Abstract
Chlamydia trachomatis is an obligate intracellular bacterial pathogen that infects hundreds of millions of individuals globally, causing blinding trachoma and sexually transmitted disease. More effective chlamydial control measures are needed, but progress toward this end has been severely hampered by the lack of a tenable chlamydial genetic system. Here, we describe a reverse-genetic approach to create isogenic C. trachomatis mutants. C. trachomatis was subjected to low-level ethyl methanesulfonate mutagenesis to generate chlamydiae that contained less then one mutation per genome. Mutagenized organisms were expanded in small subpopulations that were screened for mutations by digesting denatured and reannealed PCR amplicons of the target gene with the mismatch specific endonuclease CEL I. Subpopulations with mutations were then sequenced for the target region and plaque-cloned if the desired mutation was detected. We demonstrate the utility of this approach by isolating a tryptophan synthase gene (trpB) null mutant that was otherwise isogenic to its parental clone as shown by de novo genome sequencing. The mutant was incapable of avoiding the anti-microbial effect of IFN-γ–induced tryptophan starvation. The ability to genetically manipulate chlamydiae is a major advancement that will enhance our understanding of chlamydial pathogenesis and accelerate the development of new anti-chlamydial therapeutic control measures. Additionally, this strategy could be applied to other medically important bacterial pathogens with no or difficult genetic systems.
Keywords: genetics, mutation screen
Our understanding of microbial virulence, and hence our ability to develop effective medical therapies and prophylactic vaccines, has been guided by genetics. Chlamydiae are obligate intracellular bacteria that are important human and animal pathogens (1), but they lack systems for genetic manipulation. Chlamydia trachomatis, a strict human pathogen, is the etiological agent of blinding trachoma and sexually transmitted infections, which afflict hundreds of millions of people globally (1–3). The major impasse in the study of chlamydial pathogenesis is the lack of practical genetic tools. The development of genetic systems has been hampered by the organism's obligate intracellular lifestyle and complex biphasic developmental biology (4). C. trachomatis genomes are amenable to manipulation as shown by naturally occurring recombination (5) and by the moderate frequency of in vitro lateral gene transfer (6, 7). Chlamydiae have also been successfully transformed to antibiotic resistance by electroporation, but transformants either were unstable or successful transformation was limited to replacing the 16S rRNA with its antibiotic-resistant allele (8, 9). Thus, these advancements have not led to the development of practical methods for genetic manipulation.
We describe and demonstrate the utility of a reverse-genetic strategy for use in chlamydiae based on an approach used extensively in plant genetics called targeting-induced local lesions in genomes (TILLING) (10). TILLING combines high-density point mutations induced by standard chemical mutagenesis with a sensitive DNA screening technique that identifies SNPs in the target gene. In this study, we used low-level ethyl methanesulfonate (EMS) mutagenesis in combination with CEL I digestion, one of the most popular mutation detection assays in TILLING projects (11). As proof of principle, we isolated and characterized an isogenic C. trachomatis trpB nonsense mutant.
Results and Discussion
Mutagenesis of C. trachomatis.
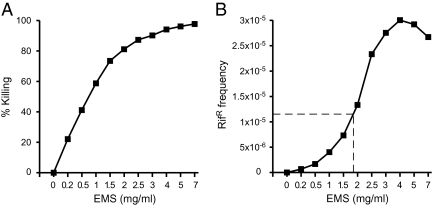
EMS was used as a mutagen because it primarily introduces C-G to T-A transition mutations (12) capable of generating nonsense mutations, and mutational rates can be estimated by determining the frequency of rifampicin-resistant (RifR) mutants. Thus, frequency of RifR was used as a proxy for overall frequency of transition events. Total and RifR chlamydiae generated in cells infected with EMS-treated C. trachomatis serovar D (CTD) were titered by parallel plaque assay performed in the presence or absence of rifampicin (Fig. 1). A direct relationship between concentration of EMS and RifR frequency was observed up to 4 mg/mL EMS. Because the goal of our study was to generate isogenic C. trachomatis mutants, the desired mutation frequency was one mutation per genome. The C. trachomatis genome contains ∼4.3 × 105 C-G base pairs, and transitions of five of these C-G pairs can yield RifR (6, 13–17). Thus, we estimated that a RifR frequency of 1.16 × 10−5 [1/(4.3 × 105/5) = 1.16 × 10−5] corresponded to a predicted level of mutagenesis that would result in a single C-G base pair mutation per genome (Table S1). This frequency fell between what we observed when C. trachomatis–infected cells were treated with 1.5 and 2 mg/mL EMS (Fig. 1B). Mutagenesis was repeated on a larger population of infected cells by using 1.5 mg/mL EMS to produce sufficient numbers of mutants for generating a library. The measured RifR frequency in this library was 5.2 × 10−6, which corresponds to ∼0.5 mutations per chlamydial genome (Table S1).
Fig. 1.
Effects of EMS mutagenesis on chlamydial survival and frequency of rifampicin resistance (RifR). C. trachomatis–infected McCoy cells were treated with various concentrations of EMS at 19 h postinfection. Chlamydiae were harvested 28 h after treatment and assayed for infectivity and resistance to rifampicin. (A) EMS killing curve. (B) The frequency of RifR after EMS mutagenesis. Plaque assays were performed on EMS-treated organisms in the presence or absence of rifampicin (0.1 μg/mL) to quantify resistant organisms. The frequency of RifR increased proportionately with increasing concentrations of EMS. A calculated mutagenesis frequency that would result in one mutation per chlamydial genome corresponded to 1.16 × 10−5 RifR frequency (indicated by the dashed line).
Identification of trpBA Mutants.
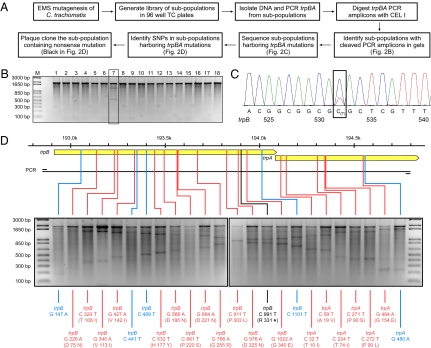
Mutagenized chlamydiae were expanded in monolayers of McCoy cells grown in 96-well tissue-culture plates in subpopulations consisting of ∼10 organisms per well. The subpopulations were expanded by three sequential rounds of cell lysis and reinfection (Fig. 2A). The expanded subpopulations were divided, with one portion frozen at −80 °C and the other used for genomic DNA preparation. A 1,900-bp fragment of the C. trachomatis trpBA operon was PCR-amplified from each subpopulation. PCR amplicons were heat-denatured, slowly reannealed, and digested with the mismatch-specific endonuclease CEL I (11). If multiple alleles are present in the PCR amplicons, these alleles form heteroduplexes during reannealing that are specifically cleaved by CEL I at positions of base-pair mismatching. Subpopulations containing trpBA mutants were identified by the appearance of these digestion products after agarose gel electrophoretic analysis. Fig. 2B depicts results of CEL I–digested PCR amplicons from 18 subpopulations; the presence of a trpBA mutant in the subpopulation analyzed in lane 7 is indicated by the appearance of two lower-mass DNA digestion products. PCR amplicons from this and other subpopulations yielding similar results were capillary-sequenced to confirm and identify the specific mutation. For example, in Fig. 2C, the small secondary peak in the chromatogram indicates that this subpopulation contained a mutant that had undergone a C to T transition mutation in base 532 of the trpB gene.
Fig. 2.
The reverse-genetic approach used to generate targeted mutations in the trpBA operon. (A) Schematic for generating and screening libraries. (B) Identification of subpopulations harboring trpBA mutations by CEL I digestion of the PCR-amplified trpBA operon followed by DNA gel electrophoretic analysis. The gel shows an example of the result of CEL I digestion of 18 subpopulations. Subpopulation in lane 7 contains a trpBA mutant, as is clearly indicated by the appearance of CEL I digestion products. (C) Confirmation and identification of the trpBA mutation by capillary sequencing. The trpBA PCR amplicon from subpopulation lane 7 was capillary sequenced. The chromatogram revealed a small secondary peak at the mutational site, confirming the presence of a SNP in trpB and identifying it as a C to T transition at nucleotide 532. (D) Summary of the 24 trpBA mutations identified in the analysis of the library of subpopulations. CEL I digestion of the 24 subpopulations harboring trpBA mutations are shown on the gels in the order of their genomic locations. The trpB and trpA ORFs are illustrated by solid yellow arrows. Synonymous (blue) and nonsynonymous (red) SNPs identified by capillary sequencing are indicated below each sample. Locations of SNPs are indicated in the trpBA operon above the gel image. Genomic scale and the region corresponding to the PCR amplicon are also shown. The nonsense mutation (black) in trpB truncates the ORF by 186 bp.
The size of the library that would need to be screened to identify a trpB or trpA nonsense mutant was estimated based on our original calculation of 0.5 mutations per genome. In the trpBA target region, transitions of 37 C or G nucleotides would result in nonsense mutations. Thus, we estimated that, within the trpBA target region, the nonsense mutant frequency would be approximately one nonsense mutation in 2.3 × 104 organisms [2.3 × 104 = (4.3 × 105/0.5)/37]. We screened ∼2,800 subpopulations theoretically containing 2.8 × 104 mutagenized organisms. From this screen, we identified 24 subpopulations that contained trpBA mutants confirmed by capillary sequencing. These 24 mutations corresponded to 5 synonymous and 19 nonsynonymous changes (Fig. 2D). One of the 19 nonsynonymous mutations was a nonsense mutation located at nucleotide position 991 in trpB. Notably, the mutation frequency observed from our library screening was similar to the 0.5 mutations per genome predicted by calculations based on RifR frequency.
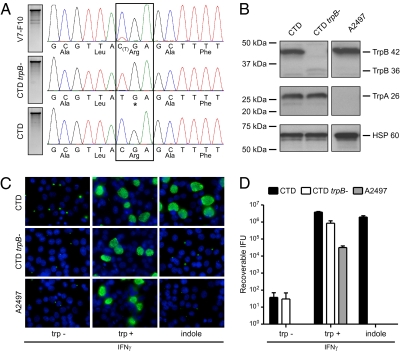
The CTD trpB nonsense mutant (CTD trpB−) was isolated by two rounds of plaque cloning. The C to T transition at nucleotide 991 in trpB was reconfirmed by capillary sequencing (Fig. 3A). The genomes of the mutant and the CTD parental strain were sequenced with the 454 FLX Titanium platform, and the genomic sequences were compared with the CTD/UW-3/CX reference sequence (18). Twenty SNP mutations were identified relative to the reference sequence, 19 of which were conserved between the two strains (Table 1). Importantly, de novo genome sequencing revealed no additional mutations between CTD trpB− and the CTD parental strain, confirming that the CTD trpB− was a true isogenic mutant.
Fig. 3.
Genetic and phenotypic characterization of the CTD trpB− nonsense mutant. (A) Chromatograms corresponding to the region of the trpB nonsense mutation in the original subpopulation (V7-F10) containing the trpB nonsense mutant, the plaque-cloned trpB nonsense mutant (CTD trpB−), and the parental clone (CTD) with the wild-type trpB genotype. Asterisk indicates a stop codon. (B) Western blotting with anti-TrpA and -TrpB antibodies of HeLa cell lysates infected with CTD, CTD trpB−, and a C. trachomatis ocular strain (A2497) with a frameshift mutation in trpA. CTD expressed both the 42-kDa TrpB and 26-kDa TrpA, whereas CTD trpB− expressed TrpA but not the 42-kDa TrpB. Strain A2497 expressed TrpB but not TrpA. Anti-HSP60 mAb served as a loading control. (C) Chlamydial inclusion formation during IFN-γ–induced tryptophan starvation and supplementation of the culture medium with no tryptophan, tryptophan, or indole detected by immunofluorescent staining with anti-chlamydial antibody (green) and DAPI (blue) for DNA. (D) Recoverable IFU as measured after a similar rescue experiment (n = 3). Infectivity of all strains was reduced by 4.5–5 log10 in the absence of tryptophan compared with IFU titers after tryptophan supplementation. The infectivity of CTD was fully rescued from tryptophan starvation by indole, whereas the isogenic CTD trpB− mutant and A2497 were not rescuable by indole. Shown are means and SD.
Table 1.
De novo genome sequencing of the CTD trpB− nonsense mutant and its parental clone (CTD)
| CTD/UW-3/CX (reference sequence) |
CTD (parental clone) |
CTD trpB− (mutant clone) |
|||
| SNP location | Gene | Nucleotide change | Amino acid change | Nucleotide change | Amino acid change |
| 30572 | CT025 | C → G | Thr → Ser | C → G | Thr → Ser |
| 55047 | CT049 | G → C | Gly → Ala | G → C | Gly → Ala |
| 55099 | CT049 | A → G | — | A → G | — |
| 152301 | CT135 | G → – | Frameshift | G → – | Frameshift |
| 193902 | CT170 | – | – | C → T | Arg → * |
| 403784 | CT352 | A → C | Ile → Leu | A → C | Ile → Leu |
| 450477 | CT394 | A → G | Lys → Glu | A → G | Lys → Glu |
| 517109 | IG (CT446–CT447) | G → T | — | G → T | — |
| 588168 | CT511 | G → T | Thr → Lys | G → T | Thr → Lys |
| 621054 | CT551 | – → G | Frameshift | – → G | Frameshift |
| 628883 | IG (CT556–CT557) | C → T | — | C → T | — |
| 706330 | CT621 | T → C | Thr → Ala | T → C | Thr → Ala |
| 717493 | CT630 | T → C | Gln → Arg | T → C | Gln → Arg |
| 720059 | IG (CT632–CT633) | A → T | — | A → T | — |
| 727542 | CT638 | – → G | Frameshift | – → G | Frameshift |
| 733706 | CT640 | T → C | Thr → Ala | T → C | Thr → Ala |
| 752502 | IG (CT655–CT656) | – → C | — | – → C | — |
| 858691 | 23S rRNA | G → A | — | G → A | — |
| 858694 | 23S rRNA | G → A | — | G → A | — |
| 858698 | 23S rRNA | C → – | — | C → – | — |
The two genomes were compared with the CTD/UW-3/CX reference genomic sequence (GenBank accession no. NC_000117.1) (18). CTD and CTD trpB− are clonal derivatives of the CTD/UW-3/CX reference strain (18). The two strains share 19 SNPs compared with CTD/UW-3/CX. Eighteen of these 19 SNPs were also found and described previously in other CTD/UW-3/CX derivative clones (24). Bold type indicates the single unique mutation identified between CTD and CTD trpB− [193,902-bp genomic location in trpB (ORF CT170)]. SNP location refers to the location of the mutation in the CTD/UW-3/CX reference genomic sequence; for single base-pair insertion, the location of the preceding nucleotide is given. IG, intergenic region with adjacent ORFs indicated. Asterisk indicates a stop codon.
CTD trpB− Cannot Be Rescued by Indole.
C. trachomatis strains are tryptophan auxotrophs. Paradoxically, genitotropic but not oculotropic strains have retained a functional trpBA operon (18–21). The trpBA operon is negatively regulated and expresses the TrpBA tryptophan synthase that uses exogenous indole as substrate to synthesize tryptophan and escape the anti-microbial effect of IFN-γ–mediated tryptophan starvation (20, 21). We used these phenotypic and genetic markers of the trpBA operon to characterize the CTD trpB− mutant. Western blots using specific anti-TrpA and -TrpB antibodies were done with lysates of HeLa cells infected with CTD, CTD trpB−, and a C. trachomatis ocular strain A2497 (22) that has a frameshift mutation in trpA. As expected, the ocular strain A2497 expressed the 42-kDa TrpB but not the 26-kDa TrpA, whereas CTD expressed both polypeptides (Fig. 3B). In contrast, lysates of CTD trpB− expressed TrpA but not full-length TrpB. A weakly immunoreactive polypeptide of 36 kDa was detected, likely representing the truncated form of TrpB. The infectivity of CTD trpB− under tryptophan starvation and indole rescue was assessed in HeLa 229 cells. Shown in Fig. 3C are immunofluorescent images of HeLa cells infected with CTD, CTD trpB−, and A2497 after IFN-γ treatment and supplementation of the culture medium with no tryptophan, tryptophan, or indole. Predictably, none of the strains grew in IFN-γ–treated tryptophan-starved cells, and all three strains produced large intracellular inclusions when the culture medium was supplemented with tryptophan. Only CTD growth and typical inclusion formation was rescued in IFN-γ–treated cells when the culture medium was supplemented with indole, the TrpBA synthase substrate. The quantity of infectious chlamydial progeny produced under the same experimental conditions is shown in Fig. 3D. When grown in the presence of tryptophan, all strains were highly infectious and generated large numbers of recoverable inclusion-forming units (IFU). In contrast, the infectivity of each strain was reduced by 4.5–5 log10 in the absence of tryptophan. Although the infectivity of the CTD parent was fully rescued from the effects of tryptophan starvation by indole, the infectivity of the isogenic CTD trpB− mutant was not rescued. These results prove that the CTD trpB− strain is both a genetic and phenotypic null mutant.
Conclusions
The TILLING-based reverse-genetic approach used in this study can target any gene in the C. trachomatis genome. Although the isolation of a null mutant is labor-intensive, the time, cost, and effort involved is comparable to that required to generate a hybridoma producing a monoclonal antibody against a specific polypeptide. The obvious limitation of the approach is that genes essential for in vitro growth cannot be targeted for null mutations. However, genes functioning primarily in in vivo infection and survival represent logical targets. Mutation of these genes is highly desirable because it will yield novel findings about chlamydial pathogenesis and provide potential new targets for the future generation of live-attenuated vaccines. An excellent example of such genes are those encoded by the C. trachomatis cryptic plasmid; even the loss of the entire plasmid does not alter the chlamydial growth rate in vitro but has a significant attenuating phenotype in vivo (23). Because it is unknown which genes are essential for in vitro growth, identifying a target gene as nonessential before screening a large library of organisms with a single mutation per genome would be valuable. Constructing and prescreening a smaller, heavily mutagenized library with high-density point mutations could identify such genes. Additionally, essential genes could be studied by isolation of conditional lethal mutants. For example, temperature-sensitive mutants could be isolated from a library mutagenized and constructed at temperatures below 37 °C and screened for the inability to grow at 37 °C. In summary, the ability to create isogenic C. trachomatis null mutants will provide important new information about chlamydial pathogenesis that will ultimately bring us closer to the development of chlamydial vaccines. Moreover, this approach could be applied to other chlamydial or bacterial species for which current methods of genetic modification are limited or impractical.
Materials and Methods
Chemical Mutagenesis.
McCoy cells were infected at a multiplicity of infection of 0.5 with a plaque-cloned CTD/UW-3/CX strain. Infected cells were exposed for 1 h to EMS at concentrations between 0.2 and 7 mg/mL at 19 h postinfection. Chlamydiae were harvested 28 h after mutagenesis, and recoverable IFU were determined by titration on McCoy cells. Frequency of RifR was evaluated by plaque assay in McCoy cells in the presence and absence of 0.1 μg/mL rifampicin added at 18 h postinfection.
Library Construction.
McCoy cells infected with 1 × 107 IFU of C. trachomatis were mutagenized with 1.5 mg/mL of EMS. Mutants were titered in McCoy cells, and the frequency of RifR was determined. McCoy cells grown in 96-well tissue-culture plates (5 × 104 cells per well) were infected with 10 IFU per well. Chlamydiae were harvested into sucrose phosphate glutamate buffer at 48 h postinfection by vigorous pipetting with a multichannel pipette and were immediately used to reinfect McCoy cell monolayers grown in 96-well tissue-culture plates. Infected cells were harvested and passaged for a third time, and half of the harvested chlamydiae was frozen at −80 °C. DNA was extracted from this material by treatment with 0.5 N NaOH followed by the addition of an equal volume of 1 M Tris·HCl, pH 8.
Mutation Screen with CEL I.
Target regions were PCR-amplified, heat-denatured at 94 °C, and reannealed by slow cooling to promote formation of dsDNA heteroduplexes. These denatured and reannealed PCR amplicons were digested by CEL I (Transgenomic Inc.) as previously described (11, 24). Digestion products were visualized by DNA agarose gel electrophoresis. Table S2 list the PCR and sequencing primers used for trpBA.
De Novo 454 Genome Sequencing.
The genomes of the parental CTD clone and the CTD trpB− nonsense mutant clone were sequenced with the 454 FLX Titanium platform (Roche Applied Science) as previously described (24). Assembled contigs from these strains were compared with each other and to the published CTD reference genomic sequence (18). Confirmation of mutations were performed by PCR and capillary sequencing as previously reported (24). Table S3 shows the primer sets used for confirming the genomic sequences. Additionally, the two repetitive genomic regions in C. trachomatis (hctB and tarP) were also PCR-amplified and capillary-sequenced for confirmation as previously described (24). Both strains contained the intact wild-type alleles at these loci. The PCR and sequencing primers are listed in Table S4.
Western Blot Analysis.
Western blot analysis of TrpB and TrpA was performed as described previously (20, 21). Anti–HSP-60 antibody was included in the analysis as a loading control.
Indole Rescue.
The in vitro indole-rescue assays were performed as described previously (20, 21). After indole rescue, chlamydiae-infected cells were harvested and titered on HeLa cell monolayers or analyzed by immunofluorescence after staining of fixed cells with anti-chlamydial antibody or DAPI for DNA.
Supplementary Material
Acknowledgments
We thank Robert Heinzen and Paul Beare for critical review of the manuscript, Anita Mora for assistance with graphic arts, the Genomics Unit of the Rocky Mountain Laboratories Research Technologies Section for the genome sequencing and analysis, and Kelly Matteson for manuscript preparation. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. D.V. was supported by the European Research Area-NET Pathogenomics ChlamyTrans project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the NCBI Sequence Read Archive under study no. SRP030617 (454 reads).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102229108/-/DCSupplemental.
References
- 1.Schachter J. Chlamydial infections (first of three parts) N Engl J Med. 1978;298:428–435. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- 2.Schachter J. Chlamydial infections (third of three parts) N Engl J Med. 1978;298:540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- 3.Schachter J. Chlamydial infections (second of three parts) N Engl J Med. 1978;298:490–495. doi: 10.1056/NEJM197803022980905. [DOI] [PubMed] [Google Scholar]
- 4.Moulder JW. The relation of basic biology to pathogenic potential in the genus Chlamydia. Infection. 1982;10(Suppl 1):S10–S18. doi: 10.1007/BF01640709. [DOI] [PubMed] [Google Scholar]
- 5.Gomes JP, Bruno WJ, Borrego MJ, Dean D. Recombination in the genome of Chlamydia trachomatis involving the polymorphic membrane protein C gene relative to ompA and evidence for horizontal gene transfer. J Bacteriol. 2004;186:4295–4306. doi: 10.1128/JB.186.13.4295-4306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMars R, Weinfurter J, Guex E, Lin J, Potucek Y. Lateral gene transfer in vitro in the intracellular pathogen Chlamydia trachomatis. J Bacteriol. 2007;189:991–1003. doi: 10.1128/JB.00845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suchland RJ, Sandoz KM, Jeffrey BM, Stamm WE, Rockey DD. Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob Agents Chemother. 2009;53:4604–4611. doi: 10.1128/AAC.00477-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binet R, Maurelli AT. Transformation and isolation of allelic exchange mutants of Chlamydia psittaci using recombinant DNA introduced by electroporation. Proc Natl Acad Sci USA. 2009;106:292–297. doi: 10.1073/pnas.0806768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam JE, Davis CH, Wyrick PB. Expression of recombinant DNA introduced into Chlamydia trachomatis by electroporation. Can J Microbiol. 1994;40:583–591. doi: 10.1139/m94-093. [DOI] [PubMed] [Google Scholar]
- 10.McCallum CM, Comai L, Greene EA, Henikoff S. Targeted screening for induced mutations. Nat Biotechnol. 2000;18:455–457. doi: 10.1038/74542. [DOI] [PubMed] [Google Scholar]
- 11.Oleykowski CA, Bronson Mullins CR, Godwin AK, Yeung AT. Mutation detection using a novel plant endonuclease. Nucleic Acids Res. 1998;26:4597–4602. doi: 10.1093/nar/26.20.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashburner M. Drosophila: A Laboratory Handbook. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 13.DeMars R, Weinfurter J. Interstrain gene transfer in Chlamydia trachomatis in vitro: Mechanism and significance. J Bacteriol. 2008;190:1605–1614. doi: 10.1128/JB.01592-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreses-Werringloer U, Padubrin I, Köhler L, Hudson AP. Detection of nucleotide variability in rpoB in both rifampin-sensitive and rifampin-resistant strains of Chlamydia trachomatis. Antimicrob Agents Chemother. 2003;47:2316–2318. doi: 10.1128/AAC.47.7.2316-2318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutlin A, Kohlhoff S, Roblin P, Hammerschlag MR, Riska P. Emergence of resistance to rifampin and rifalazil in Chlamydophila pneumoniae and Chlamydia trachomatis. Antimicrob Agents Chemother. 2005;49:903–907. doi: 10.1128/AAC.49.3.903-907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rupp J, Solbach W, Gieffers J. Variation in the mutation frequency determining quinolone resistance in Chlamydia trachomatis serovars L2 and D. J Antimicrob Chemother. 2008;61:91–94. doi: 10.1093/jac/dkm447. [DOI] [PubMed] [Google Scholar]
- 17.Suchland RJ, Bourillon A, Denamur E, Stamm WE, Rothstein DM. Rifampin-resistant RNA polymerase mutants of Chlamydia trachomatis remain susceptible to the ansamycin rifalazil. Antimicrob Agents Chemother. 2005;49:1120–1126. doi: 10.1128/AAC.49.3.1120-1126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephens RS, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 19.Carlson JH, Porcella SF, McClarty G, Caldwell HD. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun. 2005;73:6407–6418. doi: 10.1128/IAI.73.10.6407-6418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldwell HD, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest. 2003;111:1757–1769. doi: 10.1172/JCI17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fehlner-Gardiner C, et al. Molecular basis defining human Chlamydia trachomatis tissue tropism. A possible role for tryptophan synthase. J Biol Chem. 2002;277:26893–26903. doi: 10.1074/jbc.M203937200. [DOI] [PubMed] [Google Scholar]
- 22.Kari L, et al. Pathogenic diversity among Chlamydia trachomatis ocular strains in nonhuman primates is affected by subtle genomic variations. J Infect Dis. 2008;197:449–456. doi: 10.1086/525285. [DOI] [PubMed] [Google Scholar]
- 23.Carlson JH, et al. The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun. 2008;76:2273–2283. doi: 10.1128/IAI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturdevant GL, et al. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun. 2010;78:3660–3668. doi: 10.1128/IAI.00386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.