Abstract
Osteocytes are well evidenced to be the major mechanosensor in bone, responsible for sending signals to the effector cells (osteoblasts and osteoclasts) that carry out bone formation and resorption. Consistent with this hypothesis, it has been shown that osteocytes release various soluble factors (e.g. transforming growth factor-β, nitric oxide, and prostaglandins) that influence osteoblastic and osteoclastic activities when subjected to a variety of mechanical stimuli, including fluid flow, hydrostatic pressure, and mechanical stretching. Recently, low-magnitude, high-frequency (LMHF) vibration (e.g., acceleration less than <1g, where g=9.98 m/s2, at 20-90 Hz) has gained much interest as studies have shown that such mechanical stimulation can positively influence skeletal homeostasis in animals and humans. Although the anabolic and anti-resorptive potential of LMHF vibration is becoming apparent, the signaling pathways that mediate bone adaptation to LMHF vibration are unknown. We hypothesize that osteocytes are the mechanosensor responsible for detecting the vibration stimulation and producing soluble factors that modulate the activity of effector cells. Hence, we applied low-magnitude (0.3g) vibrations to osteocyte-like MLO-Y4 cells at various frequencies (30, 60, 90 Hz) for 1 hour. We found that osteocytes were sensitive to this vibration stimulus at the transcriptional level: COX-2 maximally increased by 344% at 90 Hz, while RANKL decreased most significantly (-55%, p<0.01) at 60 Hz. Conditioned medium collected from the vibrated MLO-Y4 cells attenuated the formation of large osteoclasts (≥10 nuclei) by 36% (p<0.05) and the amount of osteoclastic resorption by 20% (p=0.07). The amount of soluble RANKL (sRANKL) in the conditioned medium was found to be 53% lower in the vibrated group (p<0.01), while PGE2 release was also significantly decreased (-61%, p<0.01). We conclude that osteocytes are able to sense LMHF vibration and respond by producing soluble factors that inhibit osteoclast formation.
Keywords: osteocyte, osteoclast, low-magnitude, high-frequency vibration, RANKL/OPG, PGE2
Introduction
Bone is a dynamic organ that perceives and adapts to the mechanical forces it experiences through the processes of bone resorption and bone formation. This is evident in athletes, where strenuous physical activity leads to increased bone mass [1], whereas in the case of bed rest patients [2-4] or astronauts under microgravity conditions [5; 6], diminished functional loading leads to decreased bone quantity. Considering the strong correlation between bone architecture and its surrounding mechanical environment, some researchers have turned to a biomechanical approach in treating skeletal disorders in an attempt to harness the native mechanosensitivity of bone tissue to enhance bone structure.
Recently, low-magnitude (LM; <1g, where g=9.98 m/s2), high-frequency (HF; 20-90 Hz) vibrations have gained interest as studies show that such a mechanical signal can positively influence skeletal homeostasis. Studies in animals have demonstrated that LMHF vibration stimulated an anabolic response in both weight-bearing [7; 8] and non-weight-bearing [9] bone. Further, LMHF vibration is able to rescue mice from ovariectomy-induced osteoporosis [10] and decreases osteoclast activity in adolescent mouse skeleton [11], providing evidence of LMHF vibration's anti-resorptive potential. Humans have also shown response to LMFH vibration treatment, as postmenopausal women treated with LMFH vibration stimulation gained higher bone mineral density (BMD) in the hip and spine compared to the placebo group after 6-12 months [12; 13]. Children with cerebral palsy, a disabling condition, also accrued higher BMD in the trabecular bone of the tibial and spinal regions after 6 months of vibration intervention [14]. Although the anabolic and anti-resorptive potential of LMHF vibration is becoming apparent, the underlying cellular and molecular regulation of this phenomenon is currently unknown.
The adaptation process of bone to mechanical forces is well evidenced to be the result of a series of cellular events orchestrated by osteocytes, the most abundant cells in bone. Osteocytes populate the bone tissue through a fluid-filled network comprised of cavities (termed lacunae) that house the cell bodies, and interconnect with each other and with cells on the bone surface via cellular projections running through narrow channels (termed canaliculi). Because of their unique location, osteocytes appear to be suitably placed for detecting mechanical loading [15-18] and sending signals to the effector cells at the bone surface, osteoblasts and osteoclasts, which carry out the formation and resorption of bone, respectively. Indeed, osteocytes have been found to communicate with effector cells through gap junctions [19] and soluble factors [20; 21], and such communication is mechanically regulated [22-25]. In particular, fluid flow-induced shear stress stimulated osteocytic production of anti-osteoclastic soluble factors [22; 23].
Among the many signals that osteocytes release in response to mechanical stimulation, receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG) are the two critical molecules in regulating osteoclasts. A requisite for osteoclast formation and activation is the binding of RANKL to the RANK receptor on osteoclast precursor cells [26; 27]. RANKL is expressed primarily as a transmembrane protein in osteoblastic/stromal cells, but may be converted to a soluble form through ectodomain shedding [28-30]. Both the membrane-bound and soluble forms of RANKL are biologically active, although the former has been found to be more potent [30]. OPG, also secreted by osteoblastic cells, is a decoy receptor of RANKL. By blocking the RANKL-RANK ligand interaction, OPG acts to antagonize the formation and survival of osteoclasts. Thus, the balance between RANKL and OPG defines the number of osteoclasts formed and their activity, which determines the rate of bone resorption.
Prostaglandin E2 (PGE2), the synthesis of which is catalyzed by the enzyme cyclooxygenase-2 (COX-2), is another important signaling molecule that osteocytes release in response to mechanical stimuli [32; 31]. PGE2 has been reported to act on both osteoblasts and osteoclasts, and have both stimulatory and inhibitory effects. In particular, PGE2 promotes the differentiation of osteoclasts in bone marrow cultures and spleen cell cultures in a manner that depends on its dosage, stage of osteoclast maturation, and supporting cell type [33-36]. PGE2 released by bone cells has been found to increase upon fluid flow stimulation and mediate downstream responses such as increased expression of gap junction protein connexin (Cx) 43 [37; 38] and decreased expression of OPG [39-41].
Osteoclast formation and activity have been demonstrated to be regulated by mechanical signals. In vitro, mechanical strain [42] and physiological levels of compression [43] applied directly to primary murine marrow cells inhibited osteoclast formation, while microgravity upregulated expression of genes involved in osteoclast maturation and activity, and increased bone resorption [44]. Mechanical stimulation can also modulate osteoclastogenesis activated by cell-cell contact with stromal/osteoblastic cells via changes in their RANKL/OPG ratio: pressurization on stromal cells [45] and microgravity on osteoblasts [46] increased their ability to initiate osteoclast formation and activity, concomitant with an up-regulation of RANKL/OPG ratio. Conversely, fluid flow downregulated the RANKL/OPG ratio in murine bone marrow stromal cells and mitigated their osteoclastogenic potential [47]. Furthermore, recent studies suggest that osteoclast formation can be mechanically regulated without direct contact between osteoclast precursors and supporting cells. Soluble factors produced by osteocytes in response to fluid flow were able to decrease osteoclast formation and activity in vitro [22; 23], suggesting that osteocytes deeply embedded in the mineralized tissue of bone may mediate bone resorption via diffusible biochemical factors.
In the present study, we hypothesized that osteocytes are able to sense LMHF vibration, and respond by secreting soluble signals that modulate the formation and activity of osteoclasts. To test this hypothesis, we subjected osteocyte-like MLO-Y4 cells to vibration at a magnitude of 0.3g and a frequency of 30, 60, or 90 Hz. We quantified the mRNA levels of COX-2, OPG, and RANKL and the release of several soluble factors (PGE2, OPG, and sRANKL). Finally, we tested how soluble factors released by osteocytes under LMHF vibration may influence the formation and activity of osteoclasts.
Methods and Materials
Cell cultures
Two cell lines – MLO-Y4 and RAW264.7 – were used in this study. MLO-Y4 (gift of Dr. Linda Bonewald, University of Missouri-Kansas City, Kansas City, MO) is a murine cell line that exhibits osteocyte-like morphology and several important molecular osteocyte markers [48]. RAW264.7 (American Type Culture Collection) is a murine monocyte/macrophage cell line that differentiates into multinucleated, bone-resorbing osteoclasts under RANKL stimulation alone.
MLO-Y4 cells were maintained in α-MEM (Invitrogen) supplemented with 2.5% fetal bovine serum (FBS) (HyClone), 2.5% calf serum (CS) (HyClone), and 100 U/ml Penicillin and 100 μg/ml Streptomycin (1% P/S) (Invitrogen) on type I rat tail collagen (BD Biosciences)-coated plates at 37°C and 5% CO2. Two days prior to vibration experiment, MLO-Y4 cells were plated in collagen-coated 6-well plates at a density of 4×105 cells/well for mRNA quantification and 3×105 cells/well for conditioned medium (CM) collection. This ensured that a maximum confluency of 80% was reached at the end of the experiment.
RAW264.7 cells were maintained in DMEM (Invitrogen) supplemented with 10% FBS (HyClone).To induce osteoclast formation, 20 ng/ml soluble RANKL (sRANKL) (R&D Systems) was added to RAW264.7 cells seeded at a density of 1×104 cells/well in a 24-well plate (day 0). The effect of soluble factors released by osteocytes on osteoclastogenesis was studied using the CM collected from MLO-Y4 cells. After 24 hours, the medium was replaced with a 1:1 (v:v) mixture of CM and growth medium. This procedure was repeated every subsequent day until day 5, when the cultures were fixed and stained for tartrate-resistant acid phosphatase (TRAP). Osteoclasts were identified as TRAP-positive cells containing three or more nuclei (RAW-OC). To quantify the number of RAW-OCs and the number of nuclei in each cell, five random fields of view per well were captured under microscope (100× magnification). To assess osteoclast activity, the same culture protocol described above was repeated on a synthetic calcium-phosphate surface (BD BioCoat™ Osteologic™, BD Biosciences) at a density of 1×103 cells/well for 7 days. Cells were then removed with bleach and the surface was treated with von Kossa staining. Resorbed areas were identified as regions that did not stain for von Kossa, and imaged under microscope (200× magnification). Image analysis was performed using ImageJ (NIH, version 1.42) in which the resorbed areas were defined manually and the number of pixels within each resorbed area was obtained.
Low-magnitude, high-frequency vibration
The system consisted of a custom-made vibration platform attached to a shaker (ET-127, Labworks Inc) (Figure 1) that delivered vertical vibrations. The amplitude, waveform, and frequency of the vibration provided by the shaker were controlled with VibeLab computer program (Labworks Inc). Peak-to-peak acceleration was measured at the centre of the vibration plate with a piezoelectric accelerometer (8632C5, Kistler), which output a voltage signal to the computer for feedback control between the desired and measured waveforms. We evaluated the signal's consistency by taking an average of the accelerometer reading at six random time points. The accelerometer was verified to measure 0.3g within less than 0.1% error. The system was housed in an ambient gas condition. To bring the temperature to approximately 37°C, a heater placed in the vicinity of the shaker was turned on for at least 15 minutes before the cells were placed on the vibration platform and throughout the duration of the experiment.
Figure 1. Experimental set-up.
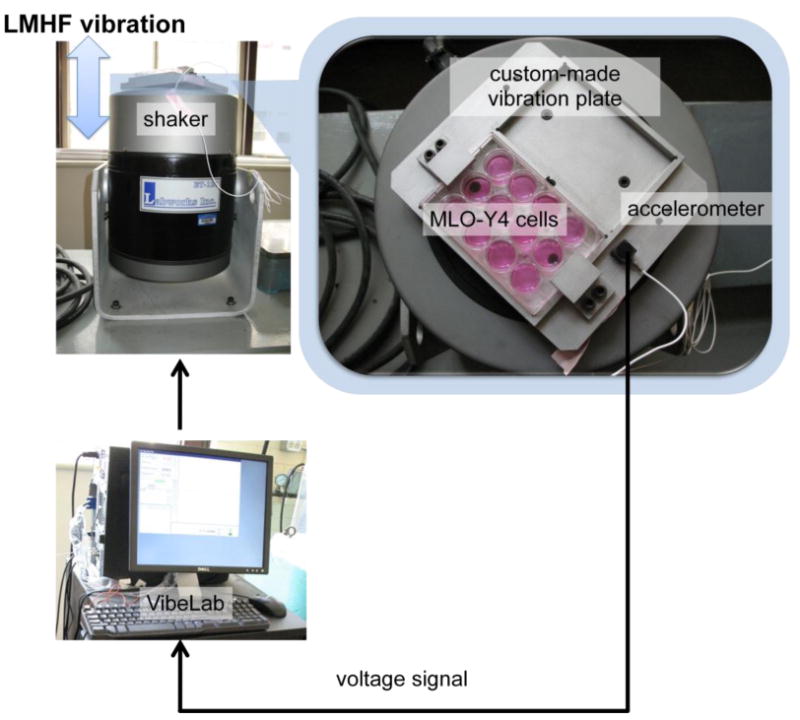
A rigid vibration plate was custom-made to fit two standard multi-well tissue culture plates. LMHF vibration was delivered through a vibration shaker controlled by a feedback loop consisting of an accelerometer attached to the vibration plate and a computer equipped with a VibeLab user interface.
To prevent fluid perturbation within the wells (and thus possible fluid shear stimulation on the cells) when vibrations were applied, culture wells containing MLO-Y4 cells of both the vibrated and non-vibrated groups were completely filled with working medium (α-MEM supplemented with 1% FBS, 1% CS, and 1% P/S) and tightly sealed with gas permeable sealing film (Excel Scientific) immediately prior to vibration. To confirm that there was no significant vibration-induced shear stress on osteocytes, we estimated the possible level of shear stress τ (Equation 1) assuming there was a very small displacement of fluid arising from vibrations. A sinusoidal vibration signal at 0.3g and 30 Hz (i.e. ∼10-2 s/cycle) generates a peak-to-peak displacement of ∼10-7 m (the largest displacement engendered compared to signals at 60 or 90 Hz). Thus, the velocity of the fluid u, or displacement over time, was estimated to be on the order of 10-5 m/s. The dynamic viscosity μ of the liquid was approximated by water's viscosity at room temperature as 10-3 Pa·s. Finally, the characteristic length of the fluid movement dy was defined as the width of the well in a 6-well plate, which is on the order of 10-2 m. Based on the estimated magnitudes of the relevant parameters, the maximum level of vibration-induced shear stress was estimated to be 10-6 Pa, which is at least five orders of magnitude lower than those that are known to excite bone cells in vitro [22; 49].
| (Equation 1) |
MLO-Y4 cells in the experimental group were subjected to 30, 60, or 90 Hz of sinusoidal vibrations at 0.3g for 1 hour. The frequencies and acceleration amplitude were chosen based on various animal and human vibration studies that reported positive bone remodeling [7-9; 12; 14]. Cells in the non-vibrated group were cultured and sealed in a similar manner, but were placed on a stationary plate for 1 hour. Immediately following the completion of vibration procedure, the cells were lysed for RNA isolation and mRNA analysis. For the Conditioned Medium (CM) study, following the 1 hour of vibration or control stationary period, cells in both the vibrated and non-vibrated groups were incubated with fresh growth medium (0.5 ml/well for 0.5 h time point, 1 ml/well for other time points as indicated), which was then collected as CM and stored at -20°C until subsequent analysis or use.
mRNA Quantification
Total RNA was isolated from MLO-Y4 cells using RNeasy Mini kit (Qiagen) and treated with DNase I (Fermentas) to remove any contaminating genomic DNA. Reverse transcription was performed on 2 μg RNA using SuperScript III (Invitrogen). The resulting cDNA samples were subjected to quantitative PCR (qPCR) using gene-specific primers and SYBR Green I Master Mix (Roche) in LightCycler 480 (Roche). Standards and samples were run in triplicate. mRNA levels of each gene of interest were normalized to 18S levels. Mouse-specific primers for COX-2, OPG, RANKL and 18S are listed in Table 1.
Table 1. Mouse gene-specific primer sequences.
| Gene | Forward | Reverse | Product size (bp) |
|---|---|---|---|
| COX-2 | 5′-TCCTCCTGGAACATGGACTC-3′ | 5′-CCCCAAAGATAGCATCTGGA-3′ | 173 |
| OPG | 5′-GGGCGTTACCTGGAGATCG-3′ | 5′-GAGAAGAACCCATCTGGACATTT-3′ | 125 |
| RANKL | 5′-CAGCATCGCTCTGTTCCTGTA-3′ | 5′-CTGCGTTTTCATGGAGTCTCA-3′ | 107 |
| 18S | 5′-GAGAAACGGCTACCACATCC-3′ | 5′-CCTCCAATGGATCCTCGTTA-3′ | 158 |
Quantification of Prostaglandin E2 (PGE2)
Supernatant levels of PGE2 were quantified using enzyme-linked immunoassay (Cayman Chemicals) per manufacturer's instructions. Values were normalized to total protein content, measured using BCA™ Protein Assay Kit (Pierce).
ELISA
Supernatant levels of RANKL and OPG in CM were measured using Quantikine Mouse RANKL Immunoassay and Quantikine Mouse OPG Immunoassay (R&D Systems), respectively, per manufacturer's instructions. Values were normalized to total protein content.
Statistical Analysis
Two-tailed t-test was used to compare means between two groups. One-way ANOVA was used to compare means of more than three groups, followed by Tukey post-hoc test. A significance level of 0.05 was employed. Data presented are representative of one experiment and reported as mean ± standard deviation. All experiments have been repeated for two to three times.
Results
LMHF vibration upregulates COX-2 mRNA level but decreases PGE2 release
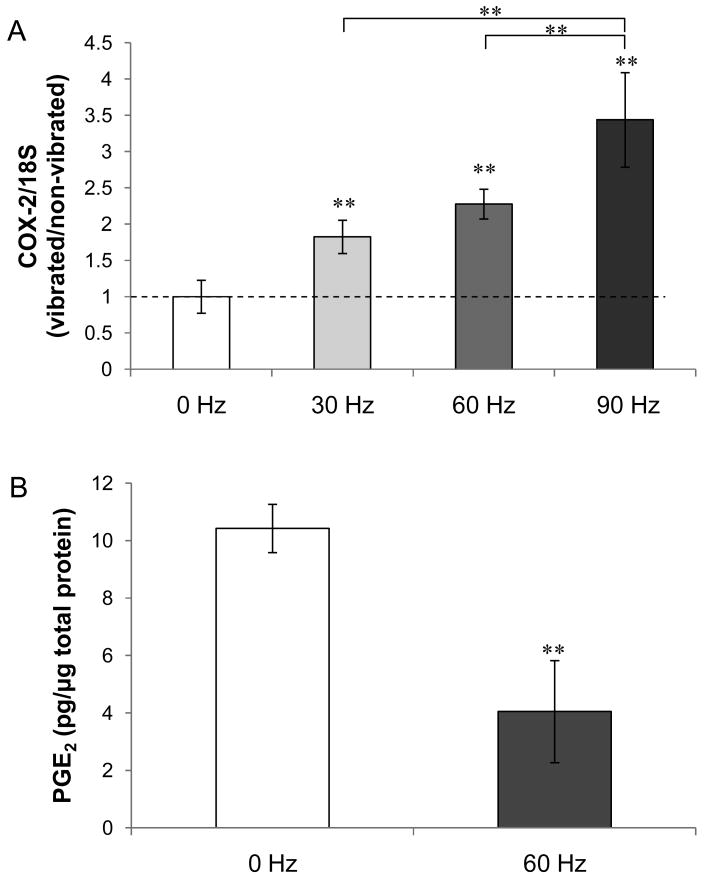
As an upregulation in COX-2 gene expression has been found in bone cell's early response to fluid flow stimulation [50], we measured COX-2 mRNA levels in MLO-Y4 osteocytes under LMHF vibration. One hour of LMHF vibration loading on MLO-Y4 cells induced significant changes in the mRNA level of COX-2 at all frequencies compared to 0 Hz stationary control (Figure 2A). Specifically, there is a trend of increasing COX-2 mRNA level as the frequency increased, indicating a dose-dependent relationship between mRNA expression and vibration frequency. At 90Hz, the COX-2 mRNA level was elevated by 3.4-fold (p<0.01) compared with the stationary control.
Figure 2. Effect of LMHF vibration on COX-2 mRNA level and PGE2 release in MLO-Y4 cells.
(A) The ratio of normalized COX-2 mRNA level between vibrated group and non-vibrated control (0 Hz) increased as the frequency of vibration increased, with the highest fold increase seen in 90 Hz (3.4-fold). (B) A 61% decrease in the amount of PGE2 accumulated in the medium during 1 hour of vibration loading was observed. **p<0.01 compared to 0 Hz control unless otherwise indicated (n=5).
Since fluid flow is known to upregulate COX-2-mediated PGE2 production in osteocytes [31; 49; 51], we next determined whether LMHF vibration had a similar effect. We collected CM immediately following 1 hour of vibration and found a significant decrease in the levels of PGE2 in the vibration group (-61%, p<0.01) compared with the stationary control (Figure 2B).
LMHF vibration downregulates RANKL mRNA and protein levels
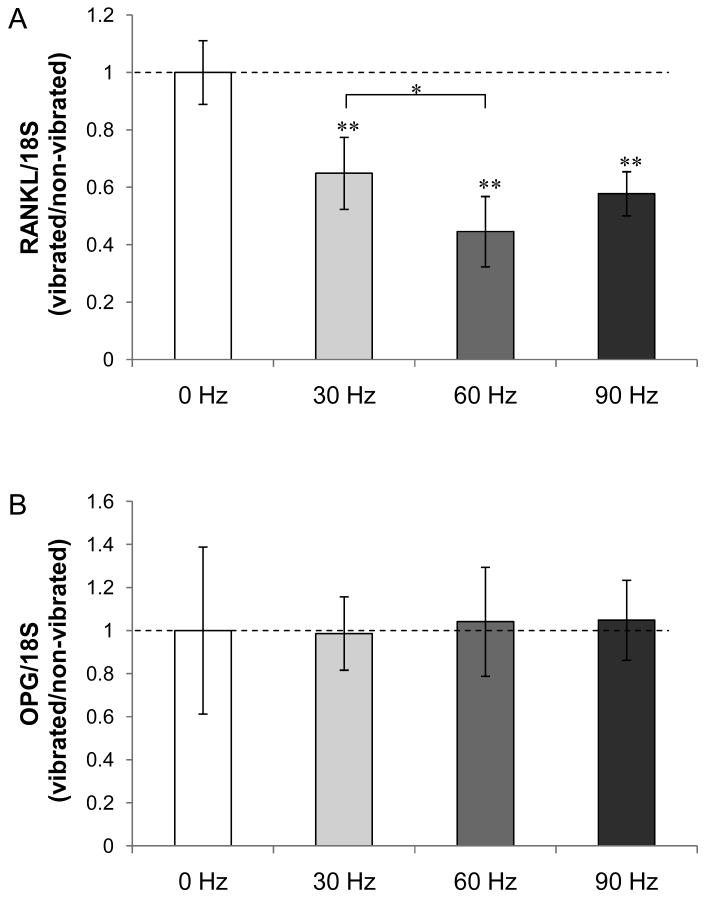
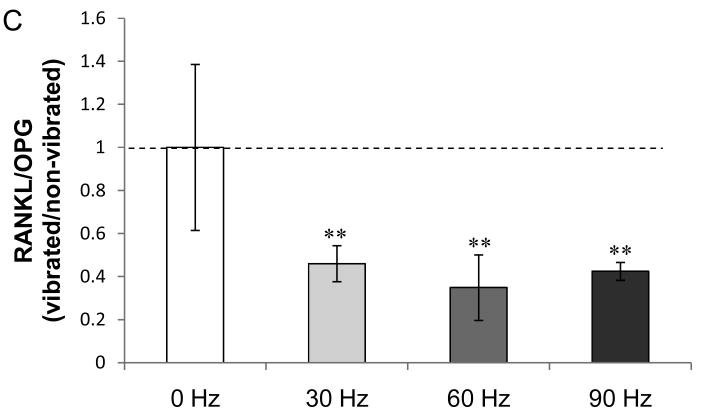
We investigated whether LMHF vibration alters the RANKL/OPG signaling axis in osteocytes. One hour of LMHF vibration loading on MLO-Y4 cells decreased RANKL mRNA levels at all frequencies, and most significantly at 60 Hz (−55%; p<0.01) (Figure 3A). Although the mRNA level of OPG was not altered (Figure 3B), the decrease in RANKL contributed to a significant decrease in RANKL/OPG ratio at all frequencies (Figure 3C). Together, these data suggest that LMHF vibration drives mRNA expression changes towards an anti-resorptive effect.
Figure 3. Effect of LMHF vibration on RANKL/OPG mRNA expression.
(A) LMHF vibration did not alter OPG expression. (B) RANKL expression decreased as the frequency increased and most significantly at 60 Hz (-55%). *p<0.05; **p<0.01 compared to 0 Hz control (n=5).
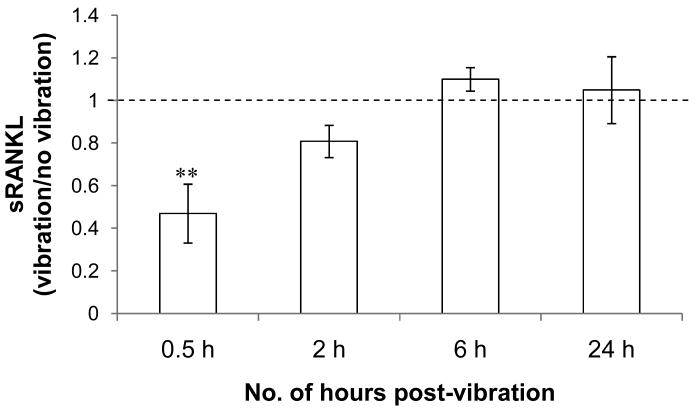
Considering our observed changes in RANKL mRNA expression and previous reports of altered sRANKL/OPG protein production under fluid flow, we next investigated whether LMHF vibration may cause MLO-Y4 cells to produce a lower amount of sRANKL. We collected CM at various time points (0.5, 2, 6, 24 hours) post-vibration and measured the amount of OPG and sRANKL using ELISA. Despite previous reports of OPG release by MLO-Y4 cells [22], we were not able to detect any OPG at all time points. For sRANKL, the amount was decreased by 53% in the CM collected at 30 minutes post-vibration from the vibrated MLO-Y4 cells (Figure 4). At as early as 2 hours post-vibration, the amount of sRANKL returned to control levels.
Figure 4. Amount of sRANKL in MLO-Y4 conditioned medium.
LMHF vibration caused a transient decrease in the amount of sRANKL produced by MLO-Y4 cells. At 0.5 hour post-vibration, there was a 53% decrease in the amount of sRANKL produced by vibrated MLO-Y4 cells. The amount of sRANKL in the vibrated groups returned to levels comparable to static control at as early as 2 hours post-vibration. The average sRANKL values in static controls (represented by a normalized value of 1) were 600±120, 1720±590, 2750±380, and 7710±1180 pg/mg total protein at 0.5, 2, 6, and 24 hours post-vibration, respectively. **p<0.01 compared to 0 Hz control (n=6).
Conditioned medium from the vibrated MLO-Y4 cells inhibits formation of large osteoclasts
Previous studies have shown that fluid flow caused osteocytes to secrete soluble factors that inhibited osteoclast formation and activity [22; 23]. To investigate whether LMHF vibration has similar effects, CM was collected from MLO-Y4 cells 30 minutes after the completion of 1 hour of LMHF vibration at 60 Hz. RAW264.7 monocytes were induced to form osteoclasts with sRANKL in the presence of 50% CM from either the vibrated or the non-vibrated MLO-Y4 osteocytes. After 5 days in culture, TRAP-positive, multinuclear cells were observed (Figure 5A). In the vibration group, there was a small but insignificant decrease in the total number of RAW-OCs containing 3 or more nuclei per cell (Figure 5B). However, upon closer examination, the population of RAW-OCs containing 10 or greater number of nuclei was found to be significantly lower in the cultures containing CM from the vibrated osteocytes (-36%, p<0.05) (Figure 5C).
Figure 5. Effect of conditioned medium from vibrated MLO-Y4 cells on osteoclast formation.
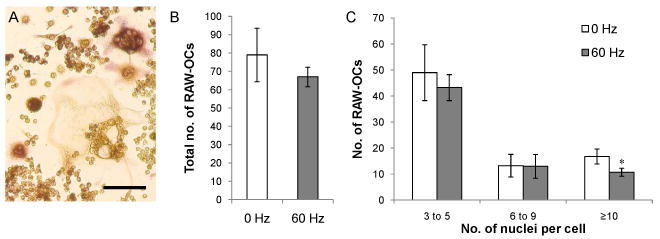
(A) TRAP-positive, multinuclear RAW-OCs after 5 days in medium supplemented with 20 ng/ml sRANKL. Bar = 100 μm. (B) LMHF vibration caused MLO-Y4 cells to secrete soluble factors that moderately decreased the total number of osteoclasts formed. (C) Osteoclasts that formed in the presence of conditioned medium from the vibrated MLO-Y4 contained fewer number of large RAW-OCs (≥10 nuclei) (-36%). *p<0.05 compared to 0 Hz control (n=4).
Since the number of nuclei in an osteoclast has been linked to the cells' bone-resorbing potential [52; 53], we next investigated whether the vibrated MLO-Y4 osteocytes produced diffusible factors that affected the activity of osteoclasts. RAW-OCs were formed under the same condition as described above but on a synthetic calcium-phosphate surface. After 7 days in culture, areas of calcium-phosphate resorption were observed (Figure 6A). Osteoclasts cultured in the presence of CM from the vibrated MLO-Y4 cells resorbed less calcium-phosphate than the control counterpart containing CM from the non-vibrated cells (-20%, p=0.07) (Figure 6B).
Figure 6. Effect of conditioned medium from the vibrated MLO-Y4 cells on osteoclast activity.
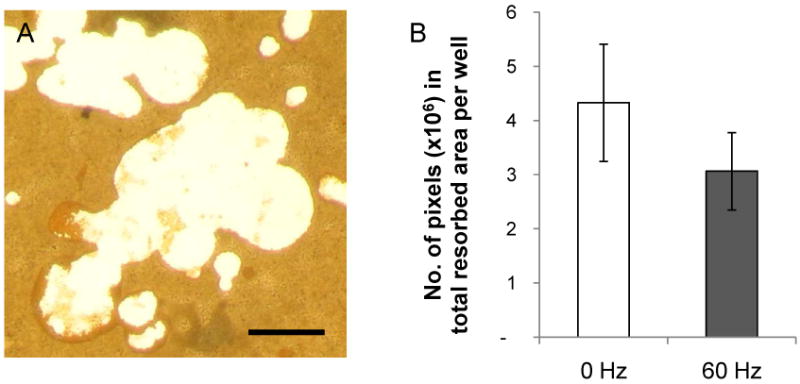
(A) Areas of calcium-phosphate resorbed by RAW-OCs (white) did not stain for von Kossa (brown). Bar = 50 μm. (B) There was a trend of decreased calcium-phosphate resorption by RAW-OCs in the presence of conditioned medium from the vibrated MLO-Y4 cells (p=0.07, n=5).
Discussion
In this study, we aimed to investigate the underlying cellular and molecular mechanism by which LMHF vibration plays an anti-resorptive role in bone. We found that MLO-Y4 osteocytes responded to LMHF vibration at both the transcript and protein levels, and secreted soluble factors that inhibited osteoclast formation. There was also a trend of decreased osteoclast resorption activity.
Consistent with results from the studies of bone cells under fluid flow, exposure to 1 hour of LMHF vibration led to a significant upregulation of COX-2 mRNA expression [54-56]. Interestingly, we found a significant decrease in the amount of PGE2 secreted by MLO-Y4 cells under vibration loading, which is consistent with the results by Bacabac et al., who showed that vibration loading decreased PGE2 release in osteoblasts while COX-2 was upregulated in a frequency-dependent manner [57]. Cherian et al. recently showed that fluid-flow induced PGE2 release in osteocytes was mediated through the opening of Cx43 hemichannels [58]. Future studies will help elucidate possible mechanisms of reduced PGE2 release under vibration stimulation. As PGE2 is known to stimulate osteoclast formation and activation [33-36], the biological significance of inhibited PGE2 release by vibration may be an anti-resorptive effect.
As RANKL/OPG is the major signaling axis controlling osteoclast formation, activation, and survival, we measured RANKL and OPG mRNA expression changes in the vibrated MLO-Y4 cells. Cells under vibration expressed a significantly lower level of RANKL mRNA. Due to an unaltered level of OPG, the overall RANKL/OPG ratio was decreased. In contrast, You et al. demonstrated that oscillatory fluid flow induced an increase in the expression of both OPG and RANKL in MLO-Y4 cells, but also an overall decrease in the RANKL/OPG ratio [22]. This suggests that the two mechanical stimuli may have a similar functional outcome on bone (e.g. decreased osteoclast activity) via regulation of the RANKL/OPG axis, but the immediate response to the two different forms of mechanical stress may be exerted via different pathways upstream of RANKL/OPG signaling depending on the particular mechanical signal.
We continued to measure the amount of sRANKL secreted by MLO-Y4 osteocytes under vibration loading. RANKL exists primarily as membrane-bound form, but may be released from the plasma membrane through ectodomain shedding. Our ELISA data indicated that at 30 minutes post-vibration, the amount of sRANKL significantly decreased in the CM collected from the vibrated MLO-Y4 cells compared with the non-vibrated MLO-Y4 cells. Because of the relatively short time span over which the phenomenon was observed, we speculate that vibration loading likely inhibited RANKL release from the membrane as opposed to inhibiting de novo protein synthesis. Future studies investigating the effect of LMHF vibration on recently proposed candidates of RANKL sheddases (e.g. TNF-α converting enzyme (TACE) [28], metalloproteinase(MMP)-7 [59], and MMP-14 [30]) may elucidate the precise mechanism.
The physiologic and pathologic significance of RANKL shedding are currently unclear. An overexpression of soluble RANKL in transgenic mice induced severe osteoporosis by accelerated osteoclastogenesis. Furthermore, in a mouse model of prostate cancer metastasis in bone, solubilization of RANKL by MMP-7 contributes to tumor-induced osteolysis [59]. In contrast, Hikita et al. proposed that ectodomain shedding in osteoblasts may work as a negative regulator of local bone resorption by reducing membrane-bound RANKL [30], which was shown to be more potent than the soluble form [29]. In the case of osteocytes, however, their anatomical location likely provides limited cell-cell contact with osteoclast precursors and thus a lower level of membrane-bound RANKL-RANK interaction compared with osteoblasts at the bone surface. Thus, osteocytes may contribute to the RANKL/OPG balance via regulation of soluble RANKL, and inhibit osteoclastogenesis by decreasing the amount of RANKL cleaved from the membrane.
We attempted to measure the amount of OPG in the CM of MLO-Y4 cells, but the levels of OPG at all post-vibration time points (0.5, 2, 6, 24 h) were below the detection threshold of the ELISA kit (31.2pg/ml) for both the vibrated and non-vibrated groups. To determine whether the lack of OPG was due to the manipulation of the cells associated with the experiment, we sampled the CM from the MLO-Y4 cells maintained in culture, but again could not quantify any OPG. The reason for the absence of OPG release is unclear, although others have previously reported OPG release from MLO-Y4 cells [22]. The possibility of over-subculturing leading to altered protein expression profile is unlikely, as the passage number of the cells used in the experiments was relatively low (passage no. 24-30). Despite the lack of OPG release, the behavior of our MLO-Y4 cells appeared normal, as they exhibited the typical dendritic morphology and the same cellular responses reported by other groups when stimulated by fluid flow [22; 60].
Based on our observed differential levels of PGE2 and sRANKL, we expected that osteocytes might detect LMHF vibration and respond by secreting diffusible signals that act on osteoclastic cells. Indeed, when cultured in the presence of CM from the vibrated MLO-Y4 cells, the number of TRAP-positive, multinuclear cells was moderately decreased, concomitant with a significantly lower number of large osteoclasts formed (those containing ≥10 nuclei per cell). As large osteoclasts are associated with higher resorbing capability [53; 61], the data suggests that the vibrated MLO-Y4 osteocytes secrete soluble signals that inhibit not only the formation of osteoclasts, but also the amount of bone resorption. This was supported by the finding that RAW-OCs cultured in the presence CM from the vibrated MLO-Y4 osteocytes resorbed a moderately lower amount of calcium-phosphate (-20%, p=0.07).
In our study, the observed vibration-induced changes in mRNA expression were frequency-dependent, as a greater fold change was observed as the frequency increased. COX-2 mRNA expression peaked at 90 Hz, while RANKL mRNA expression was decreased most significantly at 60 Hz, suggesting that there is an optimal frequency at which the signal is most anabolic/anti-resorptive. This possibility is supported by in vivo LMHF vibration studies, where vibration at 90 Hz was more anabolic than vibration at 45 Hz in ovariectomized rats [8]. Interestingly, in an animal model involving orthodontic tooth movement, Nishimura et al. found that when teeth were subjected to vibrations at their resonance frequency (i.e. the frequency that produced the largest amplitude of vibration to the periodontal tissue, which in this case occurred at approximately 60 Hz), the speed of tooth movement was accelerated, along with an increased number of osteoclasts and enhanced expression of RANKL in the alveolar bone. These data confirm the existence of a frequency-dependent effect of vibration stimulus on the skeleton, and suggest that the exact outcome on bone remodeling may be largely dependent on the structure and function of different bones.
The mechanism behind the frequency dependency of osteocyte response to vibration is not well understood. In a previous in vitro study involving application of fluid flow to bone cells, a larger percentage of rat osteoblasts responded in the form of intracellular calcium oscillations when loading was applied at a lower frequency (0.2 Hz) compared to at 1 Hz [62], while another study showed that nitric oxide (NO) production in MC3T3-E1 cells increased linearly with the rate of fluid shear stress (where frequency was varied between 5-9 Hz) [63]. A possible explanation is that due to the viscoelasticity of cells, cells are less stiff and thus more deformable (and responsive) at lower rates of loading (below 1 Hz). However, at higher frequencies, the mechanical signal may excite bone cells via different mechanotransducing machinery independent of cellular deformation. Bacabac et al. proposed that the cellular component responsible for sensing high-frequency vibration might be the nucleus [57].
The effects observed in our current study are a direct result of MLO-Y4 cells being subjected to vibration loading, as our experimental set-up delineated fluid flow-induced effects from vibration effects by minimizing the movement of fluid in the system. In vivo, the precise mechanical stimulus pattern and magnitude experienced by osteocytes at the cellular level when the whole body is subjected to LMHF vibration are currently unknown. However, the transmissibility of whole-body vibration to the axial skeleton in human subjects was confirmed by Rubin et al., who showed that the vibration signal was maximally transmitted (∼80%) to the hip and spine when subjects were in a standing position. There is ongoing effort in using mathematical modeling to better characterize the signal at the cellular level [57].
Recent animal studies suggest that LMHF vibration has the ability to direct the lineage commitment of bone marrow-derived mesenchymal stromal cells (BM-MSCs) [64; 65] by biasing cell fate in favor of osteogenesis over adipogenesis. However, currently there is no direct evidence demonstrating the role of BM-MSCs in the mechanotransduction of LMHF vibration. Considering the cellular heterogeneity of the bone marrow compartment in which BM-MSCs reside, heterotypic cellular interactions are likely to influence the cell fate of this progenitor population [66; 67]. As osteocytes have been shown to affect osteogenesis via soluble factors [20], osteocytes may play a role in directing the MSC fate via soluble factors under the stimulation of LMHF vibration.
In conclusion, our data provides a first glimpse at how osteocytes respond to low magnitude, high-frequency vibration. Osteocytes appear to mediate the anti-resorptive effect associated with LMHF vibration found in vivo. At the transcript level, LMHF vibration drives gene changes that favor anti-resorptive effects. This conclusion is further supported by the observation that under LMHF vibration, MLO-Y4 cells secrete soluble factors that inhibit the number and the size of the osteoclasts formed. A decrease in sRANKL and PGE2 secreted by vibrated osteocytes suggest that RANKL and PGE2 may be involved in the inhibitory effects of LMHF vibration on osteoclastogenesis.
Acknowledgments
We would like to thank Qiming Pang (University of Toronto) for her assistance in establishing the vibration system, Emily Walker (University of Toronto) for her technical support on qPCR, and Dr. William Stanford (University of Toronto) for the use of the qPCR machine in his laboratory. This research is supported by NSERC DG and CFI LOF as well as funding from US National Institute of Health (AR054385 and P20RR016458).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones H, Priest J, Hayes W, Tichenor C, Nagel D. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204–208. [PubMed] [Google Scholar]
- 2.Leblanc A, Schneider V, Evans H, Engelbretson D, Krebs J. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5(8):843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc A, Schneider V, Krebs J, Evans H, Jhingran S, Johnson P. Spinal bone mineral after 5 weeks of bed rest. Calcif Tissue Int. 1987;41(5):259–261. doi: 10.1007/BF02555226. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson C, Hulley S, Vogel J, Hattner R, Bayers J, McMillan D. Effect of prolonged bed rest on bone mineral. Metabolism. 1970;19(12):1071–1084. doi: 10.1016/0026-0495(70)90032-6. [DOI] [PubMed] [Google Scholar]
- 5.Rambaut P, Johnston R. Prolonged weightlessness and calcium loss in man. Acta Astronautica. 1979;6(9):1113–1122. doi: 10.1016/0094-5765(79)90059-6. [DOI] [PubMed] [Google Scholar]
- 6.Vico L, Collet P, Guignandon A, Lafage-Proust M, Thomas T, Rehailia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355(9215):1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 7.Rubin C, Turner AS, Müller R, Mittra E, McLeod K, Lin W, Qin Y. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17(2):349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 8.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40(6):1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Garman R, Gaudette G, Donahue L, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25(6):732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 10.Oxlund BS, Ørtoft G, Andreassen TT, Oxlund H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32(1):69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 11.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39(5):1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19(3):343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 13.Verschueren SMP, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19(3):352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 14.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 15.You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–1386. doi: 10.1016/s0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- 16.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci U S A. 2007;104(40):15941–15946. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A. 2004;101(47):16689–16694. doi: 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2007;292(1):C545–552. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 20.Heino TJ, Hentunen TA, Väänänen HK. Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into osteoblasts. Exp Cell Res. 2004;294(2):458–468. doi: 10.1016/j.yexcr.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Heino TJ, Hentunen TA, Vaananen HK. Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: Enhancement by estrogen. J Cell Biochem. 2002;85(2002):185–197. doi: 10.1002/jcb.10109. [DOI] [PubMed] [Google Scholar]
- 22.You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2007 doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone. 2007;41(5):745–751. doi: 10.1016/j.bone.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Heino TJ, Hentunen TA, Väänänen HK. Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into osteoblasts. Exp Cell Res. 2004;294(2):458–468. doi: 10.1016/j.yexcr.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun. 2006;348(3):1082–1088. doi: 10.1016/j.bbrc.2006.07.146. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacey DL, Timms E, Tan H, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian Y, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin Ligand Is a Cytokine that Regulates Osteoclast Differentiation and Activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 28.Lum L, Wong BR, Josien R, Becherer JD, Erdjument-Bromage H, Schlöndorff J, Tempst P, Choi Y, Blobel CP. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem. 1999;274(19):13613–13618. doi: 10.1074/jbc.274.19.13613. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, Sakai H. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275(3):768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- 30.Hikita A, Yana I, Wakeyama H, Nakamura M, Kadono Y, Oshima Y, Nakamura K, Seiki M, Tanaka S. Negative Regulation of Osteoclastogenesis by Ectodomain Shedding of Receptor Activator of NF- B Ligand. J Biol Chem. 2006;281(48):36846–36855. doi: 10.1074/jbc.M606656200. [DOI] [PubMed] [Google Scholar]
- 31.Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX. Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278(44):43146–43156. doi: 10.1074/jbc.M302993200. [DOI] [PubMed] [Google Scholar]
- 32.Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating Fluid Flow Increases Prostaglandin Production by Cultured Chicken Osteocytes--A Cytoskeleton-Dependent Process. Biochem Biophys Res Commun. 1996;225(1):62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 33.Quinn J, Sabokbar A, Denne M, de Vernejoul M, McGee J, Athanasou N. Inhibitory and stimulatory effects of prostaglandins on osteoclast differentiation. Calcif Tissue Int. 1997;60(1):63–70. doi: 10.1007/s002239900187. [DOI] [PubMed] [Google Scholar]
- 34.Collins DA, Chambers TJ. Prostaglandin E2 promotes osteoclast formation in murine hematopoietic cultures through an action on hematopoietic cells. J Bone Miner Res. 1992;7(5):555–561. doi: 10.1002/jbmr.5650070512. [DOI] [PubMed] [Google Scholar]
- 35.Kaji H, Sugimoto T, Kanatani M, Fukase M, Kumegawa M, Chihara K. Prostaglandin E2 stimulates osteoclast-like cell formation and bone-resorbing activity via osteoblasts: role of cAMP-dependent protein kinase. J Bone Miner Res. 1996;11(1):62–71. doi: 10.1002/jbmr.5650110110. [DOI] [PubMed] [Google Scholar]
- 36.Ono K, Kaneko H, Choudhary S, Pilbeam CC, Lorenzo JA, Akatsu T, Kugai N, Raisz LG. Biphasic Effect of Prostaglandin E2 on Osteoclast Formation in Spleen Cell Cultures. J Bone Miner Res. 2005;20(1):23–29. doi: 10.1080/14041040510033842. [DOI] [PubMed] [Google Scholar]
- 37.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, Jiang JX. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283(39):26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16(2):249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 39.Tanabe N, Maeno M, Suzuki N, Fujisaki K, Tanaka H, Ogiso B, Ito K. IL-1[alpha] stimulates the formation of osteoclast-like cells by increasing M-CSF and PGE2 production and decreasing OPG production by osteoblasts. Life Sci. 2005;77(6):615–626. doi: 10.1016/j.lfs.2004.10.079. [DOI] [PubMed] [Google Scholar]
- 40.Coetzee M, Haag M, Kruger MC. Effects of arachidonic acid, docosahexaenoic acid, prostaglandin E2 and parathyroid hormone on osteoprotegerin and RANKL secretion by MC3T3-E1 osteoblast-like cells. J Nutr Biochem. 2007;18(1):54–63. doi: 10.1016/j.jnutbio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Kirschenbaum A, Yao S, Levine AC. Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann N Y Acad Sci. 2006;1068:225–233. doi: 10.1196/annals.1346.047. [DOI] [PubMed] [Google Scholar]
- 42.Rubin J, Murphy T, Nanes MS, Fan X. Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol Cell Physiol. 2000;278(6):C1126–1132. doi: 10.1152/ajpcell.2000.278.6.C1126. [DOI] [PubMed] [Google Scholar]
- 43.Klein-Nulend JJ, Veldhuijzen Paul, Van Strien Michael E, De Jong Marcel, Burger Elisabeth H. Inhibition of osteoclastic bone resorption by mechanical stimulation in vitro. Arthritis Rheum. 1990;33(1):66–72. doi: 10.1002/art.1780330108. [DOI] [PubMed] [Google Scholar]
- 44.Tamma R, Colaianni G, Camerino C, Di Benedetto A, Greco G, Strippoli M, Vergari R, Grano A, Mancini L, Mori G, Colucci S, Grano M, Zallone A. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J. 2009;23(8):2549–2554. doi: 10.1096/fj.08-127951. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Zhao Z, Zou L, Li J, Wang F, Li X, Zhang J, Liu Y, Chen S, Zhi M, Wang J. Pressure-loaded MSCs during early osteodifferentiation promote osteoclastogenesis by increase of RANKL/OPG ratio. Ann Biomed Eng. 2009;37(4):794–802. doi: 10.1007/s10439-009-9638-9. [DOI] [PubMed] [Google Scholar]
- 46.Rucci N, Rufo A, Alamanou M, Teti A. Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J Cell Biochem. 2007;100(2):464–473. doi: 10.1002/jcb.21059. [DOI] [PubMed] [Google Scholar]
- 47.Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39(5):1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Bonewald LF. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. J Bone Miner Metab. 1999;17(1):61–65. doi: 10.1007/s007740050066. [DOI] [PubMed] [Google Scholar]
- 49.Klein-Nulend J, Van der Plas A, Semeins CM, Ajubi NE, Frangos JA, Nijweide PJ, Burger EH. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9(5):441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 50.Klein-Nulend J, Burger EH, Semeins CM, Raisz LG, Pilbeam CC. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res. 1997;12(1):45–51. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- 51.Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiol. 1999;276(1 Pt 1):E171–178. doi: 10.1152/ajpendo.1999.276.1.E171. [DOI] [PubMed] [Google Scholar]
- 52.Lees RL, Sabharwal VK, Heersche JN. Resorptive state and cell size influence intracellular pH regulation in rabbit osteoclasts cultured on collagen-hydroxyapatite films. Bone. 2001;28(2):187–194. doi: 10.1016/s8756-3282(00)00433-6. [DOI] [PubMed] [Google Scholar]
- 53.Piper K, Boyde A, Jones SJ. The relationship between the number of nuclei of an osteoclast and its resorptive capability in vitro. Anat Embryol. 1992;186(4):291–299. doi: 10.1007/BF00185977. [DOI] [PubMed] [Google Scholar]
- 54.Norvell SM, Ponik SM, Bowen DK, Gerard R, Pavalko FM. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol. 2004;96(3):957–966. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]
- 55.Wadhwa S, Godwin SL, Peterson DR, Epstein MA, Raisz LG, Pilbeam CC. Fluid flow induction of cyclo-oxygenase 2 gene expression in osteoblasts is dependent on an extracellular signal-regulated kinase signaling pathway. J Bone Miner Res. 2002;17(2):266–274. doi: 10.1359/jbmr.2002.17.2.266. [DOI] [PubMed] [Google Scholar]
- 56.Bakker AD, Klein-Nulend J, Burger EH. Mechanotransduction in bone cells proceeds via activation of COX-2, but not COX-1. Biochem Biophys Res Commun. 2003;305(3):677–683. doi: 10.1016/s0006-291x(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 57.Bacabac RG, Smit TH, Van Loon JJWA, Doulabi BZ, Helder M, Klein-Nulend J. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 2006;20(7):858–864. doi: 10.1096/fj.05-4966.com. [DOI] [PubMed] [Google Scholar]
- 58.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical Strain Opens Connexin 43 Hemichannels in Osteocytes: A Novel Mechanism for the Release of Prostaglandin. Mol Biol Cell. 2005;16(7):3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B, Shirai T, Matrisian LM, Futakuchi M. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7(5):485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 60.Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. PGE2 Is Essential for Gap Junction-Mediated Intercellular Communication between Osteocyte-Like MLO-Y4 Cells in Response to Mechanical Strain. Endocrinology. 2001;142(8):3464–3473. doi: 10.1210/endo.142.8.8338. [DOI] [PubMed] [Google Scholar]
- 61.Lees RL, Heersche JN. Differences in regulation of pH(i) in large (>/=10 nuclei) and small (</=5 nuclei) osteoclasts. Am J Physiol Cell Physiol. 2000;279(3):C751–761. doi: 10.1152/ajpcell.2000.279.3.C751. [DOI] [PubMed] [Google Scholar]
- 62.Donahue SW, Jacobs CR, Donahue HJ. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol. 2001;281(5):C1635–1641. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- 63.Bacabac RG, Smit TH, Mullender MG, Dijcks SJ, Van Loon JJWA, Klein-Nulend J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem Biophys Res Commun. 2004;315(4):823–829. doi: 10.1016/j.bbrc.2004.01.138. [DOI] [PubMed] [Google Scholar]
- 64.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104(45):17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24(1):50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem. 1999;72(3):396–410. [PubMed] [Google Scholar]
- 67.Martelli F, Verrucci M, Migliaccio G, Zingariello M, Rana RA, Vannucchi AM, Migliaccio AR. Removal of the spleen in mice alters the cytokine expression profile of the marrow micro-environment and increases bone formation. Ann N Y Acad Sci. 2009;1176:77–86. doi: 10.1111/j.1749-6632.2009.04968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]