Abstract
The prostaglandin and leukotriene families of lipid mediators are formed via two distinct biosynthetic pathways that are initiated by the oxygenation of arachidonic acid by either cyclooxygenase-2 (COX-2) or 5-lipoxygenase (5-LOX), respectively. The 5-LOX product 5S-hydroxyeicosatetraenoic acid, however, can also serve as an efficient substrate for COX-2, forming a bicyclic diendoperoxide with structural similarities to the arachidonic acid-derived prostaglandin endoperoxide PGH2 [Schneider C, et al. (2006) J Am Chem Soc 128:720–721]. Here we identify two cyclic hemiketal (HK) eicosanoids, HKD2 and HKE2, as the major nonenzymatic rearrangement products of the diendoperoxide using liquid chromatography–mass spectrometry analyses as well as UV and NMR spectroscopy. HKD2 and HKE2 are furoketals formed by spontaneous cyclization of their respective 8,9-dioxo-5S,11R,12S,15S-tetrahydroxy- or 11,12-dioxo-5S,8S,9S,15S-tetrahydroxy-eicosadi-6E,13E-enoic acid precursors, resulting from opening of the 9S,11R- and 8S,12S-peroxide rings of the diendoperoxide. Furthermore, the diendoperoxide is an efficient substrate for the hematopoietic type of prostaglandin D synthase resulting in formation of HKD2, equivalent to the enzymatic transformation of PGH2 to PGD2. HKD2 and HKE2 were formed in human blood leukocytes activated with bacterial lipopolysaccharide and calcium ionophore A23187, and biosynthesis was blocked by inhibitors of 5-LOX or COX-2. HKD2 and HKE2 stimulated migration and tubulogenesis of microvascular endothelial cells, implicating a proangiogenic role of the hemiketals in inflammatory sites that involve expression of 5-LOX and COX-2. Identification of the highly oxygenated hemiketal eicosanoids provides evidence for a previously unrecognized biosynthetic cross-over of the 5-LOX and COX-2 pathways.
Leukotrienes and prostaglandins are lipid mediators derived from oxidative modification of arachidonic acid. Both families of eicosanoids exert inflammatory and immunomodulatory functions in disease, as well as homeostatic functions in normal physiological processes (1). Atherosclerosis, asthma, and many types of cancer are prototypical inflammatory diseases that are characterized by concomitant formation of both leukotrienes and prostaglandins (2). The formation of leukotrienes and prostaglandins diverges at the point of the initial oxygenation of the common arachidonic acid substrate by either 5-lipoxygenase (5-LOX) or cyclooxygenase-2 (COX-2), respectively. From there, biosynthesis proceeds along separate and distinct pathways, each utilizing specific enzymes that catalyze complex reactions using highly unstable substrates (3, 4).
The possibility of a biosynthetic convergence of the 5-LOX and COX-2 pathways was implicated when the 5-LOX product 5S-hydroxyeicosatetraenoic acid (5S-HETE) was identified as an efficient and specific substrate for oxygenation by recombinant COX-2 (5). The COX-2 oxygenation product of 5S-HETE is a bicyclic diendoperoxide with structural similarities to the prostaglandin (PG) endoperoxide PGH2, including its chemical instability and potential for further transformation. Both PGH2 and the diendoperoxide possess a 9S,11R-endoperoxide and a 15S-hydroxyl (6). The typical cyclopentyl ring present in PGH2 and all prostaglandins is extended to a seven-membered ring in the diendoperoxide by incorporation of a second peroxide, connecting carbons 8 and 12. Thus, during transformation of 5S-HETE, COX-2 catalyzes stereospecific insertion of three molecules of oxygen into the fatty acid substrate. Notably, the “unnatural” or nonenzymatically formed enantiomer 5R-HETE is only a poor substrate for COX-2, and the COX-1 isoform does not react with 5S-HETE or 5R-HETE (5).
The transformation of PGH2 to PGE2, PGD2, PGF2α, thromboxane A2, and prostacyclin is catalyzed by a diverse family of prostaglandin synthase enzymes acting to rearrange the unstable 9,11-peroxide moiety (7, 8). Spontaneous nonenzymatic rearrangement of the endoperoxide ring also gives rise to PGE2 and PGD2 and, in addition, can lead to levuglandins and cleavage of the carbon chain into malondialdehyde and 12S-hydroxy-5Z,8E,10E-heptadecatrienoic acid (9–11). Here, we show that rearrangement of both peroxides of the diendoperoxide results in the formation of two hemiketal (HK) eicosanoids, identified as HKE2 and HKD2, with structural similarities to PGE2 and PGD2. The hemiketals are present in activated human leukocytes and stimulate tubulogenesis of murine pulmonary endothelial cells.
Results
Nonenzymatic transformation of the diendoperoxide.
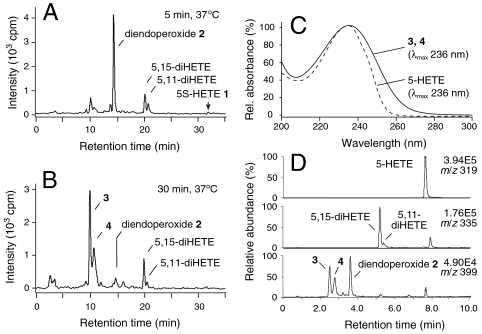
RP-HPLC analysis of short-time incubation (< 5 min) of [1-14C]5S-HETE 1 with recombinant COX-2 gave the diendoperoxide 2 as the main product together with 5,11-diHETE and 5,15-diHETE as by-products (Fig. 1A) (5, 12). When the same incubation was extracted 30 min after the addition of [1-14C]5S-HETE, the diendoperoxide was no longer present, and instead two more polar peaks 3 and 4 were detected (Fig. 1B). Products 3 and 4 had identical UV spectra with a λmax of 236 nm that were unusually broad and extended beyond 260 nm (Fig. 1C). The UV spectra showed similarity to a conjugated keto-ene moiety rather than the typical conjugated diene present in 5S-HETE. Using liquid chromatography–electrospray ionization–mass spectrometry (LC-ESI-MS) analysis in the negative ion mode a molecular ion [M-H]- of m/z 399 for 2, 3, and 4 was detected, equivalent to a molecular weight of 400 amu (Fig. 1D). These findings implicated 3 and 4 as stable rearrangement products of the diendoperoxide 2. Products 3 and 4 were also formed when the diendoperoxide was first isolated by HPLC and then incubated in PBS buffer indicating that COX-2 was not involved in the transformation of 2 to 3 and 4.
Fig. 1.
Nonenzymatic transformation of the 5S-HETE-derived diendoperoxide. [1-14C]-5S-HETE was reacted with COX-2 for 5 min (A) or 30 min (B) followed by extraction and RP-HPLC analysis with radiodetection. (C) Normalized UV spectra of 5S-HETE and products 3 and 4. (D) LC-ESI-MS analysis (negative ion mode) of an incubation of 5S-HETE with recombinant COX-2. The extracted ion chromatograms corresponding to 5S-HETE (m/z 319), diHETEs (m/z 335), and the diendoperoxide (m/z 399) are shown.
Structural Identification of 3 and 4 as Hemiketal Eicosanoids.
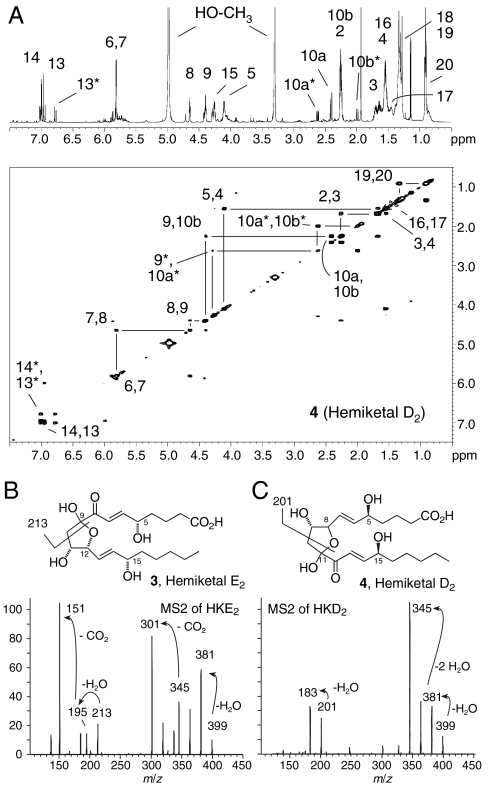
Products 3 and 4 were synthesized in a large-scale incubation of 600 μg 5S-HETE with COX-2 and isolated using RP-HPLC and further purified using straight-phase HPLC. The covalent structures of 3 and 4 were determined using 1H and 2D homo- and heteronuclear NMR analyses (Fig. 2A, Tables S1 and S2, and Figs. S1–S4). The NMR spectra of both 3 and 4 each showed mixtures of two diastereomers with partially overlapping signals in a ratio of 4∶1 to ≈1∶1 depending on the NMR solvent used (d4-methanol, d3-acetonitrile, or d3-chloroform). The structural identification of the major isomer of 4 will be discussed as representative.
Fig. 2.
Identification of products 3 and 4. (A) NMR analysis of product 4 (hemiketal D2). The 1H NMR spectrum is shown with the H,H-COSY spectrum below. The product is a mixture of two isomers in ≈5∶2 ratio. Signals from the minor isomer with a distinct chemical shift are marked with an asterisk. In the H,H-COSY spectrum only the prominent cross-peaks are visible. Weaker signals like the couplings of H5 to H6 and H14 to H15 were discernible at lesser signal to noise. (B and C) LC-ESI-MS2 spectra of 3 and 4.
The 1H NMR spectrum of 4 showed four signals with chemical shifts and coupling constants characteristic of trans double bonds, representing the 6,7-trans and 13,14-trans double bonds (Fig. 2A). The signal for H13 (6.95 ppm) showed a splitting pattern indicative of a proton with only one direct neighbor, the other proton of the double bond, H14 at 7.01 ppm. Thus, C12 does not carry a proton, implicating a keto group at this position. This was confirmed by the carbon chemical shift of 196.0 ppm for C12 determined in the heteronuclear multiple bond correlation experiment through the cross-peak with H13 (Figs. S1 and S2). H,H-COSY cross-peaks of three of the double bond protons (H6, H7, and H14) to signals between 4 and 5 ppm allowed identification of H5 (4.10 ppm), H15 (4.25 ppm), and H8 (4.64 ppm) as geminal hydroxy protons. H8 was coupled to another geminal hydroxy proton, H9 at 4.40 ppm. The two protons of the H10 methylene group were detected as dd signals at 2.40 ppm and 2.25 ppm, respectively, with a coupling constant of JH10a,b of 14.5 Hz. The large coupling constant between the methylenic protons H10a and H10b together with lack of a proton at C11 provided evidence of a furoketal ring structure in 4 encompassing carbons 8 through 11 with the hemiketal moiety located at C11. A keto group in conjugation with a double bond and oxygen functionalities on either side was consistent with the UV spectrum of 4 with λmax at 236 nm (Fig. 1C).
Product 4 was identified as the furoketal (hemiketal D2, HKD2) formed by spontaneous cyclization of the 8S-hydroxyl and 11-keto groups of 11,12-dioxo-5S,8S,9S,15S-tetrahydroxyeicosadi-6E,13E-enoic acid, one of two immediate products of opening of the 9,11- and 8,12-peroxide bridges of the diendoperoxide 2. The other immediate product is 8,9-dioxo-5S,11R,12S,15S-tetrahydroxyeicosa-6E,13E-dienoic acid, and cyclization of the 12S-hydroxyl and 9-keto gives product 3 that was designated as hemiketal E2 (HKE2) (Figs. S3 and S4 and Table S2). The absolute configuration of the chiral centers of carbons 5, 11, 12, and 15 of HKE2, and 5, 8, 9, and 15 of HKD2, respectively, was assumed to be unchanged from the diendoperoxide precursor, i.e., 5S, 8S, 9S, 11R, 12S, and 15S (5, 6). The characteristic keto-ene UV chromophore ruled out the possibility of a six-membered (pyran) ring, e.g., C8 through C12 in HKD2, or a furoketal ring of, e.g., carbons 9 through 12 in HKD2. The two isomers observed in the NMR analyses of 3 and 4 are the diastereomers stemming from the anomeric hemiketal carbons C9 or C11, respectively. The equilibrium between the diastereomers was dependent on the solvent. The NMR coupling data and chemical shifts were not sufficient to unequivocally assign the absolute configuration of the anomeric carbons in the diastereomers of HKE2 and HKD2.
Isotopic Labeling Studies.
The formation of two keto groups in the hemiketals was confirmed by mass spectrometric analyses of the transformation of unlabeled 5S-HETE, 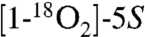 -HETE, and [5,6,8,9,11,12,14,15-d8]-5S-HETE. Incubation of a ≈1∶1 mixture of unlabeled 5S-HETE and d8-5S-HETE with recombinant COX-2 and analysis by LC-ESI-MS showed that the diendoperoxide 2 retained all eight deuterium atoms, whereas HKE2 and HKD2 had each lost two of the labels (Fig. S5). Loss of a deuterium is indicative of a change in carbon hybridization from sp3 to sp2, as it occurs, for example, when a carbon carrying a hydroxyl or peroxyl is oxidized to a keto group. The site at the molecule where loss of the deuterium label had occurred was determined by comparison of the MS2 spectra of HKE2 and HKD2 formed from unlabeled 5S-HETE, from
-HETE, and [5,6,8,9,11,12,14,15-d8]-5S-HETE. Incubation of a ≈1∶1 mixture of unlabeled 5S-HETE and d8-5S-HETE with recombinant COX-2 and analysis by LC-ESI-MS showed that the diendoperoxide 2 retained all eight deuterium atoms, whereas HKE2 and HKD2 had each lost two of the labels (Fig. S5). Loss of a deuterium is indicative of a change in carbon hybridization from sp3 to sp2, as it occurs, for example, when a carbon carrying a hydroxyl or peroxyl is oxidized to a keto group. The site at the molecule where loss of the deuterium label had occurred was determined by comparison of the MS2 spectra of HKE2 and HKD2 formed from unlabeled 5S-HETE, from 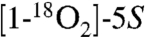 -HETE, and from d8-5S-HETE.
-HETE, and from d8-5S-HETE.
The MS2 spectrum of HKE2 showed major cleavage ions at m/z 213, 195 (loss of H2O from m/z 213), and 151 (loss of CO2 from m/z 195) (Fig. 2B). All three fragments were derived from the carboxylate end of HKE2 because the first two ions were shifted by 4 amu to m/z 217 and 199 when 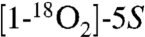 -HETE was the substrate, whereas m/z 151, formed by loss of CO2 or
-HETE was the substrate, whereas m/z 151, formed by loss of CO2 or 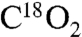 , respectively, was unchanged (Fig. S5). When d8-5S-HETE was used as substrate m/z 213 was shifted by only 2 amu to m/z 215 indicating that the loss of the two deuterium atoms in HKE2 had occurred at the side of the molecule that contained the carboxylate, implicating the oxidation of the 8- and 9-sp3 carbons to keto groups.
, respectively, was unchanged (Fig. S5). When d8-5S-HETE was used as substrate m/z 213 was shifted by only 2 amu to m/z 215 indicating that the loss of the two deuterium atoms in HKE2 had occurred at the side of the molecule that contained the carboxylate, implicating the oxidation of the 8- and 9-sp3 carbons to keto groups.
Comparison of the MS2 spectra of HKD2 derived from unlabeled 5S-HETE versus 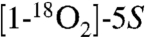 -HETE showed that the major cleavage ions m/z 201 and m/z 187 in the MS2 spectrum of HKD2 also contained the carboxylate group (Fig. 2C and Fig. S5). The fragment at m/z 201 was shifted by 4 mass units to m/z 205 when d8-5S-HETE was the substrate (Fig. S5), indicating that the loss of the two deuterium atoms in HKD2 had occurred at the methyl end of the molecule, in this case from carbons 11 and 12.
-HETE showed that the major cleavage ions m/z 201 and m/z 187 in the MS2 spectrum of HKD2 also contained the carboxylate group (Fig. 2C and Fig. S5). The fragment at m/z 201 was shifted by 4 mass units to m/z 205 when d8-5S-HETE was the substrate (Fig. S5), indicating that the loss of the two deuterium atoms in HKD2 had occurred at the methyl end of the molecule, in this case from carbons 11 and 12.
The Diendoperoxide Is a Substrate for Hematopoietic Prostaglandin D Synthase (H-PGDS).
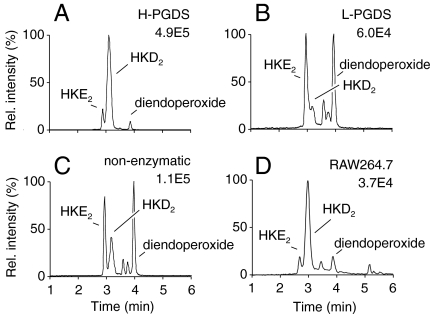
HKE2 and HKD2 appeared to be rearrangement products similar to the transformation of PGH2 to PGE2 and PGD2. We therefore tested whether H-PGDS or lipocalin PGDS (L-PGDS) was able to catalyze the transformation of the diendoperoxide into HKD2. LC-MS analysis of incubations of H-PGDS and L-PGDS with the isolated diendoperoxide showed efficient formation of HKD2 by H-PGDS, whereas L-PGDS did not react with the diendoperoxide (Fig. 3 A–C). In the H-PGDS reaction, the concentration of the cofactor glutathione was reduced from 1 mM (13) to 10 μM in order to prevent otherwise abundant formation of glutathione-HK adducts (Fig. S6). LPS-activated RAW264.7 cells that express H-PGDS (14) and show more efficient formation of PGD2 than PGE2 (15), transformed exogenous 5S-HETE mainly to HKD2 (Fig. 3D). Side-by-side incubations of 10 μM PGH2 or the diendoperoxide generated in situ using limiting amounts of recombinant H-PGDS showed that both peroxides were about equally effective substrates. The other recombinant PG synthases tested, prostacyclin synthase, thromboxane synthase, and mPGES-1, did not react with the diendoperoxide in vitro using conditions under which they readily reacted with PGH2.
Fig. 3.
Transformation of the diendoperoxide by hematopoietic prostaglandin D synthase (H-PGDS) to HKD2. LC-ESI-MS analyses of reactions of 5S-HETE with recombinant COX-2 in the presence of (A) recombinant human H-PGDS, (B) recombinant murine L-PGDS, (C) in the absence of PGDS. (D) Transformation of exogenous 5S-HETE by LPS-activated RAW264.7 cells. The extracted ion chromatograms for m/z 399 are shown.
Human Leukocytes Form HKE2 and HKD2.
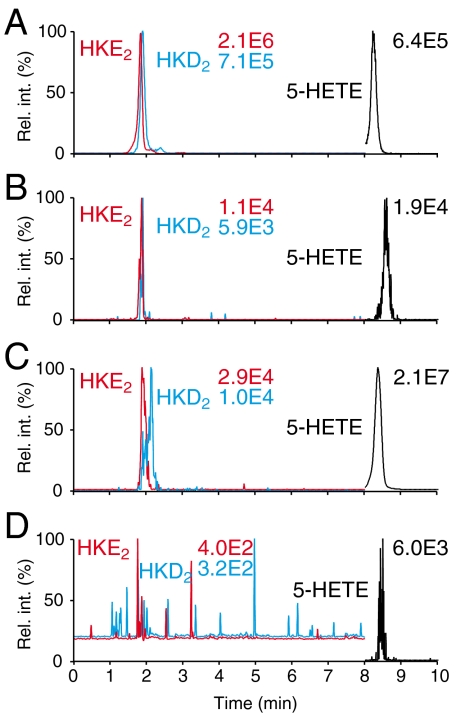
We hypothesized that human blood leukocytes would be able to biosynthesize the hemiketals in response to pertinent stimulation. 5-LOX activity in neutrophils and eosinophils can be stimulated using calcium ionophore A23187 (16), and expression of COX-2 in monocytes can be induced using LPS (17). Human peripheral blood leukocytes were isolated and treated with 10 μg/mL LPS for 6 h followed by treatment with 5 μM A23187 for 15 min and analyzed using LC-ESI-MS operated in the selected reaction-monitoring (SRM) mode. Formation of HKE2 and HKD2 was detected without addition of exogenous substrate, enhanced about 2-fold when 5 μM 5S-HETE was added, and reduced to < 3% in the presence of the COX-2 inhibitor NS-398 (Fig. 4) (18), or when the 5-LOX inhibitor AA-861 (19) or the five lipoxygenase-activating protein inhibitor MK-886 were present (20) (Fig. S7). The levels of hemiketals were ≈1–5% of the amount of 5-HETE and LTB4 (each ≈1 ng/106 cells) in the stimulated cells based on signal intensities. Hemiketals were not detected in nonstimulated leukocytes.
Fig. 4.
Isolated human leukocytes form HKE2 and HKD2. Human leukocytes were stimulated with LPS for 6 h and then with A23187 for 15 min before extraction and LC-ESI-MS-SRM analysis. (A) Standards of HKE2 (red trace) and HKD2 (blue) generated by reaction of recombinant human COX-2 with 5S-HETE (black). (B) HKE2 and HKD2 formed by the leukocytes following stimulation with LPS and A23187. (C) Addition of exogenous 5S-HETE together with A23187 to the leukocytes increased formation of HKE2 and HKD2. (D) Formation of hemiketals was attenuated by preincubation with the COX-2 inhibitor NS-398.
HKE2 and HKD2 Stimulate Endothelial Cell Tubulogenesis.
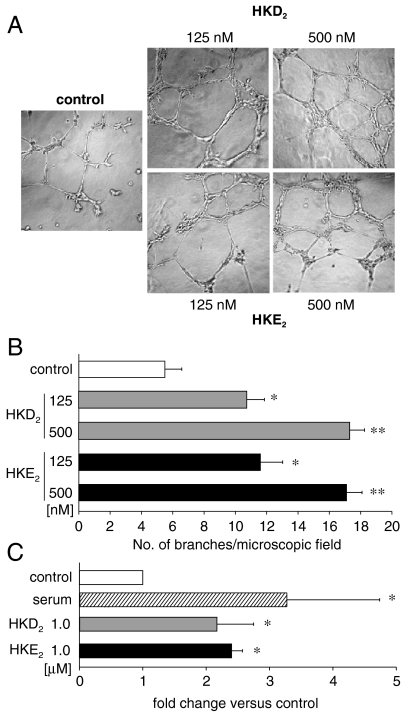
Both COX-2 and 5-LOX mediated pathways have been shown to regulate endothelial cell function and angiogenesis (21). We therefore determined whether HKD2 and HKE2 could affect endothelial cell function. Both HKD2 and HKE2 stimulated—in a dose-dependent manner—the formation of endothelial capillary-like structures, as determined by the ability of these compounds to promote endothelial cells to sprout, branch, and form ring-like structures (Fig. 5 A and B). When used at 1 μM hemiketals also stimulated endothelial cell migration (Fig. 5C). Neither HKD2 nor HKE2 (between 10 nM to 1 μM) induced changes in intracellular calcium levels of the endothelial cells nor stimulated their proliferation.
Fig. 5.
HKD2 and HKE2 induce formation of capillary-like structures and migration of endothelial cells. (A) Microvascular endothelial cells were plated onto Matrigel in the presence of 125 or 500 nM of HKD2 or HKE2. Representative images of capillary-like structures taken 6 h after plating are shown. (B) Quantification of capillary network formation. Cells incubated with HKD2 or HKE2 form tubule-like structures more efficiently than vehicle-treated cells (control). Values are the mean ± SD. calculated for 20 images per treatment. Differences between vehicle versus HKD2 or HKE2 treated cells (*), or between 125 versus 500 nM treatment (**) were significant (p < 0.05). (C) Migration of cells toward serum-free medium with or without HKD2 or HKE2 or complete medium. Values are fold changes versus serum-free medium from four experiments; (*) p < 0.05 versus control.
Discussion
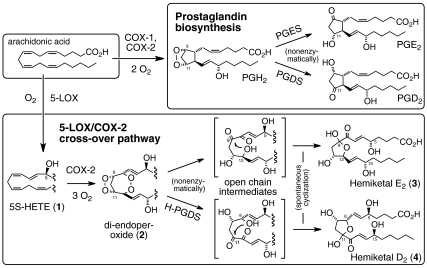
The reaction of COX-2 with the 5-LOX product 5S-HETE to form a diendoperoxide that is structurally equivalent to the prostaglandin endoperoxide suggests a previously unrecognized biosynthetic connection of the two enzymes. The specific transformation of the diendoperoxide by H-PGDS lays out a biosynthetic pathway from arachidonic acid via 5-LOX, COX-2, and H-PGDS to the furoketal eicosanoid that we named “hemiketal D2” (HKD2) (Fig. 6). The letter “D” was chosen to indicate the crucial role of H-PGDS in biosynthesis, and to acknowledge the common structural features of PGD2 (with a 9-hydroxyl and 11-keto) and HKD2 with 8- and 9-hydroxyls and 11- and 12-keto groups in the open chain form of the molecule. The 11-keto, however, is masked as the anomeric hemiketal carbon in the cyclized molecule.
Fig. 6.
Biosynthesis of hemiketals and prostaglandins. The 5-LOX metabolite 5S-HETE is converted to the diendoperoxide by COX-2 using three molecules of oxygen. The diendoperoxide is a substrate for H-PGDS opening up both endoperoxide groups into keto/hydroxy moieties to give an open chain intermediate. Hemiketal D2 results form spontaneous attack of the 8-hydroxyl at the 11-keto group. Nonenzymatic rearrangement of the diendoperoxide gives a mixture of HKE2 and HKD2. Oxygenation of arachidonic acid by COX-1 or COX-2 yields the prostaglandin endoperoxide PGH2 that rearranges to PGE2 and PGD2.
The major product formed by nonenzymatic rearrangement of the diendoperoxide was identified as a structurally related furoketal, designated “hemiketal E2” (HKE2) in analogy to PGE2. Formation of HKE2 and HKD2 upon nonenzymatic rearrangement of the diendoperoxide follows the precedent of spontaneous formation of PGE2 and PGD2 from the prostaglandin endoperoxide PGH2 (9). Unexpectedly, of the four possible permutations for two keto and two hydroxyl groups by opening of both peroxide rings of the diendoperoxide only two are manifest such that the keto groups and the hydroxyl groups are next to each other. A possible driving force for the vicinal arrangement is the stabilization gained by conjugation of the two keto groups with the double bond in a 1,2-dioxo-3-ene moiety. The open chain products undergo spontaneous ring closure between hydroxyl and keto groups to form the furoketals HKE2 or HKD2 (Fig. 6).
H-PGDS and L-PGDS are structurally unrelated enzymes that catalyze the transformation of PGH2 into PGD2 (7). The two enzymes differ markedly in their kinetic behavior with PGH2 as substrate: H-PGDS is a low-affinity, high-turnover enzyme, whereas L-PGDS is a high-affinity, low-turnover enzyme (13, 22). The high affinity of PGH2 in the L-PGDS active site is an indication of tight binding, and this might be one of the reasons why the enzyme did not catalyze the transformation of the larger diendoperoxide. H-PGDS, on the other hand, has only low binding affinity for PGH2 and efficiently catalyzed ring opening of the diendoperoxide. Both enzymes utilize nucleophilic attack of a thiolate anion at the 11-oxygen of PGH2 as their catalytic mechanism. L-PGDS uses an active site cysteine residue (22), whereas in H-PGDS the thiolate is provided by the cofactor glutathione. High concentrations of glutathione (above 100 μM) in the in vitro reactions resulted in noticeable adduct formation with the hemiketals. Adduct formation was likely due to the propensity of the oxo-ene moiety to undergo Schiff base and Michael reactions. Adduction with cellular nucleophiles like glutathione, N-acetylcysteine, and reactive amino acids like lysine and histidine (23) could also be a limiting factor for the detection of the hemiketals in tissue samples.
Human leukocytes biosynthesized both hemiketals as a result of stimulation of 5-LOX and COX-2 activities ex vivo. Inhibition of either enzyme markedly diminished formation of the hemiketals indicating the crucial role of 5-LOX and COX-2. Few cell types show concomitant expression of 5-LOX and COX-2 implicating that formation of hemiketals in the leukocyte preparation occurred through transcellular biosynthesis (24). Thus, biosynthesis of hemiketals could be physiologically relevant in sites of inflamed tissue infiltrated by neutrophils (as a source of 5-HETE) and activated macrophages (providing COX-2). This implies a possible role for hemiketals in the regulation of discrete steps during induction or resolution of inflammation (25). One such possibility is the contribution of hemiketals to tissue restoration through the stimulation of endothelial cell migration and tubulogenesis. The same effect, however, could also lead to enhanced tumor angiogenesis and an aggravation of cancer growth, when hemiketals are formed upon neutrophil invasion into COX-2 expressing tumor tissue or microenvironment.
5S-HETE is considered a by-product of 5-LOX catalysis, which is set up to further transform the initial product, 5S-HPETE, to the leukotriene (LT) epoxide, LTA4 (3). Nevertheless, 5S-HETE is formed in considerable amounts in cells that express 5-LOX (26), and it has a propensity for esterification into specific membrane phospholipids depending on the cell type (27, 28). 5S-HETE does not appear to carry out a biological function by itself, but it has a well-documented role as precursor of the eosinophil chemoattractant 5-oxo-ETE (29). Our findings suggest an additional role for 5S-HETE as a substrate for the formation of hemiketal eicosanoids in the biosynthetic convergence of the 5-LOX and COX-2 pathways, acting in tandem in the formation of biologically active lipid autacoids.
Materials and Methods
Materials.
Recombinant human COX-2 was expressed and purified as described (30). 5S-HETE was prepared by chemical synthesis (6, 31). 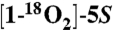 -HETE was prepared by repeated cycles of methylation and hydrolysis in H218O (> 97%) (32). [1-14C]-5S-HETE and [5,6,8,9,11,12,14,15-d8]-5S-HETE were prepared by reacting labeled arachidonic acid with recombinant human 5-LOX expressed in Sf9 insect cells. Recombinant human H-PGDS and murine L-PGDS were purchased from Cayman Chemical. In addition, H-PGDS was cloned from human colon cancer cDNA (National Center for Biotechnology Information accession number NM_014485). The cDNA encoding an N-terminal 6xHis-tag was subcloned into the pET3 expression vector, expressed in Escherichia coli, and purified using Ni-nitrilotriacetate affinity chromatography.
-HETE was prepared by repeated cycles of methylation and hydrolysis in H218O (> 97%) (32). [1-14C]-5S-HETE and [5,6,8,9,11,12,14,15-d8]-5S-HETE were prepared by reacting labeled arachidonic acid with recombinant human 5-LOX expressed in Sf9 insect cells. Recombinant human H-PGDS and murine L-PGDS were purchased from Cayman Chemical. In addition, H-PGDS was cloned from human colon cancer cDNA (National Center for Biotechnology Information accession number NM_014485). The cDNA encoding an N-terminal 6xHis-tag was subcloned into the pET3 expression vector, expressed in Escherichia coli, and purified using Ni-nitrilotriacetate affinity chromatography.
Incubations with Labeled Substrates.
Incubation of a 1∶1 mixture of 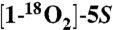 -HETE and unlabeled 5S-HETE (20 μM final) was conducted in 500 μL Tris-HCl buffer pH 8 containing 500 μM phenol, 1 μM hematin, and 1 μM COX-2 for 10 min at 37 °C. The same reaction conditions were used for the incubation of a 1∶1 mixture of d8-5S-HETE and d0-5S-HETE.
-HETE and unlabeled 5S-HETE (20 μM final) was conducted in 500 μL Tris-HCl buffer pH 8 containing 500 μM phenol, 1 μM hematin, and 1 μM COX-2 for 10 min at 37 °C. The same reaction conditions were used for the incubation of a 1∶1 mixture of d8-5S-HETE and d0-5S-HETE.
Incubations with Recombinant PGDS.
The reaction with H-PGDS was conducted in 500 μL of 100 mM Tris-HCl buffer pH 8 containing 10 μM glutathione (GSH), 10 mM MgCl2, 0.5 μM H-PGDS, and 10 μM diendoperoxide for 10 min at room temperature. The reaction with L-PGDS was conducted in the same buffer containing 10 μM GSH, 500 μg BSA, and 0.5 μM L-PGDS. Control reactions with PGH2 formed in situ were conducted using 10 μM arachidonic acid and 1 μM COX-2, and the same amount of H-PGDS, GSH, and MgCl2 or L-PGDS, GSH, and BSA as described for the reaction with the diendoperoxide. For the extraction of glutathione adducts, 30 mg Waters hydrophilic-lipophilic balanced (HLB) cartridges were conditioned with 50 mM NH4OAc (pH 3.4), and products were eluted with 95% ethanol.
Preparation and Isolation of the Hemiketals.
Twenty 2-mL reactions containing 1 μM COX-2 in 100 mM phosphate buffer pH 8, 500 μM phenol, and 1 μM hematin were conducted in parallel. The reactions were started by addition of 30 μg 5S-HETE to each vial, allowed to proceed for 30 min, acidified (pH 4), and extracted using 30 mg Waters HLB cartridges. The products were analyzed by RP-HPLC using a Waters Symmetry C18 5-μm column (4.6 × 250 mm) eluted with a gradient of acetonitrile/water/acetic acid from 20/80/0.01 to 70/30/0.01 (by volume) within 20 min at 1 mL/ min. Chromatography was monitored using an Agilent 1200 diode array detector. For isolation of HKD2 the flow rate was reduced to 0.4 mL/ min. The products were further purified using an Agilent Zorbax RX-SIL 5 μm column (4.6 × 250 mm) eluted with hexane/isopropanol/acetic acid 80/20/0.1 (by volume) at 1 mL/ min. 1H and 2D NMR spectra were recorded at 285 K using a Bruker AV-II 600-MHz spectrometer equipped with a cryoprobe. Chemical shifts are reported in parts per million relative to the signal of residual nondeuterated solvent (δ 7.25 for CDCl3, δ 3.30 for 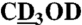 , and δ 1.93 for CD3CN).
, and δ 1.93 for CD3CN).
Human Leukocyte Isolation and Incubation.
The study was approved by the Vanderbilt Institutional Review Board, protocol #091243. Venous blood (45 mL) from healthy volunteers was collected into citric acid mixed with 10 mL of 6% dextran and allowed to settle for 1 h. Leukocytes were collected by centrifugation, and remaining red cells were removed by hypotonic lysis. Cells resuspended in PBS+ (1.5 × 107 cells) were treated with LPS (10 μg/mL) for 6 h at 37 °C followed by A23187 (5 μM) for 15 min. 5S-HETE (15 μM), NS-398 (10 μM), MK-886 (10 μM), or AA-861 (10 μM) was added to some of the incubations 30 min prior to the addition of A23187. Samples were acidified and extracted using Waters HLB cartridges. After evaporation, samples were reconstituted in 10 μL of acetonitrile and 40 μL of LC-MS column solvent A.
LC-MS Analyses.
A ThermoFinnigan Quantum Access triple quadrupole instrument with an electrospray interface was operated in the negative ion mode. Settings for sheath and auxiliary gas pressures, temperature, and interface voltage were optimized using direct infusion of a solution of PGD2. A Waters Symmetry Shield C18 3.5-μm column (2.1 × 150 mm) was eluted with a linear gradient of acetonitrile/water, 10 mM NH4OAc (5/95; solvent A) to acetonitrile/water, 10 mM NH4OAc (95/5, by volume; solvent B) at 0.2 mL/ min within 10 min. The following transitions were monitored in the negative ion SRM mode: m/z 399 → 151 for HKE2 and m/z 399 → 183 for HKD2 from 0–8 min; m/z 319 → 115 for 5-HETE from 8–10 min. The signal intensities obtained for each ion chromatogram are given in exponential format with the maximum intensity set as 100%. Data acquisition and spectral analysis was performed using ThermoFinnigan Xcalibur software.
Tubulogenesis Assay.
For the Matrigel-based tubulogenesis assay, capillary-like structure formation was analyzed as described (33). Briefly, 96-well plates were coated with 50 μL of Matrigel and incubated for 30 min at 37 °C. Mouse pulmonary endothelial cells [isolated and cultured as described (34)] were seeded over Matrigel at 1.5 × 104 cells/well in serum-free medium with or without HKD2 or HKE2. The formation of capillary-like structures was recorded hourly for the next 10 h. Shown in Fig. 5 are representative images taken 6 h after plating. Cellular nodes were defined as junctions linking at least three cells, and counted from digital images. Two independent experiments were performed in duplicate with a total of 20 images analyzed per treatment.
Migration Assay.
Cell migration was assayed in transwell plates. Lower wells were coated with matrigel (10 μg/mL) at 4 °C and then incubated 1 h at 37 °C with bovine serum albumin (1% in PBS) to inhibit nonspecific cell migration. Serum-free medium with or without HKD2 or HKE2 was added to the lower wells and endothelial cells (5 × 104 cells in 100 μL of serum-free medium) to the upper wells. Migration toward complete endothelial cell medium was used as positive control. After 6 h at 37 °C, cells on the top of the filter were removed by wiping, and the filters were fixed (4% formaldehyde in PBS). Migrating cells were stained with hematoxylin and eosin and counted. The number of migrated cells is expressed as fold change relative to control in four independent experiments.
Supplementary Material
Acknowledgments.
We thank Drs. Thomas M. Harris, Markus Voehler, and Ned Porter for careful review of the NMR data and helpful discussion. This work was supported by Award R01GM076592 from the National Institute of General Medical Sciences and Award BC063074 from the Department of Defense Breast Cancer Research Program (to C.S.), and Award 2P01DK065123 from the National Institute of Diabetes and Digestive and Kidney Diseases and a Merit Review from the Department of Veterans Affairs (to A.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge National Institutes of Health Grant S10 RR019022 for funding of the Bruker 600-MHz AV-II NMR spectrometer and accessories.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019473108/-/DCSupplemental.
References
- 1.Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 3.Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 4.Rouzer CA, Marnett LJ. Cyclooxygenases: Structural and functional insights. J Lipid Res. 2009;50(Suppl):S29–34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider C, Boeglin WE, Yin H, Stec DF, Voehler M. Convergent oxygenation of arachidonic acid by 5-lipoxygenase and cyclooxygenase-2. J Am Chem Soc. 2006;128:720–721. doi: 10.1021/ja056517y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griesser M, Boeglin WE, Suzuki T, Schneider C. Convergence of the 5-LOX and COX-2 pathways. Heme-catalyzed cleavage of the 5S-HETE-derived di-endoperoxide into aldehyde fragments. J Lipid Res. 2009;50:2455–2462. doi: 10.1194/jlr.M900181-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urade Y, Eguchi N. Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostag Oth Lipid M. 2002;68–69:375–382. doi: 10.1016/s0090-6980(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 8.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: A novel therapeutic target. Pharmacol Rev. 2007;59:207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 9.Hamberg M, Samuelsson B. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J Biol Chem. 1967;242:5344–5354. [PubMed] [Google Scholar]
- 10.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci USA. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brame CJ, Salomon RG, Morrow JD, Roberts LJ., 2nd Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. 1999;274:13139–13146. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- 12.Mulugeta S, et al. Identification and absolute configuration of dihydroxy-arachidonic acids formed by oxygenation of 5S-HETE by native and aspirin-acetylated COX-2. J Lipid Res. 2010;51:575–585. doi: 10.1194/jlr.M001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urade Y, Fujimoto N, Ujihara M, Hayaishi O. Biochemical and immunological characterization of rat spleen prostaglandin D synthetase. J Biol Chem. 1987;262:3820–3825. [PubMed] [Google Scholar]
- 14.Joo M, et al. Lipopolysaccharide-dependent interaction between PU.1 and c-Jun determines production of lipocalin-type prostaglandin D synthase and prostaglandin D2 in macrophages. Am J Physiol Lung Cell Mol Physiol. 2009;296:L771–779. doi: 10.1152/ajplung.90320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouzer CA, et al. RAW264.7 cells lack prostaglandin-dependent autoregulation of tumor necrosis factor-alpha secretion. J Lipid Res. 2005;46:1027–1037. doi: 10.1194/jlr.M500006-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Borgeat P, Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: Effects of ionophore A23187. Proc Natl Acad Sci USA. 1979;76:2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrignani P, et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J Pharmacol Exp Ther. 1994;271:1705–1712. [PubMed] [Google Scholar]
- 18.Barnett J, et al. Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim Biophys Acta. 1994;1209:130–139. doi: 10.1016/0167-4838(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimoto T, et al. 2,3,5-Trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone (AA861), a selective inhibitor of the 5-lipoxygenase reaction and the biosynthesis of slow-reacting substance of anaphylaxis. Biochim Biophys Acta. 1982;713:470–473. [PubMed] [Google Scholar]
- 20.Dixon RA, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Periz A, Claria J. New approaches to the modulation of the cyclooxygenase-2 and 5-lipoxygenase pathways. Curr Top Med Chem. 2007;7:297–309. doi: 10.2174/156802607779941378. [DOI] [PubMed] [Google Scholar]
- 22.Kumasaka T, et al. Structural basis of the catalytic mechanism operating in open-closed conformers of lipocalin type prostaglandin D synthase. J Biol Chem. 2009;284:22344–22352. doi: 10.1074/jbc.M109.018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: Focus on HNE and ONE. Drug Metab Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- 24.Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: From cell-cell interactions to in vivo tissue responses. Pharmacol Rev. 2006;58:375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 26.Powell WS, Rokach J. Biochemistry, biology and chemistry of the 5-lipoxygenase product 5-oxo-ETE. Prog Lipid Res. 2005;44:154–183. doi: 10.1016/j.plipres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Stenson WF, Parker CW. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979;64:1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards CF, Johnson AR, Campbell WB. Specific incorporation of 5-hydroxy-6,8,11,14-eicosatetraenoic acid into phosphatidylcholine in human endothelial cells. Biochim Biophys Acta. 1986;875:569–581. doi: 10.1016/0005-2760(86)90079-2. [DOI] [PubMed] [Google Scholar]
- 29.Powell WS, Gravelle F, Gravel S. Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes. J Biol Chem. 1992;267:19233–19241. [PubMed] [Google Scholar]
- 30.Schneider C, Boeglin WE, Brash AR. Identification of two cyclooxygenase active site residues, leucine-384 and glycine-526, that control carbon ring cyclization in prostaglandin biosynthesis. J Biol Chem. 2004;279:4404–4414. doi: 10.1074/jbc.M307431200. [DOI] [PubMed] [Google Scholar]
- 31.Corey EJ, Hashimoto S. A practical process for large-scale synthesis of (S)-5-hydroxy-6-trans-8,11,14,cis-eicosatetraenoic acid (5-HETE) Tetrahedron Lett. 1981;22:299–302. [Google Scholar]
- 32.Murphy RC, Clay KL. Preparation of labeled molecules by exchange with oxygen-18 water. Methods Enzymol. 1990;193:338–348. doi: 10.1016/0076-6879(90)93425-k. [DOI] [PubMed] [Google Scholar]
- 33.Pozzi A, et al. The anti-tumorigenic properties of peroxisomal proliferator-activated receptor alpha are arachidonic acid epoxygenase-mediated. J Biol Chem. 2010;285:12840–12850. doi: 10.1074/jbc.M109.081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pozzi A, et al. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci USA. 2000;97:2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.