Abstract
Amphotericin B is the archetype for small molecules that form transmembrane ion channels. However, despite extensive study for more than five decades, even the most basic features of this channel structure and its contributions to the antifungal activities of this natural product have remained unclear. We herein report that a powerful series of functional group-deficient probes have revealed many key underpinnings of the ion channel and antifungal activities of amphotericin B. Specifically, in stark contrast to two leading models, polar interactions between mycosamine and carboxylic acid appendages on neighboring amphotericin B molecules are not required for ion channel formation, nor are these functional groups required for binding to phospholipid bilayers. Alternatively, consistent with a previously unconfirmed third hypothesis, the mycosamine sugar is strictly required for promoting a direct binding interaction between amphotericin B and ergosterol. The same is true for cholesterol. Synthetically deleting this appendage also completely abolishes ion channel and antifungal activities. All of these results are consistent with the conclusion that a mycosamine-mediated direct binding interaction between amphotericin B and ergosterol is required for both forming ion channels and killing yeast cells. The enhanced understanding of amphotericin B function derived from these synthesis-enabled studies has helped set the stage for the more effective harnessing of the remarkable ion channel-forming capacity of this prototypical small molecule natural product.
Transmembrane ion conductance is a higher-order function most often performed by proteins. There are, however, several naturally occurring (1) and a growing number of unnatural (2) small molecules that demonstrate a similar capacity. The existence of these prototypes suggests that the potential for small molecules to perform ion channel-like functions in the context of living systems may extend far beyond that which is currently understood or utilized (3). The paradigmatic example is the polyene macrolide amphotericin B (AmB, Fig. 1 A and B), a very powerful but also highly toxic antifungal agent with remarkably low rates of microbial resistance despite widespread clinical utilization since the 1960s (4). Despite more than half a century of intensive study, however, the mechanism of action of AmB is poorly understood. Even the most basic components of the leading model remain unclear, including the structure/function relationships that underlie channel self-assembly (5–19), the importance of AmB/phospholipid interactions (12, 13, 15–19), the role of membrane sterols in forming ion channels (5–13, 15, 20–40), and even whether channel formation is causitively linked to antifungal activity (41–43).
Fig. 1.
Synthesis-enabled functional group deletions to probe predicted intermolecular interactions involving the C41 carboxylic acid and/or the C19 mycosamine appendages of AmB. (A) An efficient and flexible degradative synthesis pathway that transforms the natural product AmB into three functional group-deficient derivatives: MeAmB, AmdeB, and MeAmdeB (49). See SI Appendix (Schemes S1–S4) for detailed synthesis schemes and a glossary of all reagent abbreviations. (B) Bird’s eye view of the leading barrel-stave model of the AmB ion channel. Polar interactions between (C) neighboring molecules of AmB, (D) AmB and a phospholipid, and/or (E) AmB and ergosterol are predicted to be critical for AmB function.
AmB is named for its amphoteric nature, which arises from two post-PKS tailoring enzyme modifications of the polyene macrolide skeleton, namely the installation of a carboxylic acid at C41 and an amine-containing mycosamine at C19. Importantly, three different hypotheses predict specific roles for these conspicuous functional groups in the mechanism of action of AmB (Fig. 1 C–E).
First, in the now classic “barrel-stave” ion channel model (5–7, 11–13) (Fig. 1B), these appendages are predicted to form a critical ring of stabilizing polar interactions between neighboring molecules of AmB (Fig. 1C) (8, 11–14). Second, one or both of these functional groups are proposed to form critical polar interactions with phospholipid headgroups either to “anchor” AmB to the membrane (12, 13) and/or to promote channel formation (15–19) (Fig. 1D). Third, both the C41 carboxylate and mycosamine appendages are hypothesized to promote the direct binding of membrane-embedded sterols (Fig. 1E) (11–13, 30–37). An often invoked alternative model states that indirect effects on global membrane properties, rather than any direct binding interactions, are responsible for the impacts of membrane sterols on the biological activity of AmB (21–29).
Designing and executing definitive experiments that illuminate the underpinnings of AmB function has proven to be extremely challenging. For example, many computational (11–13, 29, 36, 37) and spectroscopic (9, 10, 15, 34, 39, 40) studies have been reported, but the membrane-localized, dynamic, and multimolecular nature of this small-molecule-based channel assemblage challenges the current limitations of these techniques. In addition, many studies have been performed with AmB derivatives having covalent modifications of the C41 carboxylic acid and/or C(3′) amine (14, 19, 31–35, 44–46). However, relative to many proteins and large peptides, the self-assembly of small molecules can be exquisitely sensitive to steric effects that result from even very minor covalent modifications (47, 48). Thus, the importance of all three of these putative interactions (Fig. 1 C–E) has remained unclear.
For example, N-acylation of the mycosamine appendage causes a dramatic reduction in antifungal and yeast permeabilizing activities (30), which has been used to support the prediction that a ring of polar interactions between neighboring molecules of AmB is critical (Fig. 1C) (12). However, steric clashing, rather than loss of a polar interaction, could alternatively underlie such effects (47–49). A variety of C41 esters have been found to retain yeast permeabilizing and antifungal activities (30). However, these covalently modified AmB derivatives retain a polar carbonyl group and thereby still possess the capacity to form charge-dipole interactions with the mycosamine appendage and perhaps thereby stabilize channel self-assembly (32). An attempt to probe this putative polar interaction via covalent tethering of two molecules of AmB through these two functional groups resulted in derivatives with little to no antifungal activity, but it was unclear if this was due to a lack of suitable flexibility of the covalent linker (14). Thus, the covalent modifications-based approach has yet to clarify the importance of the predicted ring of channel-stabilizing polar interactions between the mycosamine sugar and the C41 carboxylic acid.
To probe the proposal that polar interactions between AmB and the phospholipid headgroups of lipid molecules are important (Fig. 1D), a phospholipid was also covalently tethered to the mycosamine of AmB. However, this derivative also lacked antifungal activity (19), and the importance of putative interactions between the functional groups at C41 and C19 and phospholipids have also remained unclear.
The precise role of sterols in the mechanism of action of AmB has also remained ambiguous, although the importance of sterols was recognized as early as 1958 (20) and has been supported by many subsequent studies (4–13, 15, 20–40). For example, the presence of sterols is typically required for AmB to form ion channels (1, 5–7), and, albeit very rare, strains of yeast that are resistant to AmB usually have substantially modified membrane sterol content (4, 41). Two competing hypotheses have been advanced to account for these findings.
The first hypothesis states that indirect differential preorganization of membranes caused by different sterols is responsible (21–29). This model has been invoked to rationalize observations that, although AmB is typically most potent in membranes that contain sterols, increasing quantities of cholesterol (21, 22) or ergosterol (23) can actually decrease the capacity for AmB to promote membrane permeabilization. Further supporting this model, AmB can permeabilize some artificial membranes in the absence of sterols (24–28).
The competing hypothesis states that direct binding of AmB to sterols in the lipid bilayer is critical (5–13). Specifically, the C41 carboxylate and/or mycosamine appendages of AmB are proposed to form key polar interactions with the 3-β-hydroxyl group of a sterol. Combined with favorable hydrophobic interactions between the polyene and sterol cores, these polar interactions are predicted to stabilize a direct AmB/sterol complex (30–37). Studies designed to probe this putative interaction in solution or in liposomes have traditionally relied primarily on UV/Vis and/or CD spectroscopy (10). However, it has been theorized that differences in these spectra may reflect changes in AmB aggregation states rather than an AmB/sterol binding interaction (9, 34). AmB forms channels with different electrophysiological properties in membranes containing cholesterol or enantiomeric cholesterol, consistent with diastereomeric AmB/cholesterol complexes (38). It is challenging, however, to perform this same experiment in a yeast cell membrane to determine if this type of direct binding interaction is functionally relevant in vivo. More recently, solid-state NMR studies employing deuterated derivatives of AmB and cholesterol (39, 40) suggested little or no binding between these two molecules because their mobilities were dissimilar (40). Analogous experiments with AmB and ergosterol were interpreted as being alternatively consistent with similar mobilities and thus supportive of a direct binding interaction between these two molecules (40). However, in other solid-state NMR studies, through-space dephasing expected for a direct binding interaction was not observed between nonconjugated 13C-labeled AmB and 6-fluoroergosterol (34). Moreover, a covalently tethered dimer of AmB and ergosterol lacked antifungal activity (33, 34). Thus, despite extensive investigations, the question of whether AmB directly interacts with membrane-bound sterols, the structure/function relationships underlying this putative interaction, and, most importantly, the role of this putative interaction in ion channel-forming and antifungal activities in vivo have all remained unclear.
More broadly, even the question of whether channel formation is causitively linked to antifungal activity has remained a matter of substantial debate (41–43). It is frequently invoked that autooxidation of the polyene motif may alternatively underlie the antifungal effects of AmB (10, 42, 43).
Given the aforementioned complications and resulting lack of clarity associated with covalent modifications, we pursued an alternative experimental strategy involving the synthesis-enabled deletion of protic functional groups appended to the polyene macrolide skeleton, verifying retention of macrolide conformation, and determining the biological and biophysical consequences of these functional group deletions (49). Albeit much more synthetically challenging, deleting functional groups (50–52) circumvents the inherent complications associated with the exquisite sensitivity of small-molecule self-assembly to steric effects (47, 48). In addition and very importantly, the same probe reagents can be employed both in vitro and in vivo. Thus, in contrast to experiments based on perturbing membrane components, this strategy can directly link structure/function relationships determined via specific biophysical studies to the consequences of perturbing such functions in living cells.
Due to the very complex structure of polyene macrolides, their poor solubility in most organic solvents and water, and their sensitivity to light, oxygen, acid, and many other reagents, synthesizing functional group-deficient derivatives of these natural products is challenging. Nevertheless, we developed a very efficient and flexible degradative synthesis pathway (Fig. 1A) that can access three AmB derivatives, MeAmB (53, 54), AmdeB (55), and MeAmdeB, representing the systematic deletion of either or both of the post-PKS modifications predicted to be involved in each of the intermolecular interactions shown in Fig. 1 C–E. Utilizing AmB as starting material, this strategy relies on the preparation of a suitably protected common intermediate that is much more soluble and stable than the natural product. This common intermediate is then funneled into different degradative reaction sequences to access all three of our targeted functional group-deficient derivatives. Importantly, a suite of multidimensional NMR studies demonstrated that these functional group deletions have no impact on the three-dimensional shape of the polyene macrolide skeleton, thus facilitating the interpretation of biological and biophysical studies with these probe reagents (49).
In preliminary experiments (49) we found that both derivatives lacking the mycosamine unit (AmdeB and MeAmdeB) showed no antifungal activity at concentrations five- to 10-fold higher than the MICs of AmB, suggesting a potentially critical role for this sugar appendage. However, in contrast to prior studies that reported MeAmB to be fourfold less active than AmB (53) or were conducted with a mixture of polyenes (54), MeAmB was equipotent to AmB against Candida albicans. Similar results were observed with Saccharomyces cerevisiae. This discovery suggested that oxidation at C41 may not be required for channel formation (Fig. 1C) and/or channel formation may not be required for antifungal activity. It also remained unclear if these two groups are required to bind to phospholipid membranes (Fig. 1D) and/or ergosterol (Fig. 1E) and how these putative interactions might be related to channel-forming and antifungal activities. We herein report the clarification of all of these long-standing issues via harnessing the efficiency and flexibility of our degradative synthesis pathway on much larger scale and subjecting the resulting functional group-deficient probes to a systematic series of biological and biophysical studies.
Results
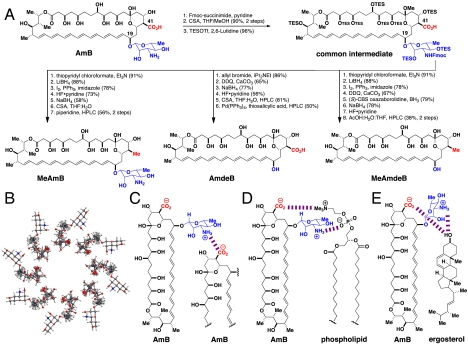
To access ≥12 mg of each of our targeted functional group-deficient derivatives of AmB, we prepared more than 24 g of our common intermediate and selectively degraded this material according to Fig. 1A. With larger quantities of each synthetic derivative in hand, we repeated MIC assays against both S. cerevisiae and C. albicans using the lowest recommended density of yeast cells (56) and the highest concentrations of AmB derivatives permitted by solubility. Even in this much more sensitive experiment, MeAmB was equipotent to AmB against both S. cerevisiae and C. albicans, whereas AmdeB and MeAmdeB were completely inactive, even at concentrations 100- to 200-fold higher than the MIC of AmB (Fig. 2A). These results confirmed that the mycosamine appendage, but not oxidation at C41, is strictly required for antifungal activity.
Fig. 2.
Mycosamine, but not an oxidized functional group at C41, is required for antifungal and ion channel activities. (A) The minimum inhibitory concentrations (MIC) for AmB and its derivatives. (B) Potassium efflux from S. cerevisiae cells after treatment with polyene macrolides (3 μM). (C) Potassium efflux from 10% ergosterol-containing LUVs after treatment with polyene macrolides (1 μM). (D) Voltage clamp recordings in ergosterol-containing planar lipid bilayers in the presence of 150 mV of applied potential following addition of AmB (10 nM), MeAmB (75 nM), AmdeB (100 nM), MeAmdeB (50 nM; planar lipid bilayers were unstable in the presence of MeAmdeB at 100 nM). All recordings are representative of at least three experiments.
To test whether the predicted ring of polar interactions between the C41 carboxylic acid and mycosamine appendages is required for ion channel formation (Fig. 1C) (8, 11–14), we initially performed potassium efflux studies with S. cerevisiae as described in the SI Appendix (57). As shown in Fig. 2B, a rapid and robust efflux of potassium ions was caused by both AmB and MeAmB, whereas no efflux was observed for the two derivatives lacking a mycosamine appendage. To probe whether these differences in ion efflux can be attributed to effects directly on the bilayer membrane, as described in the SI Appendix we performed the same studies on egg phosphatidylcholine (PC) large unilamellar vesicles (LUVs) containing 10% ergosterol, the main sterol found in yeast. As shown in Fig. 2C, AmB and MeAmB similarly demonstrated rapid permeabilization in this cell-free system whereas AmdeB and MeAmdeB were inactive.
To clarify whether these observed differences in potassium efflux were attributable to the formation of discrete ion channels versus nonspecific disruption of membrane integrity, we performed single channel recording experiments using a voltage clamp planar lipid bilayer system (1, 8). As shown with the representative traces in Fig. 2D, we routinely observed single channel activity for both AmB and MeAmB. Collectively, these findings demonstrate that the predicted ring of intermolecular polar interactions (Fig. 1C) is not required for ion channel formation. In stark contrast, channel formation was never observed with either of the derivatives lacking mycosamine (Fig. 2D). Thus, the mycosamine appendage plays an alternative and vital role in forming the AmB ion channel.
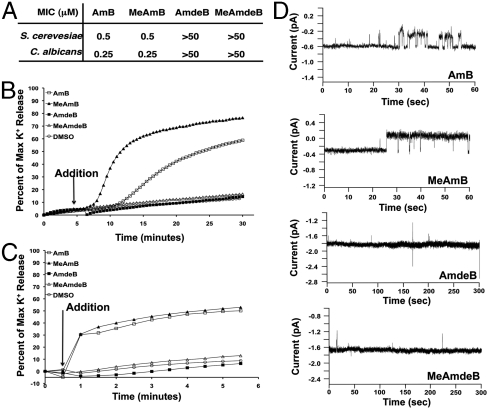
One simple hypothesis to explain these findings is that the derivatives lacking mycosamine cannot readily bind to lipid bilayers. This would be consistent with the proposal that specific interactions between the charged functional groups of AmB and the zwitterionic PC headgroups (Fig. 1D) (15–19) help anchor AmB to the membrane (12, 13). To test this proposal, we first determined the relative binding of AmB and its derivatives to yeast cells (58). As shown in Fig. 3A, both MeAmB and AmdeB bound at least as readily as AmB. To specifically probe membrane binding in the absence of potentially complicating cell wall and/or active transport systems, we also determined the binding of each compound to egg PC/ergosterol LUVs via size-exclusion chromatography (59). As shown in Fig. 3B, AmB and all three of its functional group-deficient derivatives readily bound to these cell-free membranes. Thus, neither the C41 carboxylate nor the mycosamine appendage is required for the binding of AmB to a lipid bilayer.
Fig. 3.
Neither the C41 carboxylic acid nor the mycosamine appendage is required for binding to phospholipid bilayers. (A) Binding to S. cerevisiae via centrifugation. (B) Binding to 10% ergosterol-containing LUVs via size-exclusion chromatography. All values represent the mean of at least three experiments.
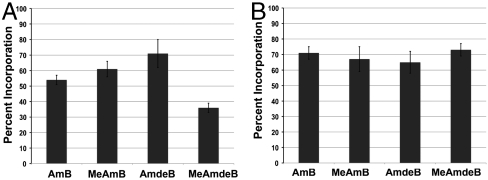
The third hypothesis predicts that the C41 carboxylic acid and/or mycosamine appendages promote a direct binding interaction between AmB and membrane-embedded ergosterol (Fig. 1E). Testing this hypothesis in a definitive manner has proven to be very challenging, but we recognized that our functional group-deficient derivatives might clarify this long-standing issue. Toward this end, as described in the SI Appendix we adapted an isothermal titration calorimetry (ITC)-based assay recently developed to probe the binding of sterols to the natural product natamycin (60). To determine whether AmB directly binds membrane-embedded ergosterol, we first titrated a solution of AmB with sterol-free LUVs and observed a small net exotherm (Fig. 4A). We then repeated the same ITC experiment using LUVs containing 10% ergosterol, and a substantially larger net exotherm was observed (Fig. 4A).
Fig. 4.
AmB directly binds to membrane-embedded ergosterol in a mycosamine-dependent fashion. (A) ITC thermograms for solutions of AmB titrated with sterol-free or 10% ergosterol-containing egg PC LUVs. (B) ITC thermograms for solutions of AmB titrated with sterol-free, 10% ergosterol-containing, and 10% lanosterol-containing POPC LUVs. (C) Differences in net exotherms for AmB, MeAmB, AmdeB, and MeAmdeB titrated with sterol-free followed by 10% ergosterol-containing egg PC LUVs. All values represent the mean of at least three experiments.
To determine whether this increase in exotherm was due to a direct binding interaction between AmB and ergosterol or alternatively to indirect sterol-mediated changes in global membrane properties (21–29), we repeated the same titrations using POPC LUVs containing no sterol, 10% ergosterol, or 10% lanosterol (Fig. 4B). Importantly, extensive solid-state NMR studies have demonstrated that the incorporation of either ergosterol or lanosterol into POPC liposomes causes very similar changes in global membrane properties (61–63). In the event, titrating AmB with sterol-free and then 10% ergosterol-containing POPC LUVs again caused a substantial increase in the observed net exotherm. In stark contrast, no increase in exotherm was observed when switching from sterol-free to 10% lanosterol-containing POPC LUVs (Fig. 4B). Collectively, these data establish that AmB directly binds membrane-embedded ergosterol.
We next tested the activity of MeAmB, AmdeB, and MeAmdeB in the same ITC-based assay to determine whether the functional groups at C41 and/or C19 are required for this small molecule–small molecule interaction. Titration of MeAmB with sterol-free followed by 10% ergosterol-containing egg PC LUVs resulted in an increase in exotherm very similar to that observed with AmB (Fig. 4C). Thus, oxidation at C41 is not required for AmB to bind ergosterol. Strikingly, however, very different results were obtained when this same pair of experiments was repeated with the two AmB derivatives lacking a mycosamine appendage (Fig. 4C). Specifically, the titration of AmdeB or MeAmdeB with sterol-free followed by 10% ergosterol-containing LUVs yielded no net increase in the observed exotherm. Thus, the mycosamine appendage is required for promoting a direct binding interaction between AmB and ergosterol. Importantly, as shown in the SI Appendix (Fig. S5), similar ITC experiments revealed that AmB also directly binds membrane-embedded cholesterol in a mycosamine-dependent fashion.
Finally, because synthetic deletion of the mycosamine appendage also causes a complete loss of the capacity to form ion channels and kill yeast cells (Fig. 2), these results are consistent with the conclusion that a direct AmB/sterol binding interaction is required for both the channel-forming and antifungal activities of AmB.
Discussion
Despite extensive studies for more than half a century, the underpinnings of the archetypal AmB ion channel have remained unclear. This is largely because the membrane-localized, dynamic, and multimolecular nature of the AmB channel assemblage challenges start-of-the-art spectroscopic and computational techniques, and readily accessible covalent modifications of the natural product are inherently linked to potentially confounding steric effects. Moreover, in vitro experiments that involve modifying the constitution of lipid bilayers are challenging to translate into living yeast cells. Overcoming these limitations, we have demonstrated an experimental strategy based on the synthesis-enabled deletion of functional groups from the polyene macrolide skeleton and determination of the biological and biophysical consequences (49, 64). In this report, we have harnessed a highly efficient and flexible degradative synthesis pathway to prepare large quantities of three functional group-deficient derivatives of AmB. Scalable access to these powerful probes has enabled us to systematically test three leading hypotheses for the role(s) of the carboxylic acid and mycosamine appendages of AmB and thereby substantially advance the fundamental understanding of this ion channel-forming natural product.
First, because MeAmB completely lacks oxygenation at C41 yet is fully competent at forming ion channels in planar lipid bilayers, LUVs, and yeast cells, a predicted ring of intermolecular polar interactions between the oppositely charged functional groups of AmB is in fact not required for channel formation. Interestingly, we did observe differences in the electrophysiological properties of ion channels formed from AmB and MeAmB, the origins of which will be the subject of a future study. Importantly, while some peptide-based ion channels may require specific intermolecular polar interactions for self-assembly (65), in many cases more general physical phenomena are alternatively proposed to drive this process (66). Thus, although other predicted specific intermolecular interaction(s) between AmB subunits (12, 13) may prove to be contributory, it is likely that the same general physical phenomena responsible for the self-assembly of channel-forming amphipathic peptides are also important for self-assembly of this small-molecule-based ion channel.
Second, because derivatives lacking the carboxylic acid and/or mycosamine appendage readily bind to LUVs and yeast cells, these two functional groups are not required to anchor AmB to the membrane. Interestingly, interactions with sterols are also frequently invoked as a requirement for membrane anchoring of AmB (7, 46). However, the aglycones AmdeB and MeAmdeB, which lack the capacity to bind ergosterol (Fig. 4C), bind very well to LUVs and yeast cells (Fig. 3). Similar to many detergents and amphipathic peptides, the membrane partitioning of AmB may alternatively be driven primarily by the classic hydrophobic effect (67, 68). Moreover, because AmdeB and MeAmdeB both contain the same putatively redox active polyene motif found in AmB and readily bind to LUVs and yeast cells (Fig. 3) yet are completely devoid of antifungal activity (Fig. 2A), polyene autooxidation is unlikely to play a primary role in the antifungal activity of AmB (42, 43). Our results do not rule out the possibility that specific interactions between AmB and phospholipids might play a constructive role in channel formation.
Third, the ITC results presented herein demonstrate that AmB directly binds membrane-embedded ergosterol and cholesterol. Moreover, deletion of the mycosamine appendage abolishes the capacity to bind both sterols; form ion channels in planar lipid bilayers, LUVs, and yeast cells; and exert antifungal activity. These results strongly support the conclusion that mycosamine-mediated direct sterol binding is strictly required for both the physiologically relevant channel-forming and antifungal activities of AmB.
Interestingly, there are two possible interpretations of these findings with respect to the mechanism(s) by which AmB kills yeast cells: Either channel formation is necessary for antifungal activity or, alternatively, sterol binding is necessary for antifungal activity and channel formation is only one of multiple sterol-binding-dependent mechanisms of action. For the following reasons, we strongly favor the latter interpretation.
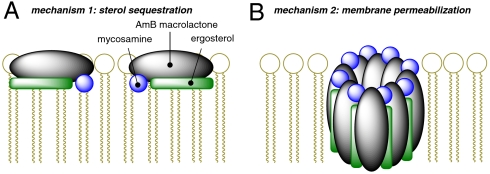
Based on the results described herein and the fact that mycosamine has only been found in polyene macrolide natural products, this glycoside in combination with a polyene may serve as a general sterol-binding motif. Importantly, however, some natural products that contain this combination of substructures demonstrate antifungal activities but do not cause substantial membrane permeabilization (7, 60, 69). For example, the mycosamine-containing tetraene macrolide natamycin, which is substantially shorter in length than AmB, was recently reported to bind ergosterol and exert antifungal activity without permeabilizing yeast cells (60). Based on these findings, it was proposed that natamycin alternatively exerts its antifungal effects via binding and sequestering sterols and thereby precluding their participation in vital cellular functions (60). It is also interesting to note, however, that natamycin is a less potent antifungal agent than AmB (69, 70). As shown in the SI Appendix (Figs. S6, S7, and S8), we have confirmed all of these findings. In addition, in some clinical isolates the membrane-permeabilizing and antifungal effects of mycosamine-bearing polyene macrolides are disconnected (41). In the light of our results, all of these findings can now be rationalized with a new model in which AmB exerts its antifungal effects via two distinct and complementary mechanisms of action: sterol-sequestration and membrane permeabilization, both of which strictly require mycosamine-mediated sterol binding (Fig. 5). This two-mechanism model may explain the antifungal activities of many if not all mycosamine-containing polyene macrolide natural products. Remarkably, an analogous pair of killing mechanisms has recently been demonstrated to underlie the antibacterial activity of the peptide nisin, which directly binds lipid II and forms ion channels in a lipid II binding dependent fashion (71). Thus, this dual killing mechanism may represent a common strategy convergently evolved in both peptide and small molecule antimicrobials. More recent studies with natamycin were accompanied by similar speculations (72).
Fig. 5.
A proposed unifying model for amphotericin B antifungal activity involving two complementary mechanisms of action that both require mycosamine-mediated binding to sterols: (A) sterol sequestration and (B) membrane permeabilization.
The results of this study stand to have several important impacts. First, this clarified picture of the archetypal AmB ion channel helps build the foundation for developing small molecules with the potential to replicate the functions of deficient protein ion channels that underlie human diseases. In addition, the conclusion that direct sterol binding is critical for the resistance-refractory antifungal activity of AmB may help guide the development of new antifungal agents. Moreover, the demonstration of direct binding of AmB to both ergosterol and cholesterol, the discovery that both of these binding interactions are mycosamine-dependent, and the connection of this direct sterol binding to ion channel and antifungal activities will help focus efforts toward the rational optimization of the therapeutic index of this clinically vital but also highly toxic antimycotic. Furthermore, to the best of our knowledge, the capacity to promote a small molecule–small molecule interaction represents a previously undescribed function for a glycosidic residue appended to a natural product (73), which may prove to be more generally operative. Finally, these studies vividly demonstrate the power of synthesis-enabled functional group deletions to illuminate highly elusive fundamental underpinnings of small molecule function.
Experimental Methods
Voltage Clamp Single Channel Recordings.
Experiments were performed using a Warner Instruments planar lipid bilayer workstation. Similar to prior studies (1), planar lipid membranes were formed from PC:phosphatidylethanolamine:ergosterol 40∶20∶1 over a 100–150 μm hole in a Teflon® sheet. The bathing solution was 2.5 M KCl 10 mM potassium phosphate (pH 7.0). Small molecules in DMSO were added, and the resulting solutions were stirred for 10 min with zero applied potential. Then, 150 mV of potential was applied across the membrane and channel formation was monitored.
Supplementary Material
Acknowledgments.
We thank Bristol-Myers Squibb for a generous gift of AmB, E. Buck and G. Fried for assistance with planar lipid bilayer studies, and R. M. Kohli and M. W. Kanan for thoughtful review of this manuscript. This work was supported by the National Institutes of Health (NIH) (GM080436). M.D.B. is a Howard Hughes Medical Institute Early Career Scientist, Beckman Young Investigator, Sloan Foundation Fellow, Dreyfus New Faculty Awardee, Bristol-Myers Squibb Unrestricted Grantee, Eli-Lilly Grantee, AstraZeneca Excellence in Chemistry Awardee, and Amgen Young Investigator. D.S.P. is an NIH Graduate Fellow (GM080436-S1), and I.D. is a Coleman Graduate Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015023108/-/DCSupplemental.
References
- 1.Ermishkin LN, Kasumov KhM, Potzeluyev VM. Single ionic channels induced in lipid bilayers by polyene antibiotics amphotericin B and nystatine. Nature. 1976;262:698–699. doi: 10.1038/262698a0. [DOI] [PubMed] [Google Scholar]
- 2.Matile S, Som A, Sorde N. Recent synthetic ion channels and pores. Tetrahedron. 2004;60:6405–6435. [Google Scholar]
- 3.Lee SJ, Gray KC, Paek JS, Burke MD. Simple, efficient, and modular syntheses of polyene natural products via iterative cross-coupling. J Am Chem Soc. 2008;262:466–468. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanglard D, Odds FC. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet Infect Dis. 2002;2:73–85. doi: 10.1016/s1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein A, Holz R. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and AmB. In: Eisenman G, editor. Membranes: Lipid Bilayers and Antibiotics. Vol. 2. New York: Marcel Dekker, Inc.; 1973. p. 377. [PubMed] [Google Scholar]
- 6.Andreoli TE. The structure and function of AmB-cholesterol pores in lipid bilayermembranes. Ann NY Acad Sci. 1974;235:448–468. doi: 10.1111/j.1749-6632.1974.tb43283.x. [DOI] [PubMed] [Google Scholar]
- 7.de Kruijff B, Demel RA. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. III. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974;339:57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- 8.Kasumov KhM, et al. How do ionic channel properties depend on the structure of polyene antibiotic molecules? Biochim Biophys Acta. 1979;551:229–237. doi: 10.1016/0005-2736(89)90001-1. [DOI] [PubMed] [Google Scholar]
- 9.Ernst C, Grange J. Structure of amphotericin B aggregates as revealed by UV and CD spectroscopies. Biopolymers. 1981;20:1575–1588. [Google Scholar]
- 10.Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 11.Khutorsky VE. Structures of amphotericin B-cholesterol complex. Biochim Biophys Acta. 1992;1108:123–127. doi: 10.1016/0005-2736(92)90015-e. [DOI] [PubMed] [Google Scholar]
- 12.Baginski M, Resat H, McCammon JA. Molecular properties of amphotericin B membrane channel: A molecular dynamics simulation. Mol Pharm. 1997;52:560–570. doi: 10.1124/mol.52.4.560. [DOI] [PubMed] [Google Scholar]
- 13.Baginski M, Resat H, Borowski E. Comparative molecular dynamics simulations of amphotericin B-cholesterol/ergosterol membrane channels. Biochim Biophys Acta. 2002;1567:63–78. doi: 10.1016/s0005-2736(02)00581-3. [DOI] [PubMed] [Google Scholar]
- 14.Umegawa Y, Matsumori N, Oishi T, Murata M. Amphotericin B covalent dimers with carbonyl-amino linkage: A new probe for investigating ion channel assemblies. Tetrahedron Lett. 2007;48:3393–3396. [Google Scholar]
- 15.Murata M, et al. Ion channel complex of antibiotics as viewed by NMR. Pure Appl Chem. 2009;81:1123–1129. [Google Scholar]
- 16.Balakrishnan AR, Easwaran KRK. Lipid-amphotericin B complex structure in solution: A possible first step in the aggregation process in cell membranes. Biochemistry. 1993;32:4139–4144. doi: 10.1021/bi00066a040. [DOI] [PubMed] [Google Scholar]
- 17.Minones J. Influence of a spreading method on the properties of amphotericin B—dipalmitoyl phosphatidic acid mixed films at the air/water interface. Langmuir. 2000;16:5743–5748. [Google Scholar]
- 18.Minones J. Mixed monolayers of amphotericin B-dipalmitoylphosphatidylcholine: Study of complex formation. Langmuir. 2002;18:2817–2827. [Google Scholar]
- 19.Matsuoka S, Matsumori N, Murata M. Amphotericin B-phospholipid covalent conjugates: Dependence of membrane-permeabilizing activity on acyl-chain length. Org Biomol Chem. 2003;1:3882–3884. doi: 10.1039/b306801c. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb D, Carter HE, Sloneker JH, Ammann A. Protection of fungi against polyene antibiotics by sterols. Science. 1958;128:361. doi: 10.1126/science.128.3320.361. [DOI] [PubMed] [Google Scholar]
- 21.HsuChen CC, Feingold DS. Polyene antibiotic action on lecithin liposomes: Effect of cholesterol and fatty acyl chains. Biochem Bioph Res Co. 1973;51:972–978. doi: 10.1016/0006-291x(73)90022-3. [DOI] [PubMed] [Google Scholar]
- 22.Shigeru M, Murata M. Cholesterol markedly reduces ion permeability induced by membrane-bound amphotericin B. Biochim Biophys Acta. 2002;1564:429–434. doi: 10.1016/s0005-2736(02)00491-1. [DOI] [PubMed] [Google Scholar]
- 23.Zumbuehl A, Stano P, Heer D, Walde P, Carreira EM. Amphotericin B as a potential probe of the physical state of vesicle membranes. Org Lett. 2004;6:3683–3686. doi: 10.1021/ol0487276. [DOI] [PubMed] [Google Scholar]
- 24.Aracava Y, Schreier S, Phadke R, Deslauriers R, Smith ICP. Effects of amphotericin B on membrane permeability—kinetics of spin probe reduction. Biophys Chem. 1981;14:325–332. doi: 10.1016/0301-4622(81)85034-x. [DOI] [PubMed] [Google Scholar]
- 25.Milhaud J, Hartman MA, Bolard J. Interaction of the polyene antibiotic amphotericin B with model membranes—differences between large and small vesicles. Biochimie. 1989;71:49–56. doi: 10.1016/0300-9084(89)90130-2. [DOI] [PubMed] [Google Scholar]
- 26.Cotero BV, Rebolledo-Antunez S, Ortega-Blake I. On the role of sterol in the formation of the amphotericin B channel. Biochim Biophys Acta. 1998;1375:43–51. doi: 10.1016/s0005-2736(98)00134-5. [DOI] [PubMed] [Google Scholar]
- 27.Ruckwardt T, Scott A, Scott J, Mikulecky P, Hartsel SC. Lipid and stress dependence of amphotericin B ion selective channels in sterol-free membranes. Biochim Biophys Acta. 1998;1372:283–288. doi: 10.1016/s0005-2736(98)00073-x. [DOI] [PubMed] [Google Scholar]
- 28.Venegas B, Gonzales-Damien J, Celis H, Ortega-Blake I. Amphotericin B channels in the bacterial membrane: Role of sterol and temperature. Biophys J. 2003;85:2323–2332. doi: 10.1016/s0006-3495(03)74656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czub J, Baginski M. Modulation of amphotericin B membrane interaction by cholesterol and ergosterol—a molecular dynamics study. J Phys Chem B. 2006;110:16743–16753. doi: 10.1021/jp061916g. [DOI] [PubMed] [Google Scholar]
- 30.Chéron M, et al. Quantitative structure-activity relationships in amphotericin B derivatives. Biochem Pharmacol. 1988;37:827–836. doi: 10.1016/0006-2952(88)90168-2. [DOI] [PubMed] [Google Scholar]
- 31.Hervé M, Debouzy JC, Borowski E, Cybulska B, Gary-Bobo CM. The role of the carboxyl and amino groups of polyene macrolides in their interactions with sterols and their selective toxicity. A 31P-NMR study. Biochim Biophys Acta. 1989;980:261–272. [Google Scholar]
- 32.Mazerski J, Bolard J, Borowski E. Effect of the modifications of ionizable groups of amphotericin B on its ability to form complexes with sterols in hydroalcoholic media. Biochim Biophys Acta. 1995;1236:170–176. doi: 10.1016/0005-2736(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 33.Matsumori N, et al. An amphotericin B-ergosterol covalent conjugate with powerful membrane permeabilizing activity. Chem Biol. 2004;11:673–679. doi: 10.1016/j.chembiol.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 34.Kasai Y, et al. Self-assembled amphotericin B is probably surrounded by ergosterol: Bimolecular interactions as evidenced by solid-state NMR and CD spectra. Chem Eur J. 2008;14:1178–1185. doi: 10.1002/chem.200701256. [DOI] [PubMed] [Google Scholar]
- 35.Matsumori N, Sawada Y, Murata M. Mycosamine orientation of amphotericin B controlling interaction with ergosterol: Sterol-dependent activity of conformation-restricted derivatives with an amino-carbonyl bridge. J Am Chem Soc. 2005;127:10667–10675. doi: 10.1021/ja051597r. [DOI] [PubMed] [Google Scholar]
- 36.Neumann A, Czub J, Baginski M. On the possibility of the amphotericin B-sterol complex formation in cholesterol- and ergosterol- containing lipid bilayers: A molecular dynamics study. J Phys Chem B. 2009;113:15875–15885. doi: 10.1021/jp905133f. [DOI] [PubMed] [Google Scholar]
- 37.Neumann A, Baginski M, Czub J. How do sterols determine the antifungal activity of amphotericin B? Free energy of binding between the drug and its membrane targets. J Am Chem Soc. 2010;132:18266–18272. doi: 10.1021/ja1074344. [DOI] [PubMed] [Google Scholar]
- 38.Mickus DE, Levitt DG, Rychnovsky SD. Enantiomeric cholesterol as a probe of ion-channel structure. J Am Chem Soc. 1992;114:359–360. [Google Scholar]
- 39.Paquet M-J, Fournier I, Barwicz J, Tancréde P, Auger M. The effects of amphotericin B on pure ergosterol- or cholesterol-containing dipalmitoylphosphatidylcholine bilayers as viewed by 2H NMR. Chem Phys Lipids. 2002;119:1–11. doi: 10.1016/s0009-3084(02)00071-3. [DOI] [PubMed] [Google Scholar]
- 40.Matsumori N, et al. Direct interaction between amphotericin B and ergosterol in lipid bilayers as revealed by 2H NMR spectroscopy. J Am Chem Soc. 2009;131:11855–11860. doi: 10.1021/ja9033473. [DOI] [PubMed] [Google Scholar]
- 41.HsuChen CC, Feingold DS. Two types of resistance to polyene antibiotics in Candida albicans. Nature. 1974;251:656–659. doi: 10.1038/251656a0. [DOI] [PubMed] [Google Scholar]
- 42.Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. Amphotericin B: Current understanding of mechanisms of action. Antimicrob Agents Chemother. 1990;34:183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Temple ME, Brady MT, Koranyi KI, Nahata MC. Periorbital cellulitis secondary to Conidiobolus incongruus. Pharmacotherapy. 2001;21:351–354. doi: 10.1592/phco.21.3.351.34210. [DOI] [PubMed] [Google Scholar]
- 44.Zumbuehl A, et al. An amphotericin B-fluorescein conjugate as a powerful probe for biochemical studies of the membrane. Angew Chem Int Edit. 2004;43:5181–5185. doi: 10.1002/anie.200460489. [DOI] [PubMed] [Google Scholar]
- 45.Zumbuehl A, et al. A novel strategy for bioconjugation: synthesis and preliminary evaluation with amphotericin B. Org Biomol Chem. 2007;5:1339–1342. doi: 10.1039/b701953j. [DOI] [PubMed] [Google Scholar]
- 46.Zumbuehl A, et al. Synthesis and investigation of tryptophan-amphotericin B conjugates. ChemBioChem. 2009;10:1617–1620. doi: 10.1002/cbic.200900096. [DOI] [PubMed] [Google Scholar]
- 47.Mathias JP, Simanek EE, Whitesides GM. Self-assembly through hydrogen bonding: peripheral crowding—a new strategy for the preparation of stable supramolecular aggregates based on parallel, connected CA3.cntdot.M3 rosettes. J Am Chem Soc. 1994;116:4326–4340. [Google Scholar]
- 48.Gorbitz CH, Etter MC. Hydrogen bonds to carboxylate groups. Syn/anti distributions and steric effects. J Am Chem Soc. 1992;114:627–631. [Google Scholar]
- 49.Palacios DS, Anderson TM, Burke MD. A post-PKS oxidation of the amphotericin B skeleton predicted to be critical for channel formation is not required for potent antifungal activity. J Am Chem Soc. 2007;129:13804–13805. doi: 10.1021/ja075739o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lemieux RU. How water provides the impetus for molecular recognition in aqueous solution. Acc Chem Res. 1996;29:373–380. [Google Scholar]
- 51.Morgan AJ, Wang YK, Roberts MF, Miller SJ. Chemistry and biology of deoxy-myo-inositol phosphates: Stereospecificity of substrate interactions within an archaeal and a bacterial IMPase. J Am Chem Soc. 2004;126:15370–15371. doi: 10.1021/ja047360x. [DOI] [PubMed] [Google Scholar]
- 52.Nam J, Shin D, Rew Y, Boger DL. Alanine scan of [l-Dap2]ramoplanin A2 aglycon: Assessment of the importance of each residue. J Am Chem Soc. 2007;129:8747–8755. doi: 10.1021/ja068573k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacPherson DT, et al. Adventures in polyene macrolide chemistry: The derivatization of amphotericin B. In: Bently PH, Ponsford R, editors. Recent Advances in the Chemistry of Antiinfective Agents. Vol. 119. Cambridge: Royal Society of Chemistry; 1993. pp. 205–222. Special Publication. [Google Scholar]
- 54.Carmody M, et al. Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups. J Biol Chem. 2005;280:34420–34426. doi: 10.1074/jbc.M506689200. [DOI] [PubMed] [Google Scholar]
- 55.Nicolaou KC, et al. Chemistry of amphotericin B. Degradation studies and preparation of amphoteronolide B. J Am Chem Soc. 1988;110:4660–4672. [Google Scholar]
- 56.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing, M27-A2, Approved Standard. 2nd Ed. 15. Vol. 22. Wayne, PA: Clinical and Laboratory Standards Institute; 2002. [Google Scholar]
- 57.Hammond SM, Lambert PA, Kliger BN. The mode of action of polyene antibiotics; induced potassium leakage in Candida albicans. J Gen Microbiol. 1974;81:325–330. doi: 10.1099/00221287-81-2-325. [DOI] [PubMed] [Google Scholar]
- 58.Kotler-Brajtburg J, Medoff G, Schlessinger D, Kobayashi GS. Characterization of the binding of amphotericin B to Saccharomyces cerevisiae and relationship to the antifungal effects. Antimicrob Agents Chemother. 1974;6:770–776. doi: 10.1128/aac.6.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breukink E, et al. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry. 1997;36:6968–6976. doi: 10.1021/bi970008u. [DOI] [PubMed] [Google Scholar]
- 60.te Welscher YM, et al. Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. J Biol Chem. 2008;283:6393–6401. doi: 10.1074/jbc.M707821200. [DOI] [PubMed] [Google Scholar]
- 61.Urbina JA, et al. Molecular order and dynamics of phosphatiylcholine bilayer-membranes in the presence of cholesterol, ergosterol, and lanosterol-A comparative study using 2-H-NMR, 13-C-NMR and 31-P-NMR spectroscopy. Biochim Biophys Acta. 1995;1238:163–176. doi: 10.1016/0005-2736(95)00117-l. [DOI] [PubMed] [Google Scholar]
- 62.Henriksen J. Universal behavior of membranes with sterols. Biophys J. 2006;90:1639–1649. doi: 10.1529/biophysj.105.067652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsueh Y, et al. Ergosterol in POPC membranes: physical properties and comparison with structurally similar sterols. Biophys J. 2007;92:1606–1615. doi: 10.1529/biophysj.106.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szpilman AM, Cereghetti DM, Manthorpe JM, Wurtz NR, Carreira EM. Synthesis and biophysical studies on 35-deoxy amphotericin B methyl ester. Chem Eur J. 2009;15:7117–7128. doi: 10.1002/chem.200900231. [DOI] [PubMed] [Google Scholar]
- 65.Urry DW, Goodall MC, Glickson JD, Mayers DF. The gramicidin A transmembrane channel: Characteristics of head-to-head dimerized π(L,D) helices. Proc Natl Acad Sci USA. 1971;68:1907–1911. doi: 10.1073/pnas.68.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee MT, Hung WC, Chen FY, Huang HW. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci USA. 2008;105:5087–5092. doi: 10.1073/pnas.0710625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heerklotz H, Seelig J. Titration calorimetry of surfactant-membrane partitioning and membrane solubilization. Biochim Biophys Acta. 2000;1508:69–85. doi: 10.1016/s0304-4157(00)00009-5. [DOI] [PubMed] [Google Scholar]
- 68.Machaidze G, Ziegler A, Seelig J. Specific binding of Ro 09-0198 (cinnamycin) to phosphatidylethanolamine: a thermodynamic analysis. Biochemistry. 2002;41:1965–1971. doi: 10.1021/bi015841c. [DOI] [PubMed] [Google Scholar]
- 69.Zygmunt WA. Intracellular loss of potassium in Candida albicans after exposure to polyene antifungal antibiotics. Appl Microbiol. 1966;14:953–956. doi: 10.1128/am.14.6.953-956.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotler-Brajtburg J, et al. Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob Agents Chemother. 1979;15:716–722. doi: 10.1128/aac.15.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasper HE, et al. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science. 2006;313:1636–1637. doi: 10.1126/science.1129818. [DOI] [PubMed] [Google Scholar]
- 72.te Welscher YM, et al., editors. Natamycin inhibits vacuole fusion at the priming phase via a specific interaction with ergosterol. Antimicrob Agents Chemother. 2010;54:2618–2625. doi: 10.1128/AAC.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walsh CT, Freel-Meyers CL, Losey HC. Antibiotic glycosyltransferases: Antibiotic maturation and prospects for reprogramming. J Med Chem. 2003;46:3425–3436. doi: 10.1021/jm030257i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.