Abstract
Biological membranes are complex, self-organized structures that define boundaries and compartmentalize space in living matter. Composed of a wide variety of lipid and protein molecules, these responsive surfaces mediate transmembrane signaling and material transport within the cell and with its environment. It is well known that lipid membrane properties change as a function of composition and phase state, and that protein-lipid interactions can induce changes in the membrane’s properties and biochemical response. Here, molecular level changes in lipid organization induced by multivalent toxin binding were investigated using grazing incidence X-ray diffraction. Structural changes to lipid monolayers at the air-water interface and bilayers at the solid-water interface were studied before and after specific binding of cholera toxin to membrane embedded receptors. At biologically relevant surface pressures, protein binding perturbed lipid packing within monolayers and bilayers resulting in topological defects and the emergence of a new orientationally textured lipid phase. In bilayers this altered lipid order was transmitted from the receptor laden exterior membrane leaflet to the inner leaflet, representing a potential mechanism for lipid mediated outside-in signaling by multivalent protein binding. It is further hypothesized that cell-surface micro-domains exhibiting this type of lipid order may serve as nucleation sites for vesicle formation in clathrin independent endocytosis of cholera toxin.
Keywords: X-ray scattering, liquid crystal, supported membrane, ganglioside, glycolipid
Interactions between proteins and the cell membrane are an integral aspect of many biological processes (1). Diverse protein-lipid complexes exist including transmembrane proteins, peripheral membrane proteins, and proteins bound to membrane associated receptor molecules. The interplay between these biological components is multifaceted: lipids can influence the structure and function of membrane proteins and at the same time membrane proteins can impact lipid organization (2). In model systems lipids are capable of adopting a variety of different ordered states with their phase behavior primarily governed by steric and van der Waals interactions between neighboring head groups and alkyl chains. Lipid organization ranges from the tightly packed gel phase to the fluid like liquid ordered (Lo) and liquid disordered (Ld) phases. Lateral heterogeneities within model membranes due to the coexistence of Ld and Lo phases have been widely used to study lipid domain formation and as analogs for lipid rafts (3, 4). In biological systems, lipid rafts are dynamic self-organized membrane microdomains that selectively recruit specific proteins and lipids while excluding others (5, 6). Typically enriched in cholesterol, sphingolipids, and glycolipids, rafts are characterized by the tighter packing of their constituent molecules in a liquid ordered phase (7). Raft microdomains offer a means to sequester proteins, enhance the local concentration of raft associated components, as well as alter the conformation of embedded proteins within the cellular membrane. Another example of membrane microdomains is the self-association of glycosphingolipids (GSL) to form a glycosynapse. Such microdomains are thought to play a role in a wide range of biological functions including cell recognition, adhesion, and signaling (8, 9). For example, in cell adhesion processes GSL-GSL interactions directly influence membrane properties and GSL membrane microdomains have also been shown to modulate the activity of cytoplasmic protein kinases. A structural mechanism has not yet been established to explain how GSL microdomains modify cellular activity. In rafts, lipid induced protein conformation changes can also influence signaling by membrane embedded protein receptors, e.g., ion-channel linked receptors, enzyme linked receptors, and G-protein coupled receptors, as they transmit signals across the membrane (outside-in signaling) through conformational or chemical changes to the protein’s intracellular domain upon small molecule binding to an extracellular domain. These changes may, for example, open a channel, activate enzymatic activity, or induce a signaling cascade that results in a cellular response. However, to our knowledge a “lipid only” mediated structural mechanism for transmembrane signaling has not yet been reported. In the work reported here, we examine if the modulation of lipid membrane by specific protein binding can provide a potential mechanism for transmembrane signaling.
Cholera toxin, which selectively binds to ganglioside glycolipids, is frequently used as a reporter for membrane rafts and as a tool to investigate protein-lipid interactions (10). The B subunit (CTB) is responsible for binding the toxin with highest affinity to ganglioside GM1 (4.61 × 10-12 M), a cell-surface receptor also associated with lipid raft domains (11). Five identical B subunits, each containing one binding site, form a pentameric ring with a vertical height of 32 Å and a radius of 31 Å (12, 13). Because binding is multivalent and of high affinity, off rates of the toxin are slow and the CTB-GM1 complex is very stable enabling CTB to effectively cross link GM1 receptors in the membrane. Receptor cross linking, in general, may act to stabilize rafts, lead to coalescence of raft domains, and is hypothesized to be involved in the exploitation of clathrin independent endocytosis pathways by multivalent toxins (14). Moreover, limiting the number of active binding pockets on cholera toxin has been shown to inhibit endocytosis, presumably due to diminished receptor cross linking (15). A mechanistic explanation for this phenomenon remains unclear.
Here we report the discovery of a unique lipid phase generated by multivalent protein binding to raft associated membrane receptors. The packing characteristics of this textured lipid phase (LT) place it intermediate between the well established Lo and gel lipid phases. Not restricted to close-packed structures, the LT phase comprises a rich variety of lipid tail tilt orientations including anisotropic and azimuthally swirled arrangements analogous to those observed in macroscopic hexatic phases of liquid crystals. Specific binding of protein to membrane embedded receptors was shown to generate the LT phase in model membranes, providing a possible window into otherwise undetectable features of lipid order within nanoscale domains in the cellular membrane. We propose that such orientationally textured domains may have biological relevance as lipid based signaling platforms and in cellular trafficking pathways. Significantly, these altered packing arrangements are transmitted from the receptor laden leaflet to the inner leaflet of the membrane providing a means for outside-in signaling. Further, the LT phase offers a mechanistic explanation for nonclathrin mediated endocytosis where altered lipid packing due to toxin binding serves as a nucleation site for vesicle formation.
Results
Perturbations to Lipid Order in Monolayers at the Air-Water Interface.
To investigate the influence of multivalent protein binding on lipid order, phase state, and membrane texture, grazing incidence X-ray diffraction (GIXD) measurements were carried out on mixed DPPE∶GM1 monolayers at the air-water interface in the presence and absence of CTB. GIXD restricts the scattering volume probed to the near surface region and is specialized for the investigation of in-plane order within thin, two-dimensional films (Fig. 1A). Using this technique, we precisely characterized the packing of gel phase lipids and subsequent perturbation and disordering induced by protein binding. With sufficient GM1 receptors in the monolayer, high coverage 2D cholera protein layers were assembled (16, 17). On a local scale, this model system is analogous to nanoscale protein aggregates and provides the advantage of amplifying the scattering signal. Reflectivity measurements were used to confirm the formation of a bound protein layer (16). Additionally, reflectivity showed that protein did not penetrate into the lipid monolayer after binding to GM1. Thus, the observed perturbations to lipid order originate from geometric constraints originating from multivalent binding and protein aggregation rather than protein penetration into the lipid layer.
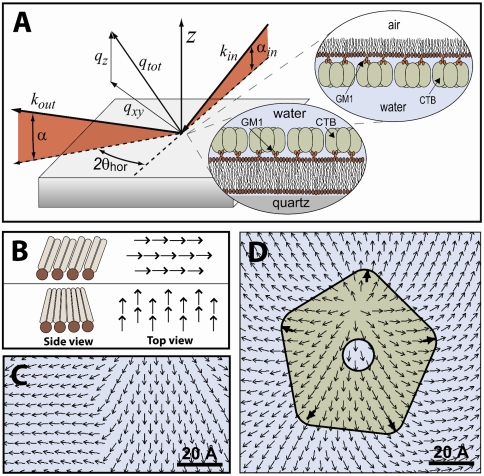
Fig. 1.
(A) The GIXD scattering geometry is shown with schematic insets representing the monolayer and bilayer lipid-CTB systems studied. qz = 2π sin α is the momentum transfer of diffracted X-rays normal to the interface and qxy = 4π sin θ is the momentum transfer perpendicular to the interface. (B) Tilt directors are vectors pointing along the lipids’ alkyl chain backbones from the head group to the methyl end. (C) A boundary between two orientations of the lipid tilt director field. (D) Perturbation to the lipid tilt director field and associated topological defect induced by pentavalent binding of a single CTB protein.
Diffraction from ordered systems of long linear amphiphilic molecules like phospholipids is traditionally described as originating from arrangements of close-packed rod-shaped molecules (alkyl chains) in 2D hexagonal or distorted hexagonal unit cells (18, 19). These molecular arrangements, typical of lipid monolayers in the gel phase, consist of uniformly oriented molecules with constant tilt magnitude and azimuthal tilt direction (Fig. 1B). Tilt directors, which are vectors pointing along the alkyl chain backbones of the lipid molecules, can be used to define their orientational order. Gel phase monolayers have a uniform tilt director field with all vectors aligned (Fig. 1B). Variations in the orientation of the molecular tilt directors due to protein binding introduce texture and topological defects into the tilt director field (Fig. 1D). Such orientational texture in lipid packing has recently been reported both at the micron and molecular scales (20, 21). Using models with close-packed rod-shaped molecules and uniform tilt director fields, we parameterized the lipid order within the monolayer at three surface pressures (spanning the range typically taken as the equivalent to a cellular membrane) and distortions resulting from protein binding.
Contour plots of diffracted intensities as a function of photon momentum transfer provide a convenient means to visualize diffraction from 2D lipid films. Fig. 2A shows diffraction from a 80∶20 DPPE∶GM1 monolayer at 45 mN/m surface pressure. The single degenerate peak at low qz (momentum transfer normal to the interface) indicates hexagonal packing and little molecular tilt of the hydrocarbon tails. In the presence of CTB the diffraction peak shifts slightly to lower qxy (momentum transfer along the interface) and higher qz corresponding to a small increase in lipid alkyl chain tilt and the area per lipid molecule (APM). At 30 mN/m (Fig. 2B), the diffraction before protein binding is qualitatively similar to 45 mN/m and, again, protein binding further increased lipid tilt and APM. In this case the lipid tilt upon protein binding is sufficiently large to clearly resolve the splitting of the degenerate peak into three distinct reflections indicative of a distorted hexagonal unit cell. We further comment that for these higher surface pressure cases, the changes in APM observed were commensurate with increased lipid tilt and conservation of the cross sectional area of the hydrocarbon chains. These findings demonstrate that at high surface pressure the geometric constraints imposed by multivalent protein binding increase the magnitude of molecular tilt and influence the positional ordering of hydrocarbon chains in the lipid monolayer.
Fig. 2.
Grazing incidence diffraction from 80∶20 DPPE∶GM1 monolayers at three surface pressures (A1, B1, and C1) and diffraction from the same systems following binding of CTB (A2, B2, and C2). At high surface pressures, protein binding causes an increase in the lipid APM that is commensurate with the increase in lipid tilt. Although APM increased after CTB binding at 20 mN/m, the lipid tilt remained approximately the same. The resulting lipid order was no longer close packed and exhibited topological defects and texture of the lipid tilt orientations.
Very different diffraction is observed at a biologically relevant surface pressure (20 mN/m) upon protein binding. The lipid diffraction in the absence of protein exhibits three nondegenerate peaks from an oblique unit cell (Fig. 2C). Protein binding, however, induces perturbations to the lipid order that greatly reduce the diffraction intensity at low qz. The diffraction is still consistent with an increase in the APM but in this case the diffraction cannot be attributed to a simple increase in lipid tilt and a concomitant change in positional order. Moreover, the scattering can no longer be described by any model based on close-packed cylindrical molecules and a uniform tilt director field. The necessary relationships between both the diffraction peak positions ( ) and peak intensities (I1 = I2 = I3) required for close-packed molecular arrangements are violated (22). In order to reduce the low qz intensity and match the measured diffraction, topological defects and orientational texture have to be introduced into the lipid tilt director vector field (Fig. 1D). These changes represent the emergence of a textured lipid phase, LT, specifically induced by multivalent protein binding. The details of this phase and modeling are described in the next section.
) and peak intensities (I1 = I2 = I3) required for close-packed molecular arrangements are violated (22). In order to reduce the low qz intensity and match the measured diffraction, topological defects and orientational texture have to be introduced into the lipid tilt director vector field (Fig. 1D). These changes represent the emergence of a textured lipid phase, LT, specifically induced by multivalent protein binding. The details of this phase and modeling are described in the next section.
Modeling GIXD from Textured Phase Monolayers.
To describe lipid order in the textured LT phase, we implemented models that perturbed the lipid order away from a close-packed gel phase and calculated the associated diffraction. By loosening the constraint imposed by close packing of lipid alkyl chains, topological defects and orientational texture could be incorporated into these models. The diffraction pattern of a 20 mN/m monolayer prior to protein binding was reproduced with a uniformly oriented tilt director field. After protein binding, no close-packed model was able to reproduce the diffraction pattern. Instead, a textured 2D liquid crystal smectic-like phase, here specified as the lipid LT phase, with azimuthal perturbations to the molecular tilt directors was required. The simplest texture, for example, can be generated from a single defect, or disclination, at the domain center yielding an arrangement of tilt directors analogous to bend domains in liquid crystals (23). Azimuthal angles of the tilt directors were described by the equation ϕ = s tan-1(y/x) + ϕ0 where x and y are the lateral coordinates of the molecules, and s is the strength of the disclination with s = ± 1 corresponding to the lowest energy symmetric textures. The LT regions have a finite size and the average length scale associated with these domains is inversely related to the width of the Bragg peaks. For a domain radius of 150 Å (corresponding to the measured Bragg peak FWHM), the scattered intensity at low qz decreased with increasing s until s ≈ 5 (Fig. 3). Increasing the strength of the texture beyond this value did not improve the quality of the fit.
Fig. 3.
Grazing incidence diffraction from monolayers with bound CTB (top) and bilayers before and after CTAB binding (bottom). Diffraction data from the monolayer-CTB complex (A) was reproduced by a textured lipid phase obtained via Monte Carlo simulation (B). (C) Bragg rod profiles extracted from (A) by integrating along qxy and fits corresponding to defect textures of strengths (s = 1, s = 2, s = 3, and s = 5) and the Monte Carlo generated domain (dark line). For a domain radius of 150 Å (corresponding to the measured Bragg Peak FWHM), the scattered intensity at low qz decreased with increasing s. The best fit to the data was found for s ≈ 5. In the bilayer case prior to protein binding (D, F), the Bragg rod FWHM indicates coupling between the membrane leaflets and exhibited limited texture. Following protein binding (E, G), cross leaflet coupling was preserved and the decreased intensity at low qz reflects an increased degree of texture.
While diffraction from textured domains with a single center defect approximated the measured data, protein crystallography of the CTB-GM1 complex shows that bound GM1 molecules orient radially outward at an oblique angle from the protein’s binding pocket (24). Using additional Monte Carlo simulations we investigated how these geometrical constraints would influence the orientation of tilt directors and lipid order. Simulations of pentavelent CTB binding to 150 Å lipid domains that take into account the geometric constraints imposed by the structure of the lipid-protein complex yielded highly textured tilt director fields containing multiple topological defects (Fig. 4). Diffraction patterns calculated from these lipid orientations were very similar to diffraction from domains with a single s = 3 disclination (Fig. 3). Using this more physically relevant model, the experimental diffraction data after CTB binding was best approximated by the scattering calculated from textured lipid phases with a molecular tilt magnitude of 20.0°, and an APM of 44.7 Å2. Compared to the 20 mN/m monolayer diffraction before protein binding, the tilt is unchanged and the small 3.7% increase in APM (43.1 to 44.7 Å2) corresponds to the degree that the lipid molecules are nonclose-packed. The modest APM expansion and loosened packing, driven by geometric constraints on the receptor molecules due to protein binding, indicates transformation to the LT phase.
Fig. 4.
Real space configurations of lipid tilt directors (vectors along the molecular backbones) exhibiting texture. Top left and center schematics show orientation of tilt directors around an s = 1, ϕ0 = π/4 and an s = 5, ϕ0 = π/4 disclination respectively. In the bottom left schematic, ∼10–15 CTB proteins are arranged beneath a 150 Å radius nano-domain corresponding to the lateral correlation size determined from the FWHM of the Bragg Peak. Magnification to the right displays the orientation of tilt directors obtained from Monte Carlo simulation. Dark arrows represent molecules with fixed orientations. A topological defect (source) can be seen near the central pore of the top left CTB molecule.
Perturbations to Bilayer Order Induced by Protein Binding.
Bilayer studies allow the impact of multivalent protein binding on both leaflets to be investigated. GIXD experiments were conducted on DPPE∶GM1 supported bilayers in the presence and absence of cholera toxin CTAB (Fig. 3 D and E) (†). For simplicity, lateral correlations between tilt director orientations (Fig. 4) were not considered in these models. Prior to protein binding the lipids had an APM of 41.3 Å2, were tilted 13.5° from the surface normal, and had a small degree of orientational texture. Molecular tilt directors spanned an azimuthal range with FWHM = 22° resulting in a texture similar to the lipid order previously observed in supported phosphocholine bilayers (21). Following protein binding, the lipid APM and tilt increased and the azimuthal variation increased to span a range with a FWHM = 30°. Thus, the geometric constraints induced by protein binding decreased lipid packing efficiency and enhanced orientational texture in the bilayer in a manner similar to that observed with monolayers. Importantly, these lipid packing changes were transmitted across the bilayer—from the exterior leaflet containing GM1 receptors to the inner lipid leaflet. The coupling between the outer and inner membrane leaflet is clearly evident from Bragg rod peak width analysis. The FWHM in the Bragg rod intensity distribution relates to the length of the diffracting lipid molecule. Assuming a rod-shaped scattering object the length over which the lipids diffract is given by the simple equation: FWHM = 2π/LZ. In the bilayer diffraction (Fig. 3 F and G), the FWHM analysis yielded a length of about 40 Å, twice the length of a lipid tail, and clearly demonstrates that the alkyl chains are coupled between the two leaflets both before and after protein binding. This molecular level coupling, which persists in the presence of cholera toxin, allows the perturbations to lipid order in one leaflet to be communicated to the opposing leaflet. Cross leaflet coupling of macrophase separated membrane domains has been previously observed in model and cellular membranes and molecular level coupling has recently been reported in gel phase phosphocholine bilayers (21, 25, 26).
Discussion
Membrane Topological Defects and Orientationally Textured Lipid Phases.
We have observed that multivalent proteins can dramatically manipulate their lipid environment upon binding to their putative cell-surface receptors. The protein does not penetrate the membrane, but imposes geometric constraints which restrict the position and orientation of bound receptors. At high lipid packing densities (high surface pressures), the perturbations to lipid order manifest themselves as changes in the lipid APM and tilt magnitude. At lower surface pressures, protein binding changes the lipid tilt director field introducing topological defects and orientational textures. A prerequisite for the formation of defects and textures is the relaxation of positional registry away from a close-packed configuration. These structural changes caused by protein-lipid complex formation reflect a phase transition from hexatic to a textured lipid phase, LT, when the surface pressure is in a biologically relevant regime.
Orientational textures of lipids are analogous to larger length scale textures observed in liquid crystal systems and represent a distinct lipid phase state, LT. This LT phase is characterized by molecular order intermediate between the gel and liquid ordered phases. Adjacent lipids cooperatively self-organize to accommodate receptors constrained by protein binding causing the emergence of textured domains. High surface pressures likely suppress the phase transition either by changing GM1’s conformation or limiting the number of bound GM1 receptors. It is interesting to note that a natural consequence of the constrained orientation of bound receptors is the localization of topological defects at or near the position of CTB’s central pore. In our Monte Carlo simulations correlation of the defect position with the center of the protein was observed in ∼50% of the cases. Because cholera toxin’s active A subunit attacks the membrane through CTB’s pore, a topological defect and associated instability of the lipid packing in this region of the membrane should enhance translocation of the protein across cellular membranes.
The textured, LT, phase and resulting topological defects may be prevalent in a variety of toxin-receptor membrane complexes featuring multivalent binding or nanoscale protein aggregation. For example, preliminary GIXD measurements indicate orientational texture in lipid monolayers following the binding of botulinum toxin. The crystal structure for this protein also suggests that the ligand fits into the receptor’s binding pocket at an oblique angle (27). Although botulinum only has a single receptor site, protein aggregation and oligomerization may impose sufficient constraints to induce texture formation, similar to pentavalent cholera toxin binding.
Structural Evidence for a Nonclathrin Mediated Endocytosis Pathway.
Cholera toxin’s infection pathway involves transport of the protein across the plasma membrane followed by trafficking to the endoplasmic reticulum. Approximately half of the uptake of cholera molecules into cells is attributed to caveolar or clathrin mediated pathways and half is associated with GM1 enriched lipid rafts (28). Raft associated endocytosis is driven by membrane dynamics and involves the formation of tubular structures with a diameter of 40–80 nm and the creation of vesicles as a vehicle to transport the protein across the membrane (28). Similar membrane invaginations have been correlated with the uptake of Shiga toxin, another pentameric protein, and with simian virus 40 (SV40) both of which bind to raft associated glycolipids (29, 30) In the case of SV40, endocytotic uptake of the capsid was found to depend on GM1’s tail structure and was enhanced when bound to GM1 with long saturated alkyl chains. The mechanism by which toxin binding initiates the formation of invaginations is unresolved. We propose that the generation of orientational texture induced by multivalent binding and protein aggregation initiates this process. Theoretical work has previously demonstrated that membrane regions incorporating textured domains can impact local membrane curvature and serve as nucleation sites for the budding of vesicles (31). Our studies show that multivalent protein binding to membrane receptors in both monolayers and bilayers generates the textured LT phase. Formation of texture through cooperative lipid rearrangement would be enhanced by long saturated alkyl tails, consistent with findings that endocytosis of SV40 is dependent on GM1’s hydrocarbon structure. Our results are also consistent with the decreased internalization of cholera toxin when the number of active binding sites is reduced from 5 to 1–2 (15). Such mutants retain their ability to bind and associate with lipid rafts but cannot cluster or cross link GM1 molecules as effectively. Mechanistically, these studies suggest that the formation of textured lipid microdomains via multivalent binding and protein aggregation into clusters are important in triggering the endocytotic pathway.
A Potential Lipid Mediated Signaling Mechanism.
Generation of orientational texture and a distinct LT lipid phase in membranes may have broad biological implications if perturbations to lipid order can be appropriated as a signaling mechanism by the cell. Analogous to Lo/Ld coexistence phases or self-association of order forming species in lipid rafts, textured lipid phases allow for new types of lateral heterogeneity in membranes. In addition, orientationally textured domains provide a means for protein binding induced changes in lipid order to be spread laterally by cooperative self-organization of adjacent lipids. Resulting alterations of membrane structure may facilitate raft clustering and potentially influence protein function (e.g., peripheral or transmembrane) at distant locations (4). On a more local scale, textured lipid phases are also capable of transmitting information across the membrane. We have shown that an extracellular molecule binding to membrane embedded receptors alters lipid packing and orientation within the receptor leaflet causing a phase transition to the LT phase and that these changes in packing and orientation are transmitted to the opposing leaflet. The resulting structural changes do not require either the translocation of a small signaling molecule through the membrane’s hydrophobic core or the transmission of the signal via conformational or chemical changes to a transmembrane protein. Rather, if we consider the bilayer as an analog to a cellular membrane, the altered lipid order at the apical leaflet induces a change in the packing of the cytoplasmic leaflet. The passing of structural changes across the bilayer represents the possibility for a fundamentally new, lipid mediated mechanism for transmembrane signal transduction.
Experimental Procedures
Materials.
Lipid monolayers and bilayers were composed of 80∶20 mole% of [1, 2-Dipalmitoyl-sn-Glycero-3-Phosphoethanolamine: monosialotetrahexosylganglioside (DPPE:GM1)] from Avanti Polar Lipids. Although limited in physiological relevance to the exoplasmic leaflet, DPPE satisfies the conditions for diffraction and bears structural similarities to a large variety of saturated phospholipids, sphingolipids, and ceramides present in cellular membranes. Previous work has shown that DPPE and GM1 are miscible and do not phase separate under these conditions. Lipids were dissolved in chloroform:methanol 90∶10 (∼1 mg/mL) and deposited on an H2O pH = 8 buffered subphase prepared using Millipore water with 170 mM NaCl, 1.4 mM Sodium Azide, 0.3 mM EDTA, 15 mM Trizma-Base from Sigma. CTAB and subunit CTB were purchased from Sigma. For monolayer experiments, CTB in powder form was dissolved in water and injected into the subphase to a final concentration of ∼4 mg/L. High receptor concentration in the monolayer and high protein concentration in the subphase yielded 2D protein crystals bound to the monolayer with surface coverage ∼50–60% (16). Surface pressure of the monolayer was adjusted from 20 to 45 mN/m and the temperature was 23 °C. Bilayer samples were deposited via Langmuir-Blodgett and Langmuir-Schaffer deposition technique at 45 mN/m and incubated with a 0.1 mg/mL CTAB solution for a minimum of 5 h before replacement with buffer.
Grazing Incident X-Ray Diffraction.
Synchrotron X-ray measurements on monolayers were carried out using a temperature controlled Langmuir trough mounted on the liquid surface diffractometer at the BW1 beam line at HASYLAB, DESY (Hamburg, Germany) at a wavelength of λ = 1.304 Å. Soller collimation yielding a lateral resolution of Δqxy = 0.0084 Å-1 and a one-dimensional position sensitive detector with vertical acceptance 0 < qz < 1.2 Å-1 were used. Bilayer measurements were conducted on beamline 6-ID at the Advanced Photon Source (Argonne National Laboratory) at a wavelength of λ = 0.545 Å and data were collected using a Mar345 image plate. The high X-ray energy used enabled measurements to be performed at the solid-liquid interface through a 1 cm thick water layer (21, 32). For GIXD experiments, the X-ray beam was adjusted to strike the surface at an incident angle corresponding to momentum transfer vector qz = 0.85 qc, where qc is the critical scattering vector for total external reflection from the interface. At this angle an evanescent wave is generated which travels along the surface and Bragg scatters from the molecular arrangements at the interface.
Supplementary Material
Acknowledgments.
We thank Dr. Kristian Kjaer for help with the monolayer measurements and Dr. Doug Robinson for assistance with the bilayer measurements. This work was supported by the National Science Foundation Chemistry Division under award 0957868, the Department of Energy (DOE) Office of Basic Energy Sciences supported travel to Argonne under award DE-FG02-OGER46340 and Los Alamos National Laboratory under DOE Contract DE-AC52-06NA25396. T.L.K. thanks the Jeff and Diane Child/Steve Whitaker fund for Distinguished Teaching and Scholarship for financial support.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
†In this case the full toxin was used. In the absence of enzymatic cleavage of the A subunit, the effects on lipid order can be attributed to binding of the B subunit only.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014579108/-/DCSupplemental.
References
- 1.White SH, Wimley WC. Membrane protein folding and stability: Physical principles. Annu Rev Bioph Biom. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 2.Phillips R, et al. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietrich C, et al. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risselada HJ, Marrink SJ. The molecular face of lipid rafts in model membranes. Proc Natl Acad Sci USA. 2008;105:17367–17372. doi: 10.1073/pnas.0807527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 6.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Bioph Biom. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 7.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 8.Hakomori S. The glycosynapse. Proc Natl Acad Sci USA. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakomori SI. Structure and function of glycosphingolipids and sphingolipids: recollections and future trends. Biochimica et Biophysica Acta. 2008;1780:325–346. doi: 10.1016/j.bbagen.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond AT, et al. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci USA. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuziemko GM, Stroh M, Stevens RC. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- 12.Zhang RG, et al. The 3-Dimensional crystal-structure of cholera-toxin. J Mol Biol. 1995;251:563–573. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]
- 13.Zhang RG, et al. The 2.4 Angstrom crystal-structure of cholera-toxin-B subunit pentamer—choleragenoid. J Mol Biol. 1995;251:550–562. doi: 10.1006/jmbi.1995.0455. [DOI] [PubMed] [Google Scholar]
- 14.Lundmark R, Carlsson SR. Driving membrane curvature in clathrin-dependent and clathrin-independent endocytosis. Semin Cell Dev Biol. 2010;21:363–370. doi: 10.1016/j.semcdb.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Wolf AA, et al. Attenuated endocytosis and toxicity of a mutant cholera toxin with decreased ability to cluster ganglioside GM(1) molecules. Infect Immun. 2008;76:1476–1484. doi: 10.1128/IAI.01286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller CE, et al. Part I: an X-ray scattering study of cholera toxin penetration and induced phase transformations in lipid membranes. Biophys J. 2008;95:629–640. doi: 10.1529/biophysj.107.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller CE, et al. Part II: diffraction from two-dimensional cholera toxin crystals bound to their receptors in a lipid monolayer. Biophys J. 2008;95:641–647. doi: 10.1529/biophysj.107.120808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alsnielsen J, et al. Principles and applications of grazing-incidence X-ray and neutron-scattering from ordered molecular monolayers at the air-water-interface. Phys Rep. 1994;246:252–313. [Google Scholar]
- 19.Kjaer K. Some simple ideas on X-ray reflection and grazing-incidence diffraction from thin surfactant films. Physica B. 1994;198:100–109. [Google Scholar]
- 20.Bernchou U, et al. Texture of lipid bilayer domains. J Am Chem Soc. 2009;131:14130, 14131. doi: 10.1021/ja903375m. [DOI] [PubMed] [Google Scholar]
- 21.Watkins EB, et al. Structure and orientational texture of self-organizing lipid bilayers. Phys Rev Lett. 2009;102:238101-1–238101-4. doi: 10.1103/PhysRevLett.102.238101. [DOI] [PubMed] [Google Scholar]
- 22.Kaganer VM, et al. Tilted phases of fatty-acid monolayers. J Chem Phys. 1995;102:9412–9422. [Google Scholar]
- 23.Ignes-Mullol J, et al. Spread monolayers: structure, flows and dynamic self-organization phenomena. Phys Rep. 2007;448:163–179. [Google Scholar]
- 24.Merritt EA, et al. Structural studies of receptor binding by cholera toxin mutants. Protein Sci. 1997;6:1516–1528. doi: 10.1002/pro.5560060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins MD, Keller SL. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc Natl Acad Sci USA. 2008;105:124–128. doi: 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lingwood D, et al. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc Natl Acad Sci USA. 2008;105:10005–10010. doi: 10.1073/pnas.0804374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenmark P, et al. Crystal structure of botulinum neurotoxin type a in complex with the cell surface co-receptor GT1b—insight into the toxin-neuron interaction. Plos Pathog. 2008;4:e1000129-1–e1000129-10. doi: 10.1371/journal.ppat.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkham M, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romer W, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 30.Ewers H, et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat Cell Biol. 2010;12:11–18. doi: 10.1038/ncb1999. [DOI] [PubMed] [Google Scholar]
- 31.Sarasij RC, Rao M. Tilt texture domains on a membrane and chirality induced budding. Phys Rev Lett. 2002;88:088101-1–088101-4. doi: 10.1103/PhysRevLett.88.088101. [DOI] [PubMed] [Google Scholar]
- 32.Miller CE, et al. Probing the local order of single phospholipid membranes using grazing incidence X-ray diffraction. Phys Rev Lett. 2008;1:058103-1–058103-4. doi: 10.1103/PhysRevLett.100.058103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.