Neri G. Differential epigenetic modifications in the FMR1 gene of the fragile X syndrome after reactivating pharmacological treatments. Eur J Hum Genet. 2005;13:641–648. doi: 10.1038/sj.ejhg.5201393. [DOI] [PubMed] [Google Scholar]
- Tahiliani M, Mei P, Fang R, Leonor T, Rutenberg M, Shimizu F, Li J, Rao A, Shi Y. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;447:601–605. doi: 10.1038/nature05823. [DOI] [PubMed] [Google Scholar]
- Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Tzschach A, Lenzner S, Moser B, Reinhardt R, Chelly J, Fryns JP, Kleefstra T, Raynaud M, Turner G, Ropers HH, Kuss A, Jensen LR. Novel JARID1C/SMCX mutations in patients with X-linked mental retardation. Hum Mutat. 2006;27:389. doi: 10.1002/humu.9420. [DOI] [PubMed] [Google Scholar]
- van Haaften G, et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41:521–523. doi: 10.1038/ng.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen M, Mulder KW, Denissov S, Pijnappel WW, van Schaik FM, Varier RA, Baltissen MP, Stunnenberg HG, Mann M, Timmers HT. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell. 2007;131:58–69. doi: 10.1016/j.cell.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Xu J, Pfau JL, Quadros PS, De Vries GJ, Arnold AP. Neonatal mice possessing an Sry transgene show a masculinized pattern of progesterone receptor expression in the brain independent of sex chromosome status. Endocrinology. 2004;145:1046–1049. doi: 10.1210/en.2003-1219. [DOI] [PubMed] [Google Scholar]
- Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, Krones A, Ohgi KA, Zhu P, Garcia-Bassets I, Liu F, Taylor H, Lozach J, Jayes FL, Korach KS, Glass CK, Fu XD, Rosenfeld MG. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446:882–887. doi: 10.1038/nature05671. [DOI] [PubMed] [Google Scholar]
- Weake VM, Workman JL. Histone ubiquitination: triggering gene activity. Mol Cell. 2008;29:653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Whitelaw NC, Whitelaw E. Transgenerational epigenetic inheritance in health and disease. Curr Opin Genet Dev. 2008;18:273–279. doi: 10.1016/j.gde.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Wissmann M, Yin N, Muller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Gunther T, Buettner R, Metzger E, Schule R. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- Wolf SS, Patchev VK, Obendorf M. A novel variant of the putative demethylase gene, s-JMJD1C, is a coactivator of the AR. Arch Biochem Biophys. 2007;460:56–66. doi: 10.1016/j.abb.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Wu J, Salido EC, Yen PH, Mohandas TK, Heng HH, Tsui LC, Park J, Chapman VM, Shapiro LJ. The murine Xe169 gene escapes X-inactivation like its human homologue. Nat Genet. 1994;7:491–496. doi: 10.1038/ng0894-491. [DOI] [PubMed] [Google Scholar]
- Wynder C, Hakimi MA, Epstein JA, Shilatifard A, Shiekhattar R. Recruitment of MLL by HMG-domain protein iBRAF promotes neural differentiation. Nat Cell Biol. 2005;7:1113–1117. doi: 10.1038/ncb1312. [DOI] [PubMed] [Google Scholar]
- Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet. 2002;11:1409–1419. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- Xu J, Taya S, Kaibuchi K, Arnold AP. Sexually dimorphic expression of Usp9x is related to sex chromosome complement in adult mouse brain. Eur J Neurosci. 2005;21:3017–3022. doi: 10.1111/j.1460-9568.2005.04134.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Watkins R, Arnold AP. Sexually dimorphic expression of the X-linked gene Eif2s3x mRNA but not protein in mouse brain. Gene Expr Patterns. 2006;6:146–155. doi: 10.1016/j.modgep.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS ONE. 2008a;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J Neurosci. 2008b;28:4521–4527. doi: 10.1523/JNEUROSCI.5382-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- Zechner U, Wilda M, Kehrer-Sawatzki H, Vogel W, Fundele R, Hameister H. A high density of X-linked genes for general cognitive ability: a run-away process shaping human evolution? Trends Genet. 2001;17:697–701. doi: 10.1016/s0168-9525(01)02446-5. [DOI] [PubMed] [Google Scholar]
- Zinn AR, Roeltgen D, Stefanatos G, Ramos P, Elder FF, Kushner H, Kowal K, Ross JL. A Turner syndrome neurocognitive phenotype maps to Xp22.3. Behav Brain Funct. 2007;3:24. doi: 10.1186/1744-9081-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]