Abstract
In Biology Oriented Synthesis the scaffolds of biologically relevant compound classes inspire the synthesis of focused compound collections enriched in bioactivity. This criterion is met by the structurally complex scaffolds of natural products (NPs) selected in evolution. The synthesis of NP-inspired compound collections approaching the complexity of NPs calls for the development of efficient synthetic methods. We have developed a one pot 4–7 step synthesis of mono-, bi-, and tricyclic oxepanes that resemble the core scaffolds of numerous NPs with diverse bioactivities. This sequence entails a ring-closing ene-yne metathesis reaction as key step and makes productive use of polymer-immobilized scavenger reagents. Biological profiling of a corresponding focused compound collection in a reporter gene assay monitoring for Wnt-signaling modulation revealed active Wntepanes. This unique class of small-molecule activators of the Wnt pathway modulates the van-Gogh-like receptor proteins (Vangl), which were previously identified in noncanonical Wnt signaling, and acts in synergy with the canonical activator protein (Wnt-3a).
Keywords: chemical biology, compound libraries
In Biology Oriented Synthesis (BIOS) biological relevance and prevalidation are employed as key criteria in the selection of compound classes for the synthesis of focused compound collections (1–4). The underlying scaffolds of natural product (NP) classes define the areas of chemical space explored by nature in evolution. NPs bind to multiple proteins in the course of their biosynthesis and when exerting their biological function. Therefore, NP scaffolds define “privileged” and biologically relevant molecular frameworks in chemical space (5). Consequently, compound collections based on and inspired by NP structure are expected to be enriched in bioactivity and to yield modulators of multiple biological processes. The use of NPs and analogues thereof has been particularly rewarding in the study of biological signal transduction events and pathways, often related to the establishment of disease (6). Thus, the synthesis of NP inspired compound collections and their evaluation in cell-based assays monitoring biological signal transduction cascades is a highly promising strategy for the identification of unique biologically relevant compound classes and probes, as well as unique candidate molecules inspiring drug discovery programs. For instance, the canonical and the noncanonical Wnt-signaling pathways play decisive roles in differentiation, patterning, and tissue regeneration (7–10). Modulators of Wnt signaling are efficient tools for the study of these processes and have been intensively searched for in drug discovery programs aimed at cancer therapy (11–14). Wnt signaling also holds promise in the development of unique methods to chemically induce stem cell differentiation and wound healing (15–20). However, Wnt-pathway activators which are required for this purpose have been identified in only very few cases (21–23).
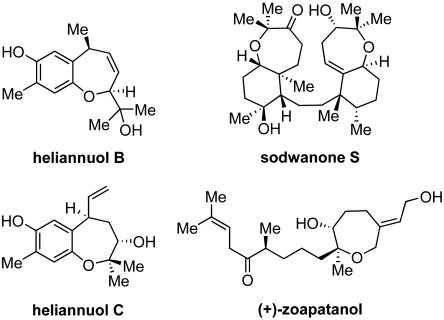
The structural complexity of NPs, and, consequently also of NP-inspired compound collections, calls for the development of efficient synthesis methods which give rapid access to NP scaffolds and allow for the flexible assembly of structural diversity. Sequential multistep reaction sequences which can be carried out as one-pot syntheses, e.g., with the help of polymer-immobilized scavenging reagents may provide advantageous solutions to the problem (24). Here we report on the development of a solution phase one-pot, 4–7 step reaction sequence that efficiently gives access to a collection of mono-, bi-, and tricyclic oxepanes. NPs with multiple bioactivities (Fig. 1) like heliannuol B and C (allelopathic and phytotoxic) (25), sodwanone S (antitumor) (26), and zoapatanol (contraceptive) (27) served as an inspiration. From this privileged library an activator of Wnt signaling was identified, which acts synergistically with the Wnt-3a protein and binds to the van-Gogh-like 1 transmembrane receptor (28).
Fig. 1.
Structures of representative bioactive NPs embodying the oxepane scaffold.
Results
Retrosynthetic Analysis.
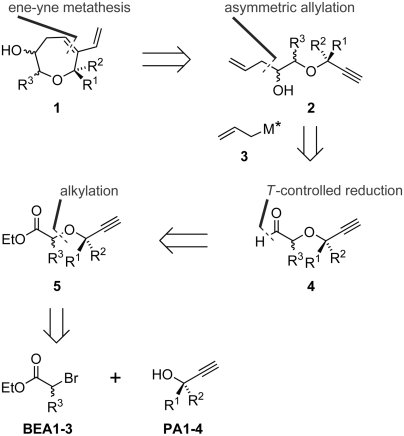
Oxepanes have been synthesized by means of different strategies (29), including ruthenium-catalyzed ring-closing metathesis of unsaturated ethers (30). For the synthesis of an NP-inspired oxepane collection a ring-closing ene-yne metathesis (RC-eneyne-M) of unsaturated linear ene-yne ethers 2 was chosen as robust key transformation with wide scope. This synthetic approach gives rise to a seven-membered oxacyclic ether embodying a diene unit with one olefin incorporated into the seven-membered ring (Scheme 1) and allows to flexibly introduce diversity at multiple intermediate steps. Homoallylic alcohols 2 should be readily accessible by means of asymmetric Brown allylation, which should proceed with predictable stereocontrol (31). The resulting secondary alcohol would remain within the ring-closing metathesis (RCM) products and allow for further functionalization. Coupling of selected propargyl alcohols (PA1-4) with α-bromo ethyl acetate derivatives (BEA1-3) would yield intermediate esters 5 and provide an opportunity for introduction of diversity. Notably, this synthesis sequence would allow for the use of solid-supported scavengers and extractive work-up procedures and thereby open up an opportunity to develop a one-pot approach.
Scheme 1.
Retrosynthetic analysis and strategic disconnection of oxepane candidates.
One-Pot Synthesis of an Oxepane Collection.
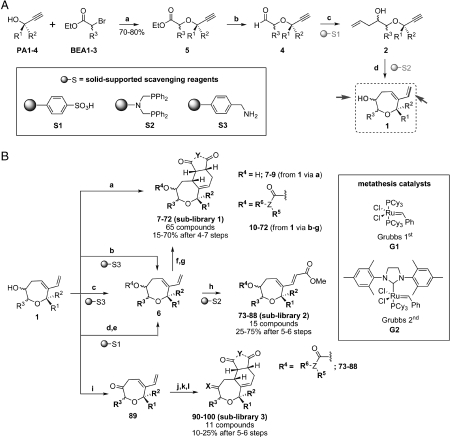
Propargyl alcohols PA1-4 were converted to aldehydes 4 by alkylation with α-bromo ethyl acetate derivatives BEA1-3 and subsequent reduction of the ester groups with diisobutylaluminium hydride (DIBAL-H) (Scheme 2A, SI Appendix: Fig. S1). Crude aldehydes 4 were allylated using allylmagnesium chloride and (+)- or (−)-diisopinocampheylboron chloride (DIPCl) as chiral auxiliary. Enantio- and/or diastereomerically enriched homoallyl alcohols 2 were obtained after scavenging of excess allyl Grignard reagent with polymer-bound sulfonic acid resin S1. As expected, matched and mismatched cases were encountered. During library synthesis we sometimes observed an unexpectedly variable stereoselectivity (8∶1 to 2∶1). This variation was obviously correlated to completeness of formation and quality of the allylation reagent. On dedicated resynthesis of hit compounds with freshly prepared reagent (see below), diastereomeric ratios of 12∶1 were regularly achieved.
Scheme 2.
(A) General synthesis of the oxepane core. Diversification sites are highlighted (arrows). Building blocks, solid-supported scavengers, reagents, and conditions: (A) NaH, THF, 0 °C to room temperature (r.t.), 10 h; (B) DIBAL-H, Et2O, 78 °C, 20 min. then aqueous HCl (1 M), -78 °C to r.t.; (C) allylmagnesium chloride (2 M in THF), (+)-DIPCl or (−)-DIPCl, THF, -78 ° > C to r.t., 4 h, then solid-supported scavenger S1; (D) First generation Grubbs catalyst (10% mol), CH2Cl2, reflux, 18 h, then S2. (B) General synthesis of the oxepane collections. Building blocks, reagents, and conditions: (A) dienophile (D2), toluene, 70 °C, 3 h; (B) acyl chloride (AC1-8), pyridine, 6 h, r.t., then S3; (C) isocyanate (I1-4), pyridine, 6 h, r.t., then S3; (D) carbonyldiimidazole, CH2Cl2, r.t. overnight; (E) amine (A1-12), K2CO3, dimethylformamide (DMF), r.t. overnight, then S1; (F) dienophile (D1-5), toluene, 70 °C, 3 h; (G) 20% H2O in THF, 10 h, r.t.; (H) methyl acrylate, second generation Grubbs catalyst (15% mol), CH2Cl2, 18 h, reflux, then S2; (I) PCC, CH2Cl2, 10 h, r.t.; (J) dienophile (D1-5), toluene, 70 °C, 3 h; (K) hydroxylamine (HA1,2), EtOH/H2O (2∶1), r.t., 10 h; and (L) 20% H2O in THF, 10 h, r.t.
In order to develop a swift one-pot synthesis, diastereomers were not separated but directly subjected to the decisive ring-closing metathesis reaction using first generation Grubbs’ catalyst (20 mol%) without purification. Upon completion of the transformation, the ruthenium catalyst was scavenged with the polymer-supported resin S2 (32) to afford oxepene-containing intermediates 1. According to NMR-spectroscopic analysis, the enantio- and diastereomeric ratio of the precursors was preserved after ring closure, as expected.
Intermediates 1 were then employed for introduction of structural diversity and synthesis of subcollections by means of chemical transformations involving the diene and the secondary alcohol (Scheme 2B). Thus, O-acylation or O-carbamoylation and subsequent Diels-Alder reaction yielded 65 compounds (subcollection 1) in 15–70% overall yield after 4–7 steps. Chromatography delivered all compounds either as single isomers or as inseparable mixtures of two isomers. p-Benzoquinone (D1, see SI Appendix) yielded hydroquinones (SI Appendix: Table S1, entries 10, 14–16, 46, 48, 49, and 51) due to aromatization of the initial Diels-Alder adducts. A nuclear Overhauser effect (NOE) study performed for compound 47 (SI Appendix: Fig. S2), indicated endo-selectivity for the Diels-Alder reaction. This finding was later confirmed by crystal structure determination of racemic compound 109 (SI Appendix: Fig. S3). The configuration of the other library members was assigned by analogy.
Further introduction of structural diversity to the oxepane scaffold was achieved through Diels-Alder cycloaddition with maleic anhydride (D5) followed by hydrolysis to diacids, which were obtained as inseparable mixtures of two isomers in 20–25% yield after 6–7 steps (SI Appendix: Table S1, entries 17–20, 26, 60–72). Cross metathesis reaction of 1 with methyl acrylate in the presence of second generation Grubbs catalyst followed by either esterification or carbamoylation of the secondary alcohol on the oxepane ring yielded 15 compounds (subcollection 2) in 25–75% overall yield after 5 or 6 steps. The cross metathesis products (SI Appendix: Table S2, entries 78–88) were formed exclusively E- configured based on the coupling constants recorded for the olefinic protons (JHa-Hb = 16.0 Hz).
In order to create a small collection of keto-oxepane derivatives (subcollection 3), the crude alcohols 1 were oxidized to the corresponding ketones using pyridinium chlorochromate (PCC) and then submitted to Diels-Alder cycloaddition with selected dienophiles (D1-5) to give keto cycloaducts as single isomers in 10–25% overall yield after 5 steps (SI Appendix: Table S3, entries 89–95). When treated with either O-methyl hydroxylamine- or O-benzyl hydroxylamine hydrochloride, a selected number of ketones were converted into oximes (1∶1 E/Z mixtures; SI Appendix: Table S3, entries 96–100) in 10–15% overall yield from intermediate 1.
Primary Screening for Wnt-Signaling Modulators in a Reporter Gene Assay.
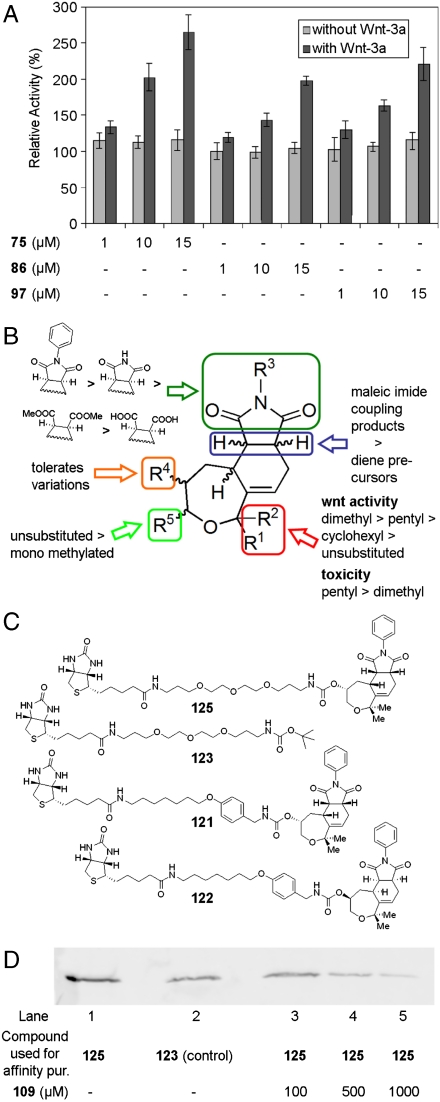
A HEK293 reporter cell line highly sensitive for stimulation by Wnt3a due to additional copies of the Frizzled receptor resulting in a 20- to 30-fold induction of a luciferase reporter (33) was used for screening of the library at 20 μM concentration. For 50 compounds the screen indicated activation of the Wnt-responsive reporter construct, resulting in an increase of the luciferase signal up to > 250% relative activity (Fig. 2A). Notably, the Wnt-pathway activators acted synergistically with the Wnt3a protein. In the absence of Wnt3a (Fig. 2A) or upon stimulation with the GSK-3β inhibitors lithium chloride or (2’Z, 3’E)-6-bromoindirubine-3’-oxime (BIO) activation (34) by the oxepanes was not observed (SI Appendix: Fig. S4). Luciferase activity was not affected by the compounds, as checked by independent control experiments.
Fig. 2.
Biological analysis of the oxepane libraries. (A) Wnt pathway modulating activity of compounds 75, 86, and 97 (see Fig. 3 for structures). Data are normalized to DMSO as control. The compounds do not activate the Wnt pathway if used in combination with cell growth media but activate if used in combination with Wnt-3a preactivated cells. (B) Structure activity relationship of the oxepanes. (C) Structures of the affinity reagents used for proteomics approaches. Compound 123 and 125 were used for proteomics and competitive Western blotting. Compound 121 and 122 represent a second generation of affinity purification reagents used for proteomics. (D) Competitive Western blot of Vangl1. The Western blot was done after affinity purification with either compound 125 (lane 1, 3–5) or compound 123 (control, lane 2) as control. Lanes 3–5 show the amount of Vangl1 protein bound on the affinity resign after elution of reversibly bound protein with the indicated concentration of compound 109.
Establishment of a Structure Activity Relationship.
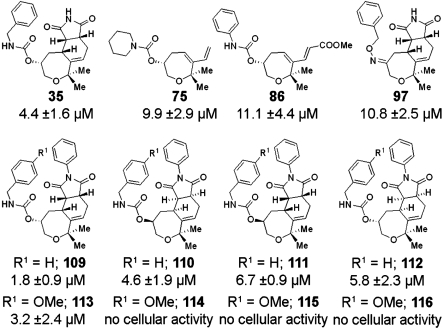
A structure activity relationship delineated from the screening results (Fig. 2B, SI Appendix: Table S5) revealed that for high activity two methyl substituents at positions R1 and R2 are beneficial. If instead of a geminal dimethyl group or a cyclohexyl group a di-n-pentyl group was installed, toxicity was found to increase. Maleic acid imide coupling products (R3 = H) and the N-phenyl analogues at the R3-position activate the Wnt signalling pathway to a higher extent than the corresponding dicarboxylic acids or oxepanes 1 and oxepane collection 3, which embody a 1,4-diene substructure. Notably, R4 could be varied widely without loss of activity (SI Appendix: Table S5) including esters and carbamates, but introducing a methyl substituent R5 already reduced the activity. Based on these results tricyclic oxepane 109 (Fig. 3) was chosen as most promising candidate for further investigation and for tracking the cellular target.
Fig. 3.
Oxepanes used in the biological investigations. For active compounds the ED50 values based on their activity in the reporter gene assay are given. Inactive compounds were measured up to 20 μM final concentration.
Synthesis of a Bait Probe for Target Identification.
For target identification by means of a chemical proteomics approach (see below) four stereoisomers 109–112 of the most active Wnt activator were synthesized separately (see SI Appendix) and investigated independently in the reporter gene assay which revealed that 109 was most active (ED50 = 1.8 ± 0.9 μM) (Fig. 2). To identify a suitable site for linker attachment compounds 113–116 carrying an additional para-methoxy group in the benzyl carbamate moiety were synthesized (see SI Appendix) and investigated as well. Activity was identical for compound 113, which has the same absolute configuration as 109. The other isomers 114–116 were inactive (up to 20 μM, Fig. 2). Therefore, the para position at the benzyl carbamate moiety was selected to attach a biotinylated linker. Probe molecule 121 was synthesized from 113, and stereoisomer 122 was prepared as control (see SI Appendix). In addition, compound 125 was synthesized as alternative probe having the linker directly attached to the oxepane core, and 123 was used as additional control substance.
Identification of Target Proteins by a Chemical Proteomics Approach.
To gain initial insight into the biological mode of action of Wnt-pathway activation, oxepane 35 (Fig. 3) was exposed to SW480 cancer cells (SI Appendix: Fig. S6). These cells harbor a mutation in the tumor suppressor gene APC (adenomatous polyposis coli) and hence show constitutively activated Wnt signaling. Treatment of SW480 cells with oxepane 35 did not additionally activate Wnt signaling suggesting that the compound must act upstream of APC. In the light of this finding and the results that 35 and 109 act synergistically with Wnt-3a but not with two glycogen synthase kinase 3 beta (GSK3-β) inhibitors (SI Appendix: Fig. S4), the cellular target was therefore assumed to be closely related to the Wnt receptor complex.
For target identification, HEK293 cell lysate was treated with affinity probes 125 or 121 immobilized on streptavidin-coated magnetic beads. Bound proteins were then released either by heating to 95 °C or by elution with increasing concentrations of nonbiotinylated ligand 109 and identified by means of PAGE, in-gel digestion with trypsin, and mass-spectrometric identification of the resulting peptides (see SI Appendix). Control pull down experiments were performed with 123 and with 122 (Fig. 3C). Proteins that were found at least three times in five independent pull down experiments with active probe 125 and could be confirmed in additional pull down experiments with probe 121 but not with the control probes 123 and 122 were considered further, if a connection to Wnt signaling had been documented in the literature. These criteria led to the proteins CDC2 (35), Pescadillo-1 (36), and van-Gogh-like receptor protein 1 (37–39) as plausible candidates. While CDC2 and Pescadillo-1 could not be eluted from the affinity beads by nonimmobilized ligand 109, van-Gogh-like receptor protein 1 (Vangl1) was specifically released with increasing concentration of compound 109 (Fig. 2D). This finding indicates that Wnt activating oxepane 109 binds reversibly to Vangl1. Because Vangl1 is a multipass hydrophobic integral membrane protein (see below), we were not surprised to find that it bound to a minor extent to the triethylene glycol control resin from which it could be released by heating and identified by means of Western blotting.
Discussion
The Wnt signal transduction pathway is of major importance in the regulation of cell differentiation and patterning as well as tissue regeneration, wound healing, and stem cell differentiation (15–20). Small-molecule modulators of Wnt signaling are considered efficient tools for the study of these processes and unique sources of inspiration for drug discovery programs aimed at e.g., cancer therapy (11–14). Notably, small-molecule activators of Wnt signaling which might for instance be invaluable as reagents for directed stem cell differentiation have been identified in only very few cases (21–23).
For the development of unique small-molecule collections enriched in bioactivity we have forwarded BIOS as a principle that builds on the selection of biologically relevant compound classes in evolution. In BIOS the underlying scaffolds of biologically relevant compound classes, in particular NPs inspire the synthesis of focussed compound collections. In order to approach NP-like diversity and structural complexity, for the synthesis of BIOS-based compound collections efficient reliable and flexible multistep synthesis sequences are required. The use of multistep, one-pot solution phase sequences is particularly promising because it minimizes synthesis-, isolation-, and purification efforts.
For an oxepane collection, we have developed a 4–7 step synthesis sequence that gives efficient access to mono-, di-, and tricyclic NP-inspired oxepanes. The synthesis proceeds in one pot and without isolation of intermediates and employs a ring-closing ene-yne metathesis as robust central step. This strategy enabled the assembly of a focussed compound collection that resembles the core structures of NPs with a mono- or polycyclic oxepane-based structural framework. The synthesis is operationally convenient and does not require elaborate instrumentation. By virtue of the use of polymer-immobilized scavenging reagents this approach yields crude products that are readily purified by means of conventional chromatography methods.
The oxepane NPs inspiring this synthesis effort display multiple biological activities and most likely target multiple proteins involved in various phenomena. Thus, we expected that the use of the oxepane collection in several cell-based assays monitoring different biological processes should yield modulators of complex biological systems at significantly enhanced frequency.
The oxepanes such derived were subjected to cell-based assays that monitor signaling through the Ras- or the Wnt-pathway, neurite outgrowth, and cell cycle progression. Much to our delight the most promising hits were activators of Wnt signaling that act synergistically with the Wnt3a protein and appeared to target the Wnt receptor complex. Employing a chemical proteomics approach we found that the most potent oxepane 109 binds to and most likely exerts its biological activity through the Vangl1.
Due to this Wnt-signaling enhancing activity we would like to term the compound class “Wntepanes” (Wnt activating oxepanes) with compound 109 being Wntepane 1. The finding that the Vangl1 is a target of the Wntepane is in accordance with the activity profile of this molecule and with reports on Vangl1 function in the literature. Vangl1 is a multipass hydrophobic integral membrane protein and serves as receptor in the noncanonical Wnt-signaling pathway. This protein can directly bind to and thereby antagonize the Wnt target protein Dishevelled (Dsh). Thus, van-Gogh-like receptors are modulators of Wnt signaling in vivo (40) and mediate cross talk between canonical and noncanonical Wnt signaling (40, 41). The antagonistic activity of van-Gogh-like receptors towards Wnt signaling had been demonstrated by knockdown with antisense oligonucleotides which led to an increase of Wnt signaling (41).
It is therefore likely that Wntepane 1 interacts with Vangl1 (and possibly Vangl2 as well) and thereby at least partially liberates and restores the signal transducing activity of Dsh. This hypothesis is supported by the observed synergistic mode of action with Wnt, because only in the case of Wnt mediated activation will the pool of Dsh be sensitive to modulation by Vangl. Modulation by a small molecule should then lead to an increase of signal. This mechanism would also be in accordance with the lack of synergism of Wntepane 1 with Li+ ions, which inhibit GSK3, and its lack of activity in SW480 cells.
Conclusion
Overall, our results demonstrate that NP inspired compound collections with an oxepane scaffold can efficiently be synthesized and that they may be rich sources for unique modulators of biological systems. The identification of the Wntepanes as ligands for the Vangl1 is notable, because small molecules targeting this protein have not been described before. In the future, Wntepane1 may serve as an advantageous tool for the study of biological processes mediated by Vangl1.
Materials and Methods
Luciferase Based Screening of Wnt-Signalling Modulators.
Reporter gene carrying cells (3,500) were seeded in a white 96 well plate (Corning) and incubated for 4 h at 37 °C and 5% CO2 in a tissue culture incubator. The medium was replaced by 100 μL of 20% Wnt-3a enriched medium supplemented with the appropriate amount of test compound. The cells were incubated for 14 h in the presence of compound and subsequently lysed. The medium was removed and 30 μL lysis buffer [25 mM Tris-HCl (pH = 7.5), 150 mM NaCl, 5 mM MgCl2, 1% NP-40, 1 mM DDT, 5% glycerol] was added and cells were incubated for 10 min. 100 μL luciferase reagent (25 mM Tris-HCl pH = 7.8, 0.5 mM coenzymeA, 0.5 mM EDTA, 0.5 mM ATP, 10 mM MgCl2, 40 mM tricine, 0.5 mM luciferine, 10 mM DTT) was added, and measurements were started immediately. An Infinite 200 M (Tecan) plate reader was used, implementing shaking at 120 rpm for 5 s before data acquisition and an integration of the signal for 1 s per well. Every concentration was measured in quadruplicate and normalized against data from DMSO-treated samples. Assessment of the assay in 96 well format using DMSO and PKF118-310 (SI Appendix: Fig. S6) as control confirmed the robustness of the experimental set-up and readout and generally resulted in Z’-factors > 0.7 (42). Six of the most active oxepanes (35, 36, 104, rac-105, rac-106, and 109) were tested at higher concentrations and showed no cytotoxicity to the liver cell line HepG2 and the endothelial cell lines HeLa and Hek293 up to a concentration of 160 μM (SI Appendix: Fig. S5).
Solution Phase Synthesis of Oxepane 1 Using Polymer-Supported Scavenging Reagents.
To a cooled suspension (0 °C) of sodium hydride [1.5 equivalent (equiv.) , 95% dispersion in mineral oil] in 50 mL of THF, a solution of a selected building block PA1-4 (1 equiv.) in THF (20 mL) was added drop wise over 20 min. The mixture was warmed to 25 °C and stirred for 15 min. After cooling to 0 °C, a solution of selected building block BEA1-3 (1.5 equiv.) in THF (10 mL) was added drop wise over 30 min and the resulting mixture was warmed to 25 °C and stirred for 6 h. Water was added (20 mL, then 100 mL), followed by Et2O (100 mL) and the layers were separated. The aqueous layer was extracted with Et2O ( ). The combined ether extracts were washed with brine (2 × 20 mL), dried with Na2SO4, filtered, and concentrated. Purification of the residue by silica gel chromatography (cyclohexane/ethyl acetate 9∶1) furnished propargyl ethers 5 (70–80% yield).
). The combined ether extracts were washed with brine (2 × 20 mL), dried with Na2SO4, filtered, and concentrated. Purification of the residue by silica gel chromatography (cyclohexane/ethyl acetate 9∶1) furnished propargyl ethers 5 (70–80% yield).
To a solution of the ester 5 in diethyl ether at -78 °C, diisobutylaluminium hydride (1 M in hexane, 1.5 equiv.) was added slowly by a syringe pump over 30 min. The mixture was stirred at -78 °C for 20 min. HCl solution was added (1 M) and the mixture was stirred for 1 h. The mixture was extracted with diethyl ether ( ,
,  ). The combined extracts were washed with water (2 × 10 mL) and brine (2 × 10 mL), dried with Na2SO4, filtered, and concentrated under reduced pressure to afford aldehydes 4 (90%-quantitative yield).
). The combined extracts were washed with water (2 × 10 mL) and brine (2 × 10 mL), dried with Na2SO4, filtered, and concentrated under reduced pressure to afford aldehydes 4 (90%-quantitative yield).
(+) or (−)-DIPCl was dissolved in THF in a two-neck round-bottom flask and cooled to 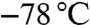 . Allylmagnesium chloride (2 M in THF, 1.5 equiv.) was added drop wise and the mixture was stirred at
. Allylmagnesium chloride (2 M in THF, 1.5 equiv.) was added drop wise and the mixture was stirred at 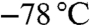 for 1 h. The reaction mixture was warmed to 20 °C and stirred for 1 h. After cooling to
for 1 h. The reaction mixture was warmed to 20 °C and stirred for 1 h. After cooling to 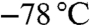 , crude aldehyde 4 (dissolved in THF) was added drop wise and the mixture was stirred at
, crude aldehyde 4 (dissolved in THF) was added drop wise and the mixture was stirred at 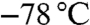 for 1 h. The reaction mixture was allowed to warm to room temperature, stirred for 1 h, and then diluted with methanol (10 mL). Sulfonic acid resin (S1) was added and the mixture was shaken at room temperature for 6 h. The resin was drained, washed with methanol and CH2Cl2 and the solvents were evaporated to afford the crude homoallylic alcohols 2.
for 1 h. The reaction mixture was allowed to warm to room temperature, stirred for 1 h, and then diluted with methanol (10 mL). Sulfonic acid resin (S1) was added and the mixture was shaken at room temperature for 6 h. The resin was drained, washed with methanol and CH2Cl2 and the solvents were evaporated to afford the crude homoallylic alcohols 2.
In a two-neck round-bottom flask with attached refluxing condenser, alcohol 2 was dissolved in CH2Cl2 (0.002 M). Ar was bubbled through the solution by a needle for 30 min. First generation Grubbs catalyst (20 mol%) was added and the reaction mixture was heated to reflux. After the reaction was complete [thin layer chromatography (TLC) control], polymer-bound ruthenium scavenger resin S2 (20 equiv. relative to the catalyst added) was added and the mixture was shaken at room temperature for 10 h. The resin was filtered off over a pad of silica gel, washed with dichloromethane, and the solvents were evaporated to obtain the crude product 1.
Supplementary Material
Acknowledgments.
We are grateful to Petra Janning and Andreas Brockmeyer for support in performing and analysis of the proteomics experiments. This research was supported by the Max-Planck-Gesellschaft, the state of Nord-Rhein-Westfalen [Zentrum für Angewandte Chemische Genomik (ZACG) Dortmund], the European Commission (FP6, RTN Endocyte), and the European Research Council (FP7, ERC Grant agreement n° 268309).
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference nos. 803785 and 803786).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015269108/-/DCSupplemental.
References
- 1.Breinbauer R, Vetter IR, Waldmann H. From protein domains to drug candidates-natural products as guiding principles in the design and synthesis of compound libraries. Angewandte Chemie International Edition. 2002;41:2878–2890. doi: 10.1002/1521-3773(20020816)41:16<2878::AID-ANIE2878>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 2.Koch MA, et al. Compound library development guided by protein structure similarity clustering and natural product structure. Proc Natl Acad Sci USA. 2004;101:16721–16726. doi: 10.1073/pnas.0404719101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch MA, et al. Charting biologically relevant chemical space: a structural classification of natural prducts (SCONP) Proc Natl Acad Sci USA. 2005;102:17272–17277. doi: 10.1073/pnas.0503647102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nören-Müller A, et al. Discovery of protein phosphatase inhibitor classes by biology-oriented synthesis. Proc Natl Acad Sci USA. 2006;103:10606–10611. doi: 10.1073/pnas.0601490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bon RS, Waldmann H. Bioactivity-guided navigation of chemical space. Acc Chem Res. 2010;43:1103–1114. doi: 10.1021/ar100014h. [DOI] [PubMed] [Google Scholar]
- 6.Hinterding K, Alonso-Díaz D, Waldmann H. Organic synthesis and biological signal transduction. Angewandte Chemie International Edition. 1998;37:688–749. doi: 10.1002/(SICI)1521-3773(19980403)37:6<688::AID-ANIE688>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7.Coombs GS, Covey TM, Virshup DM. Wnt signaling, disease and translational medicine. Curr Drug Targets. 2008;9:513–531. doi: 10.2174/138945008784911796. [DOI] [PubMed] [Google Scholar]
- 8.Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008;134:646–656. doi: 10.1016/j.cell.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tada M, Kai M. Noncanonical Wnt/PCP signaling during vertebrate gastrulation. Zebrafish. 2009;6:29–40. doi: 10.1089/zeb.2008.0566. [DOI] [PubMed] [Google Scholar]
- 10.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 11.Lepourcelet M, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 12.Chung N, et al. A 1,536-well ultra-high-throughput siRNA screen to identify regulators of the Wnt/beta-catenin pathway. Assay Drug Dev Technol. 2010;8:286–294. doi: 10.1089/adt.2009.0262. [DOI] [PubMed] [Google Scholar]
- 13.Emami KH, et al. A small molecule inhibitor of the beta-catenin/CREB binding protein transcription. Proc Natl Acad Sci USA. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen B, et al. Development of small molecules targeting the Wnt pathway for the treatment of colon cancer: a high-throughput screening approach. Nat Chem Biol. 2009;2:100–107. doi: 10.1152/ajpgi.00005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochegarov A. Small molecules for stem cells. Expert Opin Ther Pat. 2009;19:275–281. doi: 10.1517/13543770802709010. [DOI] [PubMed] [Google Scholar]
- 16.Osakada F, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–3179. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- 17.Reya T, Clevers H. Wnt signaling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 18.Zhang DL, et al. Effect of Wnt signaling pathway on wound healing. Biochem Biophys Res Commun. 2009;378:149–151. doi: 10.1016/j.bbrc.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Fathke C, et al. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol. 2006;7:4–12. doi: 10.1186/1471-2121-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silkstone D, Hong H, Alman BA. Beta-catenin in the race to fracture repair: in it to Wnt. Nat Clin Pract Rheum. 2008;4:413–419. doi: 10.1038/ncprheum0838. [DOI] [PubMed] [Google Scholar]
- 21.Coghlan MP, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 22.Park EJ, et al. Novel small molecule activators of beta-catenin-mediated signaling pathway: structure-activity relationships of indirubins. Bioorg Med Chem Lett. 2009;19:2282–2284. doi: 10.1016/j.bmcl.2009.02.083. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, et al. Small-molecule synergist of the Wnt/beta-catenin signaling pathway. Proc Natl Acad Sci USA. 2007;104:7444–7448. doi: 10.1073/pnas.0702136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ley SV, Baxendale IR. New tools and concepts for modern organic synthesis. Nat Rev Drug Discov. 2002;1:573–586. doi: 10.1038/nrd871. and references therein. [DOI] [PubMed] [Google Scholar]
- 25.Macias FA, Molinillo JMG, Varela RM, Torres A, Fronczek FR. Structural elucidation and chemistry of a novel family of bioactive sesquiterpenes: heliannuols. J Org Chem. 1994;59:8261–8266. [Google Scholar]
- 26.Funel-Le Bon C, Berrue F, Thomas OP, Reyes F, Amade P. Sodwanone S, a triterpene from the marine sponge. Journal of Natural Products. 2005;68:1284–1287. doi: 10.1021/np050100o. [DOI] [PubMed] [Google Scholar]
- 27.Kanojia RM, et al. Synthesis and antifertility activity of zoapatanol analogs. J Med Chem. 1985;28:796–803. doi: 10.1021/jm00383a018. [DOI] [PubMed] [Google Scholar]
- 28.Cantrell VA, Jessen JR. The planar cell polarity protein van Gogh-Like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett. 2010;287:54–61. doi: 10.1016/j.canlet.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Hoberg OJ. Synthesis of seven-membered oxacycles. Tetrahedron. 1998;54:12631–12670. [Google Scholar]
- 30.Crimmins MT, Choy AL. An asymmetric aldol-ring-closing metathesis strategy for the enantioselective construction of oxygen heterocycles: an efficient approach to the enantioselective synthesis of (+)-laurencin. J Am Chem Soc. 1999;121:5653–5660. [Google Scholar]
- 31.Brown HC, Jadhav PK. Asymmetric carbon-carbon bond formation via beta-allyldiisopinocampheylborane. simple synthesis of secondary homoallylic alcohols with excellent enantiomeric purity. J Am Chem Soc. 1983;105:2092–2093. [Google Scholar]
- 32.Westhus M, Gonthier E, Brohm D, Breinbauer R. An efficient and inexpensive scavenger resin for grubbs’ catalyst. Tetrahedron Lett. 2004;45:3141–3142. [Google Scholar]
- 33.Park S, et al. Hexachlorophene inhibits Wnt/beta-catenin pathway by promoting Siah-mediated beta-catenin degradation. Mol Pharmacol. 2006;70:960–966. doi: 10.1124/mol.106.024729. [DOI] [PubMed] [Google Scholar]
- 34.Meijer L, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Davidson G, et al. Cell cycle control of Wnt receptor activation. Dev Cell. 2009;17:788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Gessert S, Maurus D, Rossner A, Kuhl M. Pescadillo is required for Xenopus laevis eye development and neural crest migration. Dev Biol. 2007;310:99–112. doi: 10.1016/j.ydbio.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Torban E, et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci USA. 2008;105:3449–3454. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localized and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- 39.Katoh M. Strabismus (STB)/Vang-like gene family. Int J Mol Med. 2002;10:11–15. [PubMed] [Google Scholar]
- 40.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behavior and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 41.Axelrod JD. Strabismus comes into focus. Nat Cell Biol. 2002;4:E6–8. doi: 10.1038/ncb0102-e6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.