Abstract
p21-activated kinases (PAKs) are serine/threonine protein kinases that serve as important mediators of Rac and Cdc42 GTPase function as well as pathways required for Ras-driven tumorigenesis. PAK1 has been implicated in signaling by growth factor receptors and morphogenetic processes that control cell polarity, invasion, and actin cytoskeleton organization. To better understand the role of PAK1 in tumorigenesis, PAK1 genomic copy number and expression were determined for a large panel of breast, lung, and head and neck tumors. PAK1 genomic amplification at 11q13 was prevalent in luminal breast cancer, and PAK1 protein expression was associated with lymph node metastasis. Breast cancer cells with PAK1 genomic amplification rapidly underwent apoptosis after inhibition of this kinase. Strong nuclear and cytoplasmic PAK1 expression was also prevalent in squamous nonsmall cell lung carcinomas (NSCLCs), and selective PAK1 inhibition was associated with delayed cell-cycle progression in vitro and in vivo. NSCLC cells were profiled using a library of pathway-targeted small-molecule inhibitors, and several synergistic combination therapies, including combination with antagonists of inhibitor of apoptosis proteins, were revealed for PAK1. Dual inhibition of PAK1 and X chromosome-linked inhibitor of apoptosis efficiently increased effector caspase activation and apoptosis of NSCLC cells. Together, our results provide evidence for dysregulation of PAK1 in breast and squamous NSCLCs and a role for PAK1 in cellular survival and proliferation in these indications.
The p21-activated kinase (PAK) family consists of six members, which are subdivided into two groups: PAK1–3 (group I) and PAK4–6 (group II). This distinction is based on sequence similarities and also, on the presence of an autoinhibitory region in group I PAKs, which is not present in group II PAK proteins (1). As a major downstream effector of the Rho family small GTPases Cdc42 and Rac1, PAK1 plays a fundamental role in controlling cell motility by linking a variety of extracellular signals to changes in actin cytoskeleton organization, cell shape, and adhesion dynamics (2, 3). PAK1 is widely expressed in a variety of normal tissues, and expression is significantly increased in ovarian, breast, and bladder cancers (4–6). Functional studies have also implicated PAK1 in cell transformation (7), and transgenic overexpression of PAK1 in the mammary gland promotes the formation of malignant tumors and premalignant lesions in animal models, albeit with a long latency (8). These findings indicate that PAK1 may contribute to tumorigenesis in some disease contexts.
PAK1 has recently been shown to be involved in fundamental cellular processes beyond that of regulating the cytoskeleton, including regulation of apoptosis or programmed cell death (9). There are published examples that describe activated forms of PAK1 protecting against cell death induced by either cell detachment or chemotherapeutic agents (10, 11), but the relevant pathways downstream of PAK1 remain only partially understood. For instance, PAK1 has been shown to protect lymphoid progenitor cells from intrinsic apoptotic signals by phosphorylation of B-cell lymphoma 2 (BCL2) antagonist of cell death (BAD) to limit its interaction with BCL2 (12). In addition, PAK1-mediated phosphorylation of v-raf-1 murine leukemia viral oncogene homolog 1 (C-RAF) at Ser338 can stimulate translocation of C-RAF to the mitochondria and subsequent complex formation with BCL2 in HEK293T cells (13). However, additional mechanisms may be involved, and the effect of PAK1 inhibition on apoptosis of human tumor cells has yet to be thoroughly investigated. Herein, we use inducible shRNA, and small-molecule approaches were used to explore the dependence of tumor cells on PAK1 signaling to maintain cellular survival, proliferation, and in vivo tumor growth. PAK1 inhibition promoted tumor cell apoptosis as either single-agent treatment (in the context of tumor cells with focal genomic amplification of PAK1) or combination therapy with several targeted agents in squamous cell carcinoma. In particular, antagonists of X chromosome-linked inhibitor of apoptosis (XIAP) protein potently synergized with PAK1 inhibition to induce tumor cell death. Our results show that significant antitumor efficacy is observed after PAK1 inhibition and support further characterization of PAK1 as a therapeutic target.
Results
PAK1 Amplification and Oncogene Addiction in Breast Cancer.
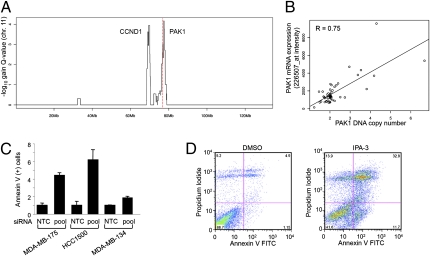
Several genomic regions with copy-number gains have been identified in breast cancer by comparative genomic hybridization approaches (14). However, the low resolution of older analysis platforms may have resulted in tumor-promoting genes being overlooked (15). As such, we assayed 51 breast tumors for DNA copy-number changes using high-resolution SNP arrays and analyzed these data using the Genomic Identification of Significant Targets in Cancer (GISTIC) method (15, 16). A chromosome 11 region of amplification is shown in Fig. 1A. Two distinct GISTIC peaks were observed at 69 and 76 Mb, suggesting that the 11q13.5 region contains two independent amplicons. The 69-Mb peak corresponds to amplification of CCND1, a very well-described genomic alteration in breast cancer (17), and the plateau of the 76-Mb peak contains the PAK1 gene (shown as a red dotted line in Fig. 1A). The frequency of PAK1 amplification was 17% (copy number ≥ 2.5) in this tumor panel, and copy-number gain was well-correlated with mRNA expression (Pearson correlation = 0.75) (Fig. 1B). Similar results were also obtained in a larger panel (n = 165) of breast tumors that were also analyzed for genomic amplification by high-resolution SNP arrays (Table S1) (18). PAK1 gene amplification was prevalent, and mean DNA copy number was greatest in luminal, hormone receptor-positive tumors (7.7 mean copy number) and was least in basal breast tumors (2.8 mean copy number) (Table S1). Taken together, this suggests that PAK1 could be a tumor-promoting driver gene in the 76-Mb amplicon of chromosome 11.
Fig. 1.
Analysis of PAK1 genomic amplification and functional role in human breast tumors. (A) GISTIC analysis of 11q13 copy-number gains. The red line represents the location of the PAK1 gene. (B) PAK1 DNA copy and mRNA expression are associated for 51 breast tumor samples (Pearson correlation = 0.75). (C) Increased Annexin V-positive cells after knockdown of PAK1 expression for three breast cancer cell lines with focal PAK1 genomic amplification and estrogen receptor-positive status: MDA-MB-175, HCC1500, and MDA-MB-134 IV. (D) FACS analysis for MDA-MB-175 cells treated with IPA-3 for 48 h. Annexin V/PI staining was then done to assess apoptosis/necrosis.
To investigate the possible contribution of PAK1 to breast cancer pathophysiology when overexpressed by genomic amplification, we determined the biological response to inhibition of PAK1 in breast cancer cells, with focal 76-Mb amplification on chromosome 11. Gene amplification and elevated expression of PAK1 were observed in luminal-subtype M.D. Anderson Metastatic Breast-175 (MDA-MB-175), human breast cancer cell 1500 (HCC1500), and MDA-MB-134IV breast cancer cell lines (5.7, 4.3, and >8.0 DNA copies, respectively). Inhibition of PAK1 catalytic activity using selective siRNA-mediated knockdown of PAK1 expression resulted in a pronounced induction of apoptosis as determined by Annexin-V/propidium iodide staining of dying cells (two- to sixfold increase in Annexin-V incorporation) (Fig. 1C). This phenotype was evident within 48–72 h and was confirmed using inhibitor targeting PAK1 activation-3 (IPA-3), an allosteric inhibitor that binds PAK1–3 and prevents activation by Rho family GTPases (19) (sevenfold increase) (Fig. 1D). Cell death induced by PAK1 inhibition was also associated with caspase activation, poly-ADP ribose polymerase (PARP) cleavage, and attenuated phosphorylation of MAPK/ERK kinase-1 (MEK1) S298 and ERK1/2 (Fig. S1 A and B). Hence, the strong induction of cell death resulting from PAK1 inhibition in breast cancer cells with PAK1 amplification suggests that this kinase can contribute to the oncogenic phenotype, at least in part, by suppressing tumor cell apoptosis. The degree to which cells were sensitive to PAK1 inhibition was also correlated with the level of PAK1 overexpression (Fig. S1 C and D). In this way, PAK1 may be an Achilles’ heel for this genetically defined subset of breast cancer (20).
PAK1 Protein Expression in Human Tumors.
To follow-up on these observations and determine the possible extent of PAK1 dysregulation across human cancers, PAK1 protein expression level and subcellular localization were ascertained by immunohistochemical (IHC) staining of tissue microarrays. Robust and selective IHC reactivity of PAK1 antibody was confirmed in cancer cell lines with immunoblot analysis of protein extracts from these cells performed in parallel (Fig. S2 A–D). PAK1 protein expression data for 226 primary breast cancers, 16 breast ductal carcinoma in situ (DCIS), 32 breast cancer lymph node metastases, 97 nonsmall cell lung cancers (NSCLC), 27 small cell lung cancers (SCLC), and 130 head and neck squamous cell carcinomas (HNSCC) are summarized in Table 1 and Fig. S2E. In the context of breast cancer, PAK1 expression was absent in normal breast epithelial cells but was detected in the malignant cells of 39% of primary breast adenocarcinomas (Fig. S2E). Cytoplasmic expression of PAK1 in early DCIS lesions was similar to expression in primary breast adenocarcinoma (P = 0.64), and PAK1 amplification could, thereby, be an early genetic event in the initiation and progression of some breast tumors. Consistent with the established function of PAK1 in modulating the actin cytoskeleton and cell motility (1), expression of cytoplasmic PAK1 in primary adenocarcinomas was associated with nodal metastasis (P = 0.03), and elevated PAK1 levels were more frequent in nodal metastases than primary tumors (P < 0.0001) (Table 1). Also, as suggested by DNA copy-number analysis (Table S1), PAK1 protein levels were higher in nonbasal breast tumors than basal tumors and independent of cyclin D1 nuclear expression as determined by IHC (Tables S2–S6). Lastly, RNA was purified from 88 primary breast cancer specimens, and cytoplasmic PAK1 IHC staining was correlated with increased mRNA expression (Fig. S2F). These data show that PAK1 expression is broadly up-regulated in breast cancer and that high expression is correlated with disease progression.
Table 1.
Frequency data for PAK1 immunohistochemistry in human cancer
| PAK1 cytoplasmic score |
PAK1 nuclear score |
|||||||
| Tumor type | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Breast ductal carcinoma in situ | 11 (69%) | 3 (19%) | 0 | 2 (13%) | 16 (100%) | 0 | 0 | 0 |
| Primary breast adenocarcinoma | 138 (61%) | 41 (18%) | 22 (10%) | 25 (11%) | 220 (97%) | 0 | 2 (1%) | 4 (2%) |
| Breast lymph node metastases | 4 (13%) | 8 (25%) | 12 (38%) | 8 (25%) | 32 (100%) | 0 | 0 | 0 |
| NSCLC adenocarcinoma | 15 (50%) | 9 (30%) | 6 (20%) | 0 | 30 (100%) | 0 | 0 | 0 |
| NSCLC squamous cell carcinoma | 24 (36%) | 8 (12%) | 16 (24%) | 19 (28%) | 47 (70%) | 3 (4%) | 4 (6%) | 13 (19%) |
| Small cell lung cancer | 10 (37%) | 8 (30%) | 9 (33%) | 0 | 27 (100%) | 0 | 0 | 0 |
| Head and neck squamous cell carcinoma | 20 (15%) | 31 (24%) | 57 (44%) | 22 (17%) | 75 (58%) | 17 (13%) | 27 (21%) | 11 (9%) |
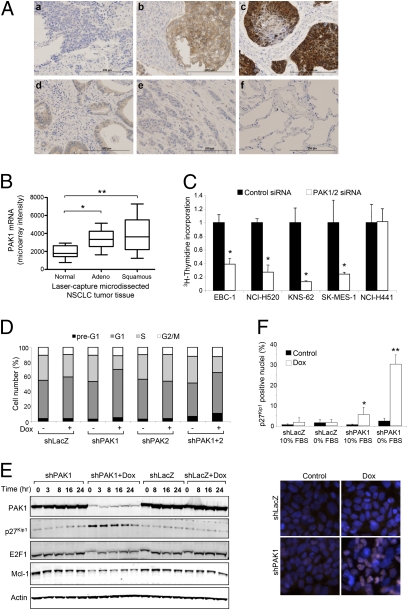
Expression of PAK1 protein was also analyzed on tissue microarrays of 27 SCLC and 97 NSCLC, the latter being comprised of 30 adenocarcinomas and 67 squamous cell carcinomas; 43 of 67 (64%) squamous NSCLC samples were positive for PAK1 expression, and 52% of all cases showed staining of moderate (2+) or strong (3+) intensity in the malignant cells (Fig. 2A, Table 1, and Tables S2–S6). Nuclear localization of PAK1 was also evident in a significant proportion of squamous NSCLCs (17/67; 25%). In contrast to squamous cell carcinoma, NSCLC adenocarcinomas (P = 0.0008) and SCLCs (P = 0.003) expressed only weak to moderate levels of PAK1 in the cytoplasm only. Adjacent normal lung tissue did not express appreciable levels of PAK1 (Fig. 2 and Fig. S2E). Interestingly, elevated PAK1 expression was also prevalent (79/130; 61%) in head and neck tumors, an additional indication of squamous cell carcinoma (Table 1 and Tables S2–S6). Additional evidence that PAK1 expression is elevated in squamous NSCLC was obtained by analyzing PAK1 mRNA expression in a distinct set of 54 laser-capture microdissected lung tissues, and PAK1 mRNA expression was highest in squamous NSCLC (P < 0.0001 compared with normal lung) (Fig. 2B). Together, these results suggest that PAK1 overexpression in squamous carcinomas may occur through multiple molecular mechanisms such as increased transcription or protein translation and stability.
Fig. 2.
PAK1 is highly expressed in human lung tumors and plays a critical role in proliferation of squamous NSCLC cell lines. (A) IHC for PAK1 was performed on (A, a–c) squamous NSCLC, (A, d and e) adenocarcinoma NSCLC, and (A, f) normal lung tissues. (Scale bar, 200 μm.) (B) PAK1 mRNA expression in laser-capture microdissected lung tissues. Relative to normal tissues (n = 9), PAK1 expression was greater in squamous (n = 16; **P = 0.0005) and adenocarcinoma NSCLC (n = 29; *P = 0.008). (C) Proliferation of squamous NSCLC cell lines transfected with siRNA oligonucleotides was measured by [3H]thymidine uptake assay. The data are normalized to control and shown as the mean ± SD. (D) Accumulation of cells in G1 phase of the cell cycle is evident after inducible PAK1 knockdown in NCI-H520.X1 cells. (E) NCI-H520.X1 cells were serum-starved for 24 h, and cell-cycle reentry was monitored by harvesting cell lysates at the indicated time points after growth in 10% serum-containing media. (F) Anti-p27Kip1 (red) and DAPI (blue) immunofluorescence after PAK1-inducible knockdown. Quantification of p27Kip1 nuclear accumulation (n = 2,000 cells). *P < 0.05. **P < 0.0001.
PAK1 Is Required for Proliferation of Squamous NSCLC Cells.
Given the histologic data for elevated PAK1 expression in squamous NSCLC tumors, we subsequently examined the effect of RNAi-mediated knockdown of PAK1 in a panel of lung cancer cell lines to clarify the contribution of PAK1 to tumor cell growth and survival. EBC-1, NCI-H520, KNS-62, SK-Mes-1, and NCI-H441 NSCLC cell lines highly express PAK1, and transient knockdown resulted in a 2.5- to 8-fold reduction in [3H]-thymidine incorporation of four of five cell lines compared with cells transfected with a nontargeting negative-control siRNA oligonucleotide (*P < 0.0001) (Fig. 2C). Furthermore, we made use of a doxycycline-inducible shRNA system (21) to deplete endogenous target protein and further study PAK1 loss of function effects in squamous NSCLC. PAK1 and/or PAK2 shRNAs were introduced into NCI-H520.X1 (a subline of NCI-H520 that was selected for robust in vivo growth) and EBC-1 cells. As a negative control, cell lines containing LacZ-specific shRNA were generated in parallel. Tightly regulated Dox-mediated knockdown of target protein expression was observed for cell lines expressing shPAK1 and shPAK2 (Fig. S3 A and B). Inhibition of PAK1 but not PAK2 significantly reduced proliferation of NCI-H520.X1 and EBC-1 cells (*P < 0.0001) (Fig. S3C), supporting a role for PAK1 as a driver of proliferation in the squamous NSCLC subtype.
To better assess the mechanism by which PAK1 contributes to cellular proliferation, the cell-cycle profile of NCI-H520.X1 cells was determined by propidium iodide staining and fluorescence-activated cell sorter analysis. After PAK1 inhibition, cells exhibited accumulation in G1 phase (Fig. 2D). In agreement with this result, levels of the E2F1 transcription factor, which is essential for G1/S progression and known to regulate gene expression associated with DNA replication and mitosis (22), were also diminished as a consequence of PAK1 ablation (Fig. 2E). Functional classification of global gene expression differences after PAK1 inhibition revealed the down-regulation of Molecular Signature Database (MSigDB) gene sets for E2F1 targets and serum-induced cell-cycle progression (Fig. S3 D and E). Furthermore, analysis of serum-starved quiescent NCI-H520.X1 cells also revealed an accumulation of the CDK1 inhibitor, p27Kip1, in the absence of PAK1 (Fig. 2E; lane 6). Even 24 h after cell-cycle reentry, the levels of p27Kip1 remained elevated in protein lysates of cells lacking PAK1 (Fig. 2E; lane 10) compared with controls. Additional quantification of p27Kip1 accumulation was performed by immunofluorescence staining and image analysis to detect nuclear p27Kip1 in cells depleted for PAK1 (Fig. 2F). An intense signal for nuclear p27Kip1 was evident for NCI-H520.X1-shPAK1 cells treated with Dox in the presence or absence of serum (P < 0.05 and P < 0.0001, respectively) (Fig. 2F). Taken together, the functional consequences of PAK1 blockade encompasses pronounced cytostatic effects in squamous NSCLC cells.
Antitumor Efficacy of PAK1 Inhibition in Preclinical Tumor Models of Squamous NSCLC.
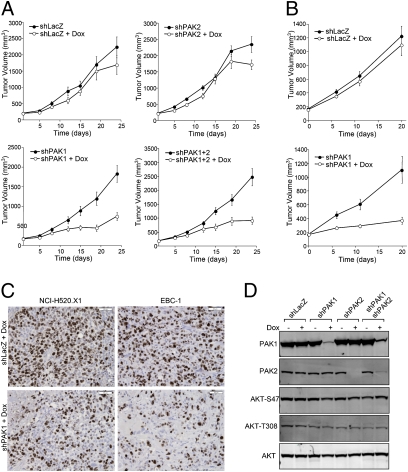
To extend our in vitro observations, the roles of PAK1 and PAK2 in tumor growth and maintenance were evaluated using NCI-H520.X1 and EBC-1 xenograft models (Fig. 3 A and B). After tumor establishment, animals were administered Dox in sucrose drinking water, and tumor growth was monitored for 21–24 d. For NCI-H520.X1 tumor-bearing animals, inhibition of PAK1 but not PAK2 significantly impaired tumor growth relative to control shLacZ mice (P < 0.0001) (Fig. 3A). Combined knockdown of PAK1 and PAK2 resulted in inhibition of tumor growth that was comparable with that of PAK1 inhibition alone (63.7% and 59.7%, respectively). Equivalent results were obtained using EBC-1 tumor xenografts, and 66.8% inhibition of tumor growth was observed after in vivo knockdown of PAK1 (Fig. 3B). Furthermore, analysis of xenograft tumors by immunohistochemistry revealed a substantial decrease in Ki-67–positive tumor cells in Dox-treated tumors expressing shPAK1 compared with shLacZ controls (Fig. 3C). The proportion of Ki-67–positive nuclei was quantified, and the antiproliferative effect of PAK1 knockdown in vivo was shown to be statistically significant (P < 0.01; NCI-H520.X1 shLacZ = 69 ± 6%, NCI-H520.X1 shPAK1 = 50 ± 4%, EBC-1 shLacZ = 91 ± 3%, EBC-1 shPAK1 = 75 ± 11%). PAK1 knockdown was not associated with decreased v-akt murine thymoma viral oncogene homolog (AKT) activation (Fig. 3D), which has been suggested in recent literature (23). PAK1-dependent changes in ERK/MAPK pathway activity or BAD protein phosphorylation were also not observed for squamous NSCLC cell lines, which is in contrast to PAK1 effector signaling in other cell types (Fig. S1) (12). As such, we further investigated PAK1 signaling in squamous NSCLC cells using a reverse-phase protein array (RPPA) phosphoproteomics platform (Fig. S4A). Decreased phosphorylation of NF-κB proteins was observed after PAK1 inhibition (Fig. S4 B and C). The importance of PAK1 in NF-κB p105-Ser933 phosphorylation and nuclear accumulation of proteolytically processed NF-κB p50 was confirmed by cell fractionation and immunoblotting (Fig. S4D). NF-κB can be induced by various signals, and the importance of this transcription factor in transformation of lung cancer cells has been recently reported (24). Together, these findings show that PAK1 is critically important not only for proliferation of PAK1-overexpressing squamous NSCLC cells in vitro but also for tumor growth in vivo. The data also support the conclusion that interfering with PAK1 signaling could have therapeutic efficacy in squamous lung cancer.
Fig. 3.
PAK1 is required for growth of established NCI-H520.X1 and EBC-1 squamous NSCLC tumors. (A) NCI-H520.X1 cells expressing inducible shRNAs against LacZ, PAK1, PAK2, or PAK1+PAK2 were implanted in the flank of athymic mice. Doxycycline was administered when tumors were established and resulted in inhibition of tumor growth for mice bearing shPAK1 and shPAK1+2 NCI-H520.X1 cells. (B) EBC-1 shLacZ or shPAK1 tumor-bearing mice were administered doxycycline to inhibit PAK1. (C) Histologic analysis of in vivo knockdown of PAK1 in NCI-H520.X1 and EBC-1 tumors. (D) AKT phosphorylation was not altered on PAK1 and/or PAK2 knockdown in squamous NSCLC tumors.
Combination of PAK1 Inhibition with Molecularly Targeted Therapeutics Induces Apoptosis of NSCLC Cells.
Given that single-agent PAK1 inhibition did not increase apoptosis of NSCLC cells and xenograft models, we hypothesized that PAK1 inhibition may synergize with other molecularly targeted agents to augment killing of tumor cells. We also reasoned that testing the combination of PAK1 inhibition plus compounds of known cellular mechanism would aid in understanding the cellular function of PAK1 in NSCLC cells as well as in rationally designing effective combination regimens that could be translated into the clinic. Hence, a cellular viability screen was performed using EBC-1-shPAK1 isogenic cells and a panel of 200 small-molecule compounds that included oncology drugs approved by the Food and Drug Administration (FDA), signaling pathway inhibitors, and DNA damaging agents (Table S7). Among the tested compounds, antagonists of inhibitor of apoptosis proteins (IAP; 12- and 57-fold), epidermal growth factor receptor (EGFR; 2.9-, 7.4-, 12.8-, and 15-fold), MEK1/2 (8.5-fold), and Src family kinases (5.4-fold) displayed dramatically enhanced efficacy in the context of combined PAK1 knockdown (Table 2). None of these agents showed a profound single-agent effect (EC50 > 6 μM) on the growth and survival of EBC-1 cells in the absence of PAK1 knockdown. Thus, PAK1 inhibition can greatly augment the efficacy of several classes of well-characterized molecularly targeted therapeutics.
Table 2.
Combination of PAK1 inhibition with pharmarray small-molecule panel
| Compound | Single-agent EC50 (mM) | Combination PAKi EC50 (mM) | Fold change in EC50 | Trade name | Mechanism of action and/or approved use |
| BV6 | 20 | 0.35 | 57.54 | n/a | Antagonist of inhibitor of apoptosis (IAP) proteins |
| Erlotinib | 20 | 1.56 | 15.02 | Tarceva | Epidermal growth factor receptor (EGFR) inhibitor for NSCLC |
| Gefitinib | 20 | 3.3 | 12.8 | Iressa | EGFR kinase inhibitor |
| G-416 | 20 | 1.67 | 12 | n/a | Antagonist of IAP proteins |
| U0126 | 19.46 | 2.3 | 8.45 | n/a | Inhibitor of MAPK/ERK kinase-1/2 (MEK1/2) |
| Lapatinib | 6.31 | 0.86 | 7.34 | Tykerb | EGFR/HER2 inhibitor used for HER2-positive breast cancer |
| Dasatinib | 8.29 | 1.53 | 5.43 | Sprycel | Dual BCR/ABL and Src family kinase inhibitor for CML |
| Altretamine | 20 | 3.94 | 5.07 | Hexalen | Alkylating chemotherapy used for refractory ovarian cancer |
| Oxaliplatin | 10.5 | 2.48 | 4.23 | Eloxatin | Platinum-based chemotherapy used for colorectal cancer |
| ZD6474 | 20 | 6.88 | 2.91 | Vandetanib | VEGF and EGF inhibitor in clinical development |
| Akt VIII | 3.21 | 2.03 | 1.58 | n/a | Akt-1/2 kinase inhibitor |
Antagonists of PAK1 and IAP Proteins Strongly Induce Apoptosis of NSCLC Cells.
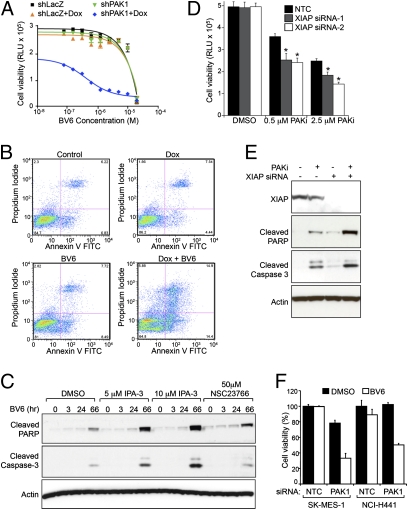
The prosurvival activity of IAP proteins is antagonized by the second mitochondrial activator of caspases (SMAC), and a number of antagonists have been described that mimic SMAC amino-terminal peptides to disrupt the association of IAP with SMAC and activated caspase-9 (25). In particular, BV6 represents one such class of small-molecule antagonists that binds to baculovirus IAP repeat (BIR) domains and promotes rapid autoubiquitination and proteasomal degradation of c-IAP1 and c-IAP2 (26). Consistent with the small-molecule screening data, strong combinatorial activity was confirmed for dual inhibition of PAK1 and IAP in EBC-1 cells (Fig. 4A). This dramatic increase in BV6 potency on EBC-1-shPAK1 cells when cotreated with Dox (EC50 = 4.1 × 10−7 μM) relative to controls (EC50 = 3.0 × 10−3 μM) translated into a strong induction of cellular apoptosis as determined by Annexin-V flow cytometry assay (Fig. 4B and Fig. S5A) and immunoblotting for cleaved caspases-3, -6, -7, and -9 (Fig. S5B). Importantly, evidence of enhanced cell killing was also observed using pharmacological inhibitors of PAK (IPA-3) or Rac1 (NSC23766) catalytic activity (Fig. 4C and Fig. S5 C and D, PF-3758309) (27–29). To further explore this apparent synergy, we investigated possible molecular mechanisms of PAK1 and IAP inhibition on the induction of apoptosis. Combined PAK1 and IAP inhibition did not involve either altered kinetics of IAP protein degradation induced by BV6 (Fig. S5E) or autocrine signaling by TNFα, a known mediator of cell death induced by IAP antagonists (Fig. S5F). Given that XIAP is not dependent on TNF receptor-mediated signaling for antiapoptotic activity and is a highly potent, endogenous inhibitor of caspases-3, -7, and -9 (30), we examined the contribution of this IAP family member to combinatorial efficacy through siRNA-mediated inhibition. Selective antagonism of XIAP together with PAK1 inhibition resulted in decreased cell viability (Fig. 4D) and prominent activation of effector caspases (Fig. 4E) that was comparable with combination with BV6 pan-IAP antagonist. Additional NSCLC cell lines, including those that are only minimally responsive to the activity of either single agent, were further examined for antitumor efficacy resulting from combined inhibition of PAK1 and IAP. Transient siRNA-mediated knockdown of PAK1 expression followed by BV6 treatment resulted in a significant reduction of SK-Mes-1 and NCI-H441 cell viability (Fig. 4F). Taken together, these studies provide strong preclinical support that NSCLC may provide several opportunities for rational combination therapies with PAK1 inhibitors.
Fig. 4.
PAK1 inhibition combines with IAP antagonists to promote apoptosis of NSCLC cells. (A) EBC-1-shPAK1 and -shLacZ cells were treated with Dox and BV6 IAP antagonist. The highest concentration of BV6 was 20 μM, and twofold serial dilutions were assessed in quadruplicate. (B) FACS analysis for Annexin V and PI staining to assess apoptosis/necrosis after PAK1 and IAP antagonism. (C) Combinatorial accumulation of cleaved PARP and caspase-3 resulting from IAP antagonism with IPA-3 or NSC23766. (D) Down-regulation of XIAP expression potentiates the proapoptotic activity of PF-3758309 PAK small-molecule inhibitor (PAK SMI; P < 0.0001). Cells were transfected with siRNA oligonucleotides for 48 h before inhibitor treatment for an additional 72 h. (E) Combined antagonism of XIAP and PAK1 promotes efficient cleavage of PARP and caspase-3. (F) Dual PAK1 and IAP inhibition results in a synergistic decrease in viability of SK-Mes-1 (squamous) and NCI-H441 (adenocarcinoma) NSCLC cells (P < 0.0001).
Discussion
PAK family kinases have been suggested to be central players in growth factor signaling networks, and this model raises the possibility that interfering with PAK1 activity could produce significant antitumor activity (31, 32). We initiated the present study to help further clarify the contribution of PAK1 signaling to tumorigenesis. As a starting point, PAK1 genomic copy number and protein expression level were ascertained in 216 and 537 human tumor samples, respectively. In the context of breast cancer, PAK1 genomic amplification was prevalent in luminal tumors (Fig. 1 and Table S1), and elevated cytoplasmic protein expression was observed across 39% of primary breast adenocarcinomas; it was associated with lymph node invasion and occurred more frequently in nodal metastases compared with primary tumors (P < 0.0001) (Tables S2–S6). It is interesting that the frequency of dysregulated expression of PAK1 was more frequent than would be predicted by genomic amplification alone, and additional regulatory mechanisms that may increase PAK1 expression in breast cancer, such as through microRNA genes (33), remain to be fully explored. Analysis of cell lines with PAK1 genomic copy-number gain revealed exquisite dependence on PAK1 expression and activity for cell survival (Fig. 1 C and D). The prosurvival function of PAK1 in breast cancers might be another contributing factor to the association of elevated PAK1 expression and reduced clinical benefit in patients treated with tamoxifen (34). Although genomic amplification of the 11q13 region has previously been reported in breast cancer (35), the high sensitivity of our SNP array analysis, correlation with IHC data, and loss of function mechanistic studies showing apoptosis after single-agent PAK1 inhibition (Fig. 1 and Table S1) significantly refine our understanding of PAK1 genomic lesions in this tumor indication. Taken together, our identification of PAK1 as an Achilles’ heel for a subpopulation of breast cancer provides evidence of oncogene addiction (20) and a rationale for PAK1-directed therapy in this disease indication.
Herein, we also show that lung and head and neck squamous carcinomas have aberrant cytoplasmic expression of PAK1 (52% and 61%, respectively) (Fig. 2 and Table 1). It is interesting that nuclear translocation of PAK1 was also observed across squamous tumor subtypes, including NSCLC (25%; n = 67) and HNSCC (42%; n = 130) (Tables S2–S6). The biological function of PAK1 in the nucleus is presently not well-understood; however, it has been shown that nuclear import of PAK1 can play a critical role in vertebrate development (36). Loss of function studies to analyze the role of PAK1 in squamous NSCLC cells revealed a requirement for PAK1 in cytoskeletal reorganization, including paxillin phosphorylation, focal adhesion turnover, and cell motility (Fig. S6) as well as cell-cycle progression (Fig. 2 C and D). E2F and p27Kip1 are known to play multifaceted roles in regulation of G0- to S-phase transitions of the cell cycle (22, 37), and the cellular levels of these proteins were significantly affected by PAK1 inhibition in squamous NSCLC cells (Fig. 2E). Altered expression of a large number of putative E2F-responsive genes, including gene clusters with well-established functions in cell-cycle regulation, DNA replication and repair, mitosis, and mitotic spindle checkpoint controls (38), further support PAK1-mediated regulation of E2F transcription factors as a proliferative mechanism for squamous NSCLC cells (Fig. S3). Inhibition of PAK1 signaling also resulted in accumulation of p27Kip1 in the nucleus (Fig. 2F). Nuclear localization of p27Kip1 is required for its tumor suppressor activity, and several oncogenic pathways are known to diminish nuclear levels of p27Kip1 to drive tumor growth and cell-cycle progression (37). Consistent with these cell-culture experiments, inducible knockdown of PAK1 in vivo also resulted in stasis of established NCI-H520.X1 and EBC-1 xenograft tumors (Fig. 3).
At present, known genetic aberrations in squamous NSCLC include p53, p16Ink4a, phosphatase and tensin homolog (PTEN), and serine-threonine kinase 11 (STK11) loss of function through mutation or methylation and activating mutations, amplification, or overexpression of protein kinases such as EGFR, MET, HER2, and PIK3CA (39). This raises the question of whether inhibition of PAK1 enzymatic activity or scaffold function might combine synergistically with therapeutic agents that target these critical growth and survival pathways to increase antitumor efficacy and tumor cell death. Small-molecule library screening was used to address this question, and PAK1 ablation rendered NSCLC cells hypersensitive to antagonists of IAP, EGFR, and Src signaling (Table 2). The mechanism of action for combinatorial efficacy of PAK1 and IAP antagonism was further investigated and involves inhibition of PAK1 enzymatic activity and synergistic induction of apoptosis (Fig. 4B and Fig. S5 A–D). The apoptotic phenotype resulting from PAK1 and IAP combined inhibition differs substantially from the antiproliferative effect that is observed after single-agent inhibition of PAK1 in squamous NSCLC cell lines (model depicted in Fig. S5G). Because small-molecule antagonists of both PAK and IAP proteins have entered clinical trials (28, 40), the potential synergy of this combination therapy can be investigated in patients. Interestingly, PAK1 signaling in squamous NSCLC cells was also associated with an accumulation of the antiapoptotic BCL2 family member, myeloid cell leukemia-1 (Mcl-1) (Fig. 2E), and suggests another mechanism for PAK1 inhibition in regulation of cell survival and therapeutic combinations with BCL2 inhibitors currently in clinical evaluation, such as Navitoclax/ABT-263 (41).
In addition to suggesting beneficial combination therapies for preclinical follow-up, the chemical–genetic approaches that we used allow for insights into the molecular mechanism of PAK1 inhibition in tumor cells. Given that the squamous histologic subtype comprises at least 30% of new NSCLC cases in the United States, our findings have important implications for the development of strategies and agents for the treatment of lung cancer. Taken together, the results from our tumor analysis and preclinical models provide evidence for potential antitumor efficacy of PAK1 inhibition.
Materials and Methods
Approval was obtained, for the use of all human tissue, from the John Radcliffe Hospital research ethics committee (C02.216). Animal experimental procedures were approved by Genentech's Institutional Animal Care and Use Committee. Detailed methods are contained in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank colleagues at Genentech for providing insightful discussions and technical assistance. A.M.J. is supported by a career development fellowship from the Pathological Society of Great Britain and Ireland. A.L.H. is supported by Cancer Research United Kingdom and the Oxford National Health Service (NHS) Biomedical Research Centre.
Footnotes
Conflict of interest statement: C.C.O., P.M.H., W.Z., V.T., T.T., T.O., D.V., M.B., L.S.F., E.M.B., H.K., and K.P.H. are employees of Genentech, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103350108/-/DCSupplemental.
References
- 1.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 2.Sells MA, et al. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 3.Delorme V, et al. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell. 2007;13:646–662. doi: 10.1016/j.devcel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasenthil S, et al. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279:1422–1428. doi: 10.1074/jbc.M309937200. [DOI] [PubMed] [Google Scholar]
- 5.Schraml P, et al. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5-q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol. 2003;163:985–992. doi: 10.1016/S0002-9440(10)63458-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito M, et al. P21-activated kinase 1: A new molecular marker for intravesical recurrence after transurethral resection of bladder cancer. J Urol. 2007;178:1073–1079. doi: 10.1016/j.juro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Vadlamudi RK, et al. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- 8.Wang RA, Zhang H, Balasenthil S, Medina D, Kumar R. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene. 2006;25:2931–2936. doi: 10.1038/sj.onc.1209309. [DOI] [PubMed] [Google Scholar]
- 9.Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menard RE, Jovanovski AP, Mattingly RR. Active p21-activated kinase 1 rescues MCF10A breast epithelial cells from undergoing anoikis. Neoplasia. 2005;7:638–645. doi: 10.1593/neo.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deacon K, Mistry P, Chernoff J, Blank JL, Patel R. p38 Mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol Biol Cell. 2003;14:2071–2087. doi: 10.1091/mbc.E02-10-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schürmann A, et al. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005;280:24698–24705. doi: 10.1074/jbc.M413374200. [DOI] [PubMed] [Google Scholar]
- 14.Climent J, Garcia JL, Mao JH, Arsuaga J, Perez-Losada J. Characterization of breast cancer by array comparative genomic hybridization. Biochem Cell Biol. 2007;85:497–508. doi: 10.1139/O07-072. [DOI] [PubMed] [Google Scholar]
- 15.Haverty PM, et al. High-resolution genomic and expression analyses of copy number alterations in breast tumors. Genes Chromosomes Cancer. 2008;47:530–542. doi: 10.1002/gcc.20558. [DOI] [PubMed] [Google Scholar]
- 16.Beroukhim R, et al. Assessing the significance of chromosomal aberrations in cancer: Methodology and application to glioma. Proc Natl Acad Sci USA. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson C, et al. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett. 1995;90:43–50. doi: 10.1016/0304-3835(94)03676-a. [DOI] [PubMed] [Google Scholar]
- 18.Kan Z, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 19.Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther. 2009;8:2559–2565. doi: 10.1158/1535-7163.MCT-09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- 21.Hoeflich KP, et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66:999–1006. doi: 10.1158/0008-5472.CAN-05-2720. [DOI] [PubMed] [Google Scholar]
- 22.Hallstrom TC, Nevins JR. Balancing the decision of cell proliferation and cell fate. Cell Cycle. 2009;8:532–535. doi: 10.4161/cc.8.4.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–1364. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 24.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Gyrd-Hansen M, Meier P. IAPs: From caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 26.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Deacon SW, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray BW, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci USA. 2010;107:9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varfolomeev E, et al. X chromosome-linked inhibitor of apoptosis regulates cell death induction by proapoptotic receptor agonists. J Biol Chem. 2009;284:34553–34560. doi: 10.1074/jbc.M109.040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 32.Arias-Romero LE, et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene. 2010;29:5839–5849. doi: 10.1038/onc.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy SD, Ohshiro K, Rayala SK, Kumar R. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res. 2008;68:8195–8200. doi: 10.1158/0008-5472.CAN-08-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holm C, et al. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006;98:671–680. doi: 10.1093/jnci/djj185. [DOI] [PubMed] [Google Scholar]
- 35.Chin K, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Lightcap CM, et al. Interaction with LC8 is required for Pak1 nuclear import and is indispensable for zebrafish development. PLoS One. 2009;4:e6025. doi: 10.1371/journal.pone.0006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wander SA, Zhao D, Slingerland JM. p27: A barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin Cancer Res. 2011;17:12–18. doi: 10.1158/1078-0432.CCR-10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren B, et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dynek JN, Vucic D. Antagonists of IAP proteins as cancer therapeutics. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.06.013. 10.1016/j.canlet.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Tse C, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.