Abstract
The syndrome of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) is the most common periodic fever disease in children. However, the pathogenesis is unknown. Using a systems biology approach we analyzed blood samples from PFAPA patients whose genetic testing excluded hereditary periodic fevers (HPFs), and from healthy children and pediatric HPF patients. Gene expression profiling could clearly distinguish PFAPA flares from asymptomatic intervals, HPF flares, and healthy controls. During PFAPA attacks, complement (C1QB, C2, SERPING1), IL-1–related (IL-1B, IL-1RN, CASP1, IL18RAP), and IFN-induced (AIM2, IP-10/CXCL10) genes were significantly overexpressed, but T cell-associated transcripts (CD3, CD8B) were down-regulated. On the protein level, PFAPA flares were accompanied by significantly increased serum levels of chemokines for activated T lymphocytes (IP-10/CXCL10, MIG/CXCL9), G-CSF, and proinflammatory cytokines (IL-18, IL-6). PFAPA flares also manifested a relative lymphopenia. Activated CD4+/CD25+ T-lymphocyte counts correlated negatively with serum concentrations of IP-10/CXCL10, whereas CD4+/HLA-DR+ T lymphocyte counts correlated positively with serum concentrations of the counterregulatory IL-1 receptor antagonist. Based on the evidence for IL-1β activation in PFAPA flares, we treated five PFAPA patients with a recombinant IL-1 receptor antagonist. All patients showed a prompt clinical and IP-10/CXCL10 response. Our data suggest an environmentally triggered activation of complement and IL-1β/-18 during PFAPA flares, with induction of Th1-chemokines and subsequent retention of activated T cells in peripheral tissues. IL-1 inhibition may thus be beneficial for treatment of PFAPA attacks, with IP-10/CXCL10 serving as a potential biomarker.
Keywords: anakinra, autoinflammatory disease, inflammasome, therapy
The syndrome of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) represents the most common autoinflammatory fever disorder in childhood (1). This clinical entity is characterized by regular occurrences of high fever (>39 °C), which are associated with at least one of the three cardinal clinical signs, including aphthous stomatitis, pharyngitis, and cervical adenitis (2, 3). Additional features, including headache, gastrointestinal symptoms, rash, and arthralgia, may be present (3–6) but are not consistently noted. The disease onset is generally before the age of 5 y, with attacks lasting 3 to 6 d and recurring every 3 to 8 wk. Patients are asymptomatic between episodes and show normal growth and development. PFAPA usually resolves in adolescence, although a small but increasing number of patients with adult-onset disease have been reported (7–9).
The etiology of PFAPA is unknown. Laboratory findings during flares show nonspecific leukocytosis with neutrophilia, elevated erythrocyte sedimentation rate (3, 4, 6), C-reactive protein (CRP) (10–12), and fibrinogen (4). In some patients serum IgD can be elevated (4, 13). PFAPA is diagnosed by exclusion of other probable causes of recurrent fevers in children, such as infectious, autoimmune, and malignant diseases. The differential diagnosis also includes cyclic neutropenia and the hereditary periodic fever syndromes (HPFs). The latter disorders are caused by genetic variants of the innate immune system (1) and include familial Mediterranean fever (FMF), TNF receptor-associated periodic syndrome (TRAPS), hyperimmunoglobulinemia D with periodic fever syndrome (HIDS), and cryopyrin-associated periodic syndromes (CAPS). The clinical overlap of PFAPA with these diseases (14–16) requires their exclusion, but at the same time raises the question of whether PFAPA is a separate entity or whether it represents a milder manifestation of HPFs or a collection of other undefined fever syndromes. Proposed concepts of the pathogenesis of PFAPA include infection, abnormal host immune responses, or a combination of both (10, 17).
To date, no completely satisfactory treatment options exist for PFAPA. Corticosteroid therapy of the flares is often associated with an increased attack frequency as well as the side effects of repeated therapy. Adenotonsillectomy is still controversial, perhaps because of differences in the cohorts that have been studied (3, 18, 19). Finally, the treatment with cimetidine is only effective in up to 30% of patients (20). Using a systems biology approach, we report data, which indicate that PFAPA flares are associated with an IL-1β/-18–mediated recruitment of activated T cells to peripheral tissues, implicating Th1-chemokines as potential biomarkers and IL-1β as a previously unexplored therapeutic target.
Results
Whole-Blood Gene Expression Profiling Differentiates PFAPA Flares from Asymptomatic Intervals and HPF Flares, and Indicates Involvement of Innate and Adaptive Immunity in the Pathogenesis of PFAPA.
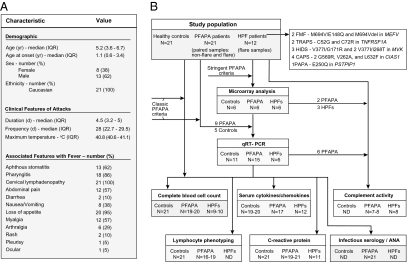
From our prospectively collected cohort of 21 PFAPA patients in whom HPFs were genetically excluded (Fig. 1A), we performed whole-blood microarray analysis in paired samples (flare, nonflare) of six patients fulfilling stringent PFAPA criteria, as noted in Materials and Methods, six healthy controls, and six HPF patients sampled during flare (Fig. 1B). Unsupervised principal component analysis of the overall messenger RNA expression revealed that PFAPA flares were remarkably distinct from asymptomatic intervals in PFAPA patients, and could be clearly differentiated from flares of patients with HPFs. In contrast, gene expression in PFAPA patients during asymptomatic periods was indistinguishable from that of healthy children (Fig. 2A).
Fig. 1.
Study population and procedures. (A) Demographic and clinical characteristics of 21 patients with PFAPA syndrome. IQR, interquartile range. (B) Schematic of the overall study population and number of patients analyzed in each different assay or experiment. Definitions of classic and stringent PFAPA criteria are provided in Materials and Methods. Infectious serology comprised antistreptolysin O, anti-DNaseB, antibody titers against HSV 1 and 2, EBV, and CMV. ANA, anti-nuclear antibody; ND, not done; PAPA, pyogenic arthritis, pyoderma gangrenosum, and acne syndrome.
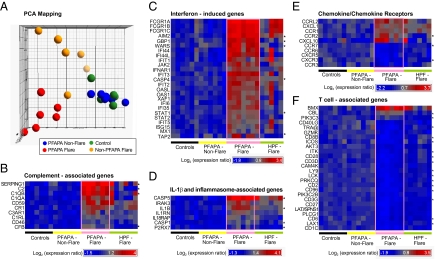
Fig. 2.
Distinct whole-blood gene expression profiles in PFAPA syndrome. Microarray analysis of messenger RNA extracted from whole blood of six PFAPA patients during and between episodes, six healthy controls and six hereditary periodic fever patients during flares [two FMF (M694V/E148Q and M694del in MEFV), one TRAPS (C52G in TNFRSF1A), three CAPS (two G569R, and L632F in NLRP3/CIAS1)]. (A) Principal component analysis of differentially expressed genes, with each dot representing one subject. (B–F) Hierarchical clustering of differentially expressed genes in the four study groups, with genes selected and listed according to their intensity of expression and canonical pathways, respectively. Gene symbols are depicted in the left column. At least twofold and statistically significant differences of probe set expression between active and inactive PFAPA disease states are shown (Wilcoxon signed-rank test P < 0.007, FDR 2%). Asterisks in the right column indicate at least 1.5-fold significantly changed transcript levels when comparing flares of PFAPA with flares of hereditary autoinflammatory diseases (Mann-Whitney U test P < 0.03, FDR 20%). Expression values were normalized per gene (row) to the mean of the healthy control group; each column represents one individual. As shown on the color bars, red indicates relative up-regulation, blue relative down-regulation.
The expression profiles of 960 probe sets changed significantly in PFAPA patients when flare and asymptomatic periods were compared [P < 0.007 at a false-discovery rate (FDR) 2.0%] (Dataset S1). Among the top up-regulated transcripts during PFAPA flares were complement genes (Fig. 2B) and transcripts typically induced by IFN or related to IFN signal transduction (Fig. 2 C and E). Furthermore, several IL-1– and inflammasome-associated genes showed elevated levels of expression during PFAPA flares (Fig. 2D). In addition to IFN-inducible protein of 10 kDa (IP-10/CXCL10), which encodes a chemokine for activated T lymphocytes (21), the transcription of various other chemokines and chemokine receptors expressed by monocytes (CCR1, CCR2) was up-regulated, whereas receptors expressed on T lymphocytes were down-regulated (CXCR3) (Fig. 2E). Accordingly, a statistically significant underrepresentation of a variety of T-cell–associated transcripts was observed during PFAPA flares compared with asymptomatic intervals (Fig. 2F).
As these findings could be simply a reflection of changes associated with fever during PFAPA, we compared the differential expression of PFAPA flare-associated transcripts with those seen during HPF flares. To maximize the possibility of detecting differentially expressed genes in this unpaired comparison of patient samples during fever, we set the fold-change and FDR criteria at 1.5 and 20% (P < 0.03), respectively (Dataset S2). Among a total of 600 differentially expressed genes, we identified the following top five biological pathways in PFAPA patients: (i) cytotoxic T-lymphocyte–mediated apoptosis of target cells, (ii) T-cell receptor signaling, (iii) IL-9 signaling, (iv) p53 signaling, and (v) TREM1 signaling, thereby suggesting the involvement of both adaptive and innate immunity. We found that 16 of 26 (61%) PFAPA flare-associated T-cell transcripts differed significantly from HPF flares, as well as 4 of 7 (57%) IL-1-inflammasome–associated genes, 4 of 10 (40%) complement genes, 3 of 10 (30%) chemokine/receptor genes, and 6 of 25 (24%) IFN-induced genes (Fig. 2 B–F).
The comparison of gene-expression levels between healthy controls with those of PFAPA patients during asymptomatic intervals showed no difference at a FDR that would correspond to a P < 0.05. As these two study populations were indistinguishable, we did not compare transcript levels of healthy controls with PFAPA patients during flares.
To validate our microarray data, we performed quantitative real-time PCR (qRT-PCR) on a larger group of PFAPA patients and controls, including PFAPA patients with less stringent (classic) criteria, as noted in Materials and Methods (Fig. 1B). We analyzed 15 paired PFAPA samples (flare, nonflare), 11 healthy controls, and 6 HPF patients during flare. The results of these experiments confirmed the complement, IL-1β– and IFN-activating processes during PFAPA flares (Fig. S1).
Taken together, our results indicate that gene-expression levels of asymptomatic PFAPA patients are indistinguishable from healthy controls, but that transcription patterns during PFAPA flares involve host defense mechanisms that comprise innate (complement cascade, IL-1-inflammasome) and adaptive (T lymphocytes, IFN-γ signaling) immunity, and support the idea of an immunologic response to environmental exposures, possibly infectious, as the trigger of PFAPA flares.
Analysis of Cell Subtype Distributions in Peripheral Blood of PFAPA Patients Reveals Activation and Peripheral Retention of Th1 Cells During PFAPA Flares.
The concurrent measurement of circulating white-cell counts and lymphocyte subpopulations during and between PFAPA flares (Fig. 1B) allowed us to elucidate the cause of reduced expression of T-lymphocyte–associated mRNA transcripts. During flares, PFAPA patients showed significant leukocytosis with neutrophilia and monocytosis, accompanied by a relative lymphopenia and eosinopenia (Table S1). The PFAPA flare-associated changes in cell counts also reached statistical significance for lymphocytes, monocytes, and eosinophils, compared with HPF patient results during flares.
Lymphocyte immunophenotyping showed that T lymphocytes were significantly reduced during PFAPA flares compared with asymptomatic intervals, affecting both CD4+ and CD8+ T-cell numbers (Table S1). Of these populations, activated CD4+ T cells, identified as CD4+HLA-DR+ (P = 0.03) and CD4+CD25+ (P = 0.004), were significantly reduced during attacks, whereas activated CD8+ T cells did not differ. Although double-negative CD3+ lymphocytes were significantly lower during PFAPA flares, there was no difference compared with healthy controls. The γ/δ T lymphocytes were slightly increased during asymptomatic periods of PFAPA relative to healthy controls, but did not show any change during flares. There were no differences in numbers of B cells, natural killer cells, and natural killer T cells when comparing PFAPA patients during flare and symptomatic periods or in healthy controls. In summary, these data indicate that in addition to the well-recognized neutrophilia, numbers of monocytes, eosinophils, and activated CD4+ lymphocytes are markedly perturbed during PFAPA attacks.
PFAPA Flares Are Associated with High Serum Levels of Proinflammatory Cytokines and Th1-Chemokines.
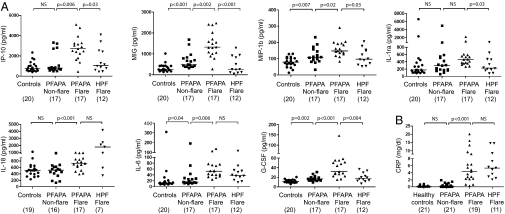
To understand the basis for the cellular distributions during flares in the context of our gene-expression results, we measured IP-10/CXCL10, in addition to 27 other chemokines and cytokines, as markers of cellular activation in sera of PFAPA patients, healthy controls, and HPF flare patients (Fig. 1B). Three chemokines [IP-10/CXCL10, monokine induced by IFN-γ (MIG/CXCL9), and macrophage inflammatory protein 1β (MIP-1β/CCL4)] that are known T-cell chemoattractants were significantly elevated during PFAPA flares compared with asymptomatic intervals (Fig. 3A), as were the levels of three proinflammatory cytokines (IL-18, IL-6, and G-CSF). In addition, IL-1–receptor (IL-1R) antagonist showed a marked increase during PFAPA flares that reached statistical significance in comparison with healthy children (P < 0.001), but not compared with asymptomatic PFAPA periods (Fig. 3A). Comparing chemokine and cytokine values during PFAPA and HPF flares, we found that IP-10/CXCL10, MIG/CXCL9, MIP-1β, IL-1R) antagonist, and G-CSF were significantly higher with PFAPA flares. During their asymptomatic intervals, PFAPA patients showed slightly, but significantly elevated levels of MIG/CXCL9, MIP-1β, IL-6, and G-CSF relative to healthy controls (Fig. 3A). CRP levels increased significantly during PFAPA episodes, but reached levels similar to those seen during HPF flares (Fig. 3B).
Fig. 3.
Distinct inflammatory protein measures in PFAPA patients. Values of (A) serum cytokines and chemokines and (B) CRP in PFAPA patients during and between flares, healthy controls, and HPF patients during flare. Results for IL-1β, IL-2, IL-4, IL-5, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, Eotaxin, FGF basic, GM-CSF, IFN-γ, MCP-1, MIP-1α, PDGF BB, RANTES (regulated upon activation, normal T cell-expressed and -secreted), TNF-α, and VEGF did not show any statistically significant differences in any of the comparisons and therefore are not shown. NS, not significant.
Given the highly elevated gene-expression levels of a variety of complement components during PFAPA flares, we next analyzed the functional activity of the classical (CH50) and alternative pathway (AH50), as well as serum and plasma components, as potential indicators of environmental PFAPA triggers. PFAPA flares were associated with a marked increase of CH50 activity, which almost reached statistical significance (P = 0.06) in comparison with asymptomatic PFAPA intervals, whereas there was no change in AH50 activity (Fig. S2). Properdin Factor B, a component of the alternative pathway but also an acute-phase reactant, was significantly elevated during PFAPA flares relative to asymptomatic intervals (P = 0.04), but did not differ from the elevated values during HPF flares. Measurements of serum C1-INH, C1q, C2, C3, C4, and C5 in two PFAPA patients during flares were normal (Dataset S3). Plasma levels of the activated complement fragments Bb, C3a, C4D, and C5a were within normal ranges when measured in one PFAPA patient during a flare.
Because the classic complement pathway is activated via antigen-antibody complexes, as well as a variety of bacterial and viral agents, we analyzed antinuclear antibody and serum titers against microbial pathogens (Fig. 1B). However, these results were not indicative for an autoimmune disease or concurrent infection (SI Results).
IP-10/CXCL10, MIG/CXCL9, and G-CSF Are Potential Biomarkers for IL-1/-18–Driven PFAPA Flares.
To identify cytokines/chemokines that could best classify PFAPA flares and therefore serve as potential biomarkers, we first examined correlations among the quantitative traits (peripheral leukocyte subsets, serum chemokines/cytokines) that were significantly elevated during PFAPA attacks across patients (n = 17). Increased IP-10/CXCL10 levels correlated strongly with a decrease of circulating CD4+CD25+ lymphocytes (r = −0.83, P < 0.001), and an increase of MIG/CXCL9 (r = 0.64, P = 0.006) and G-CSF (r = 0.51, P = 0.04) (Dataset S4). MIG/CXCL9, which is chemotactic for lymphocytes and eosinophils, correlated negatively with peripheral eosinophil counts (r = −0.58, P = 0.03), and showed a strong association with IL-18 (r = 0.63, P = 0.007). Serum concentrations of IL-1R antagonist were significantly correlated with CD4+HLA-DR+ lymphocytes (r = 0.59, P = 0.03). Furthermore, we found high correlations between concentrations of CRP and the T-cell recruiting chemokines, IP-10/CXCL10 (r = 0.64, P = 0.008) and MIG/CXCL9 (r = 0.74, P = 0.002).
To identify the strongest independent correlates of PFAPA flares among the significantly elevated serum cytokines/chemokines, we then used multivariate logistic regression models. As the serum IL-1R antagonist levels were not normally distributed, even with transformations, we excluded this variable from these calculations. PFAPA flares were independently associated with increased serum levels of IP-10/CXCL10, MIG/CXCL9, and G-CSF (Dataset S5). Furthermore, the significant association of serum IL-18 levels with PFAPA flare was linked to these three chemokines/cytokines (Dataset S5).
To analyze the interrelations of the significant flare correlates with each other and the three additional flare-associated chemokines/cytokines (MIP-1β, IL-18, IL-6), we tested associations between changes in levels of these chemokines/cytokines within each patient between flare and asymptomatic periods using multivariate linear regression. Overall, changes in IP-10/CXCL10 and MIG/CXCL9 between flare and nonflare states were closely associated with changes in G-CSF, independent of the other variables for which we adjusted (Dataset S6). Changes of IL-18 were closely related to changes in MIG/CXCL9, but less closely related to changes in IP-10/CXCL10 and G-CSF.
IL-1 Blockade Resolves PFAPA Flares and IP-10/CXCL10 Response.
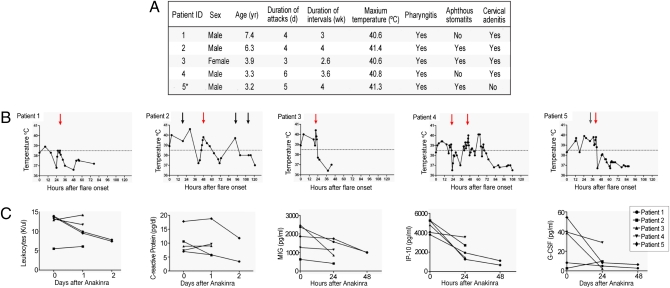
Given the overexpression of inflammasome-related genes in PFAPA flares, we hypothesized that PFAPA patients might benefit from IL-1 inhibition. Five consecutively recruited PFAPA patients fulfilling classic PFAPA criteria (Fig. 4A) received one dose of the recombinant IL-1R antagonist, anakinra, each on the second day of the flares. All children showed a prompt clinical response, with their fevers and inflammatory symptoms ceasing within hours of the injection (Fig. 4B). Two patients had a fever relapse 1 d (patient 4) and 2 d (patient 2) after treatment, respectively, whereas the other three patients remained afebrile. Patient 4 was administered a second dose of anakinra 24 h following the first dose and showed, again, a rapid decline of his fever. Leukocyte count and CRP declined 48 h after anakinra. Serum IP-10/CXCL10 concentrations, elevated before anakinra, greatly diminished by 24 h after treatment (Fig. 4C). Levels of MIG/CXCL9 and G-CSF were markedly elevated in four and three patients, respectively, and declined continuously after treatment, but IL-18 concentrations were unchanged in four of the five patients (Fig. S3). Adverse events were limited to one episode of vomiting immediately after injection in one patient.
Fig. 4.
Clinical and laboratory response of PFAPA patients treated with recombinant IL-1R antagonist (anakinra). Subcutaneous injection of five PFAPA patients with one dose of the recombinant IL-1R antagonist (anakinra) each, between 21 and 48 h following the onset of flare; one patient (patient 4) received an additional dose of recombinant IL-1R antagonist (anakinra) 24 h after the first dose. (A) Summary of demographic and clinical data of the five PFAPA patients; *Patient 5 received daily cimetidine but continued to have classic flares. (B) Fever curves before and after treatment; red arrows indicate injection of recombinant IL-1R antagonist (anakinra), black arrows indicate administration of ibuprofen, gray arrow indicates administration of acetaminophen. (C) Laboratory values and cytokine/chemokine measurements in sera before and after injection of recombinant IL-1R (anakinra).
Discussion
In this article, we used a comprehensive systems biology approach to study PFAPA, a common but poorly understood recurrent fever syndrome in children. Our data draw important distinctions between PFAPA and the HPFs at the level of gene expression and serum cytokines, suggesting biomarkers that may help to clarify the diagnosis and informing our understanding of the pathophysiology of disease flares. Molecules of the innate immune system, such as complement, IL-1β, and G-CSF, appear to figure prominently in PFAPA attacks, and several lines of evidence suggest an important effector function for activated CD-4+ T lymphocytes. Favorable clinical and laboratory responses to anakinra place IL-1β proximal in the attack cascade and suggest a previously unexplored treatment option for PFAPA.
Clinical overlap with HPFs and the lack of specific laboratory markers require the exclusion of other causes of recurrent fevers before making the diagnosis of PFAPA, which is often associated with a multitude of costly hospital admissions. Our data corroborate the concept of PFAPA as a distinct nosologic entity, which can be differentiated on the basis of gene expression and protein levels when comparing attacks with asymptomatic intervals, as well as with flares of HPF patients. The latter distinction helps to establish the PFAPA gene-expression profile as different from a more nonspecific fever-associated signature.
Although PFAPA is the most common and clinically well-defined periodic fever syndrome in children, a unifying pathophysiologic concept of this disease is lacking. Our data support a model of PFAPA in which environmental exposures and possible immunologic variants in the immature host conspire to cause recurrent febrile episodes (Fig. S4) (10, 17). PFAPA flares are by definition not associated with documented upper respiratory tract infection and—except for anecdotal reports of pathogen isolation from tonsillar or adenoidal tissues (2, 22)—no infectious processes have been identified so far. However, our findings of marked induction of genes encoding numerous innate immune molecules, including both structural and counter regulatory complement proteins, during PFAPA flares, taken together with the cardinal findings of oral and pharyngeal inflammation and cervical adenopathy, strongly suggest a microbial trigger, perhaps amplified in the susceptible host at a particular stage of development. Although the overexpression of complement genes has also been described in children with systemic onset juvenile idiopathic arthritis (SoJIA), an autoinflammatory disease with a complex genetic etiology (23, 24), the intensity of up-regulation was comparable to the results of our HPF group, therefore suggesting a nonspecific activation in SoJIA. Given the lability of complement proteins (25), it is not surprising that only one report of elevated serum complement C3 levels in PFAPA has been published (26). To better understand the trigger mechanism in PFAPA, future comparison of gene-expression profiles from PFAPA patients during flares with those of patients with other febrile illnesses should provide further information and insight as to why the immune system is stimulated with periodicity.
PFAPA flares were also characterized by evidence, at both the mRNA and protein level, of IL-1β and IL-18 activation, a further component of the innate immune system. The initiation of IL-1β and IL-18 processing is inflammasome-mediated. Inflammasomes are cytoplasmic macromolecular protein complexes that control the activation of caspase-1, which cleaves pro-IL-1β and pro-IL-18 into the biologically active, secreted forms (27). The increased expression of AIM2 and caspase-1 transcripts during flares of our PFAPA patients suggests activation of the AIM2 inflammasome (28–30), and thereby adds to the growing body of data implicating the role of IL-1β activation in periodic fever syndromes (1).
IL-1β acts as costimulator of T-cell function, usually together with an antigen or mitogen (31). Our findings correlating serum IL-1R antagonist levels (a surrogate for IL-1 levels, which are difficult to assay because of binding proteins in the serum) with activated CD4+HLA-DR+ lymphocytes support this mechanism of T lymphocyte stimulation following monocyte/macrophage activation during PFAPA flares. Activated T cells secrete IFN-γ, thereby enhancing monocyte/macrophage stimulation via a feedback loop. Accordingly, we found highly elevated serum levels of IFN-inducible chemokines, IP-10/CXCL10 and MIG/CXCL9, as well as G-CSF, a neutrophil-inducing cytokine. IP-10/CXCL10 and MIG/CXCL9 are secreted by monocytes and neutrophils, and belong to the same chemokine family that recruits activated T lymphocytes via the receptor CXCR3 (21). The strong inverse correlation of IP-10/CXCL10 with CD4+CD25+ T cells during flares of our PFAPA patients, along with a relative lymphopenia, argue for recruitment of activated T lymphocytes to inflamed peripheral tissues, as lymph nodes and adenoids. Neither gene-expression studies of TRAPS-associated TNFRSF1A mutations in an endothelial cell line (32), nor of peripheral blood mononuclear cells of SoJIA patients (33, 34), implicated these T cell-recruiting chemokines. Thus, measurement of serum Th1 chemokines could provide a novel basis for diagnostic testing of PFAPA that would be especially useful in a setting without access to genetic exclusion of HPFs.
Based on this conceptual formulation, which would place IL-1β upstream of T-cell activation in PFAPA, we treated five PFAPA patients during an attack with recombinant IL-1R antagonist. All patients showed a prompt clinical response, accompanied by a rapid decline of serum IP-10/CXCL10 and MIG/CXCL9, thus supporting a model in which IL-1β drives Th1 chemokines. Serum levels of IL-18, which acts upstream of the IL-1R antagonist, slightly increased. The fact that two patients had a fever relapse following anakinra injection is not surprising, given the short half-life of the medication. When we administered two doses of anakinra in one patient, the episode was shortened to half the regular duration. Given the fact that many patients experience an increased frequency of attacks following steroids (5, 6), and that adenotonsillectomy is an invasive and therefore risk-associated procedure, our results support IL-1 inhibition as an alternative treatment option of PFAPA attacks. Future randomized treatment trials of PFAPA patients will help to determine efficacy and safety of anakinra, which so far has been well tolerated when administered daily in CAPS patients (35, 36).
In summary, a multiparameter analytic approach clearly differentiates flares of PFAPA from exacerbations of the HPFs. Key features of PFAPA attacks include complement and IL-1β/-18 activation, an IFN gene signature, Th1-chemokinemia, and marked reductions in circulating activated CD4+ T cells and eosinophils. We propose a model in which microbial triggers activate a cascade that begins with the innate immune system and ultimately recruits activated T cells to the periphery. Because the immunologically immature host appears to play at least a permissive role, longitudinal studies of patients with PFAPA may provide additional insights into the ontogeny of how these pathways are regulated. The findings reported here may also provide the basis both for the improved diagnosis and treatment of PFAPA.
Materials and Methods
PFAPA Patients.
Patients less than 18 y of age with a diagnosis of PFAPA fulfilled the following classical criteria: (i) recurrent fever episodes (>38.5 °C) with aphthous stomatitis or pharyngitis or cervical lymphadenitis; (ii) exclusion of infectious, rheumatic, and autoimmune disorders, immunodeficiency, and cyclic neutropenia (exclusion of mutations in the ELA2 gene); (iii) exclusion of the hereditary periodic fever syndromes FMF, TRAPS, HIDS, and CAPS; and (iv) no treatment with systemic corticosteroids 10 d before enrollment, and no treatment with cimetidine, colchicine, montelukast or immune response modulators 3 mo before enrollment.
We analyzed by microarray whole-blood samples obtained from six PFAPA patients (three females, three males; median age 5.6 y, range 2.8–9.7) who fulfilled—in addition to our study inclusion criteria—the following stringent PFAPA requirements: (i) fever episodes associated with at least two of the classic clinical features, (ii) leukocytosis with neutrophilia or elevated CRP during the flare, and (iii) no clinical or laboratory signs of inflammation during the asymptomatic interval of blood sampling.
During an attack, administration of nonsteroidal anti-inflammatory drugs, such as acetaminophen or ibuprofen, was not permitted within 24 h, naproxen within 48 h, and steroids at no time point before specimen sampling, except in the case of the five patients who also received anakinra and were allowed to take acetaminophen or ibuprofen. We aimed to collect blood samples from each PFAPA patient when asymptomatic and within 48 h following the onset of an attack.
Collection and Analysis of Blood Samples.
A detailed description of the materials and methods used for DNA sequence analysis, microarray analysis, real-time quantitative RT-PCR, cytokine and chemokine measurements, lymphocyte immunophenotyping, complement system analyses, and statistical analyses is provided in SI Materials and Methods.
Treatment with IL-1R Blockade.
After parental informed consent, five PFAPA patients received anakinra, a recombinant human IL-1R antagonist, at 1 mg per kilogram body weight once subcutaneously within 48 h following the onset of an attack. One patient received a second dose 24 h after the initial dose. Patients were monitored clinically for 24 to 48 h after the injection. Blood samples were collected immediately before treatment and 24 h after therapy in all subjects, with some patients also providing samples at 48 h. Complete blood cell counts, CRP, serum IP-10/CXCL10, MIG/CXCL9, G-CSF, IL-18, IL-6, and MIP-1β were measured on all available samples.
Supplementary Material
Acknowledgments
We thank Katherine McLaurin for excellent technical assistance, Dr. William Mackenzie and Dr. Geraldine Neiss for recruitment of healthy children at the Nemours A. I. duPont Hospital for Children (Wilmington, DE), Marcia Vital for editorial assistance in preparation of the manuscript, Dr. Elaine Remmers for critical review of the manuscript, and all the children and their families for participation in this study and continuous support of our research endeavors. This work was supported by the Intramural Research Programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Human Genome Research Institute, the National Institute of Allergy and Infectious Diseases, and the Department of Laboratory Medicine of the Clinical Center of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Data deposition: GeneChip data have been deposited with the Gene Expression Omnibus of the National Center for Biotechnology Information (NCBI), accession no. GSE17732.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103681108/-/DCSupplemental.
References
- 1.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall GS, Edwards KM, Butler J, Lawton AR. Syndrome of periodic fever, pharyngitis, and aphthous stomatitis. J Pediatr. 1987;110:43–46. doi: 10.1016/s0022-3476(87)80285-8. [DOI] [PubMed] [Google Scholar]
- 3.Thomas KT, Feder HM, Jr., Lawton AR, Edwards KM. Periodic fever syndrome in children. J Pediatr. 1999;135:15–21. doi: 10.1016/s0022-3476(99)70321-5. [DOI] [PubMed] [Google Scholar]
- 4.Padeh S, et al. Periodic fever, aphthous stomatitis, pharyngitis, and adenopathy syndrome: Clinical characteristics and outcome. J Pediatr. 1999;135:98–101. doi: 10.1016/s0022-3476(99)70335-5. [DOI] [PubMed] [Google Scholar]
- 5.Tasher D, Somekh E, Dalal I. PFAPA syndrome: New clinical aspects disclosed. Arch Dis Child. 2006;91:981–984. doi: 10.1136/adc.2005.084731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feder HM, Salazar JC. A clinical review of 105 patients with PFAPA (a periodic fever syndrome) Acta Paediatr. 2010;99:178–184. doi: 10.1111/j.1651-2227.2009.01554.x. [DOI] [PubMed] [Google Scholar]
- 7.Padeh S, Stoffman N, Berkun Y. Periodic fever accompanied by aphthous stomatitis, pharyngitis and cervical adenitis syndrome (PFAPA syndrome) in adults. Isr Med Assoc J. 2008;10:358–360. [PubMed] [Google Scholar]
- 8.Cavuoto M, Bonagura VR. Adult-onset periodic fever, aphthous stomatitis, pharyngitis, and adenitis. Ann Allergy Asthma Immunol. 2008;100:170. doi: 10.1016/S1081-1206(10)60428-0. [DOI] [PubMed] [Google Scholar]
- 9.Ridder GJ, Fradis M, Berner R, Löhle E. PFAPA syndrome: Current standard of knowledge and relevance for the ENT specialist. Laryngorhinootologie. 2002;81:635–639. doi: 10.1055/s-2002-34450. [DOI] [PubMed] [Google Scholar]
- 10.Stojanov S, et al. Cytokine profile in PFAPA syndrome suggests continuous inflammation and reduced anti-inflammatory response. Eur Cytokine Netw. 2006;17:90–97. [PubMed] [Google Scholar]
- 11.Førsvoll JA, Oymar K. C-reactive protein in the periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis (PFAPA) syndrome. Acta Paediatr. 2007;96:1670–1673. doi: 10.1111/j.1651-2227.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 12.Yoshihara T, et al. Potential use of procalcitonin concentrations as a diagnostic marker of the PFAPA syndrome. Eur J Pediatr. 2007;166:621–622. doi: 10.1007/s00431-006-0281-2. [DOI] [PubMed] [Google Scholar]
- 13.Ogose T. Tonsillectomy for periodic fever, aphthous stomatitis, pharyngitis, and adenitis syndrome is not always successful. J Pediatr. 2008;152:742–, author reply 743. doi: 10.1016/j.jpeds.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Ataş B, et al. PFAPA syndrome mimicking familial Mediterranean fever: Report of a Turkish child. J Emerg Med. 2003;25:383–385. doi: 10.1016/s0736-4679(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 15.Saulsbury FT, Wispelwey B. Tumor necrosis factor receptor-associated periodic syndrome in a young adult who had features of periodic fever, aphthous stomatitis, pharyngitis, and adenitis as a child. J Pediatr. 2005;146:283–285. doi: 10.1016/j.jpeds.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Gattorno M, et al. Differentiating PFAPA syndrome from monogenic periodic fevers. Pediatrics. 2009;124:e721–e728. doi: 10.1542/peds.2009-0088. [DOI] [PubMed] [Google Scholar]
- 17.Long SS. Syndrome of periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA) – What it isn't. What is it? J Pediatr. 1999;135:98–101. doi: 10.1016/s0022-3476(99)70316-1. [DOI] [PubMed] [Google Scholar]
- 18.Garavello W, Romagnoli M, Gaini RM. Effectiveness of adenotonsillectomy in PFAPA syndrome: A randomized study. J Pediatr. 2009;155:250–253. doi: 10.1016/j.jpeds.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Licameli G, Jeffrey J, Luz J, Jones D, Kenna M. Effect of adenotonsillectomy in PFAPA syndrome. Arch Otolaryngol Head Neck Surg. 2008;134:136–140. doi: 10.1001/archoto.2007.7. [DOI] [PubMed] [Google Scholar]
- 20.Feder HM., Jr. Cimetidine treatment for periodic fever associated with aphthous stomatitis, pharyngitis and cervical adenitis. Pediatr Infect Dis J. 1992;11:318–321. doi: 10.1097/00006454-199204000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 22.Pignataro L, et al. Outcome of tonsillectomy in selected patients with PFAPA syndrome. Arch Otolaryngol Head Neck Surg. 2009;135:548–553. doi: 10.1001/archoto.2009.56. [DOI] [PubMed] [Google Scholar]
- 23.Barnes MG, et al. Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:2102–2112. doi: 10.1002/art.24601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Berg CW. Purification of complement components, regulators, and receptors by classical methods. Methods Mol Biol. 2000;150:15–52. doi: 10.1385/1-59259-056-X:15. [DOI] [PubMed] [Google Scholar]
- 26.Kawashima H, et al. Highly suspected case of FAPA (periodic fever, aphthous stomatitis, pharyngitis and adenitis) syndrome. Pediatr Int. 2001;43:103–106. doi: 10.1046/j.1442-200x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 27.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bürckstümmer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 32.Rebelo SL, et al. Novel markers of inflammation identified in tumor necrosis factor receptor-associated periodic syndrome (TRAPS) by transcriptomic analysis of effects of TRAPS-associated tumor necrosis factor receptor type I mutations in an endothelial cell line. Arthritis Rheum. 2009;60:269–280. doi: 10.1002/art.24147. [DOI] [PubMed] [Google Scholar]
- 33.Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 34.Allantaz F, et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J Exp Med. 2007;204:2131–2144. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman HM, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.