Abstract
Functional diversity of protein phosphatase 2A (PP2A) enzymes mainly results from their association with distinct regulatory subunits. To analyze the functions of one such holoenzyme in vivo, we generated mice lacking PR61/B’δ (B56δ), a subunit highly expressed in neural tissues. In PR61/B’δ-null mice the microtubule-associated protein tau becomes progressively phosphorylated at pathological epitopes in restricted brain areas, with marked immunoreactivity for the misfolded MC1-conformation but without neurofibrillary tangle formation. Behavioral tests indicated impaired sensorimotor but normal cognitive functions. These phenotypical characteristics were further underscored in PR61/B’δ-null mice mildly overexpressing human tau. PR61/B’δ-containing PP2A (PP2AT61δ) poorly dephosphorylates tau in vitro, arguing against a direct dephosphorylation defect. Rather, the activity of glycogen synthase kinase-3β, a major tau kinase, was found increased, with decreased phosphorylation of Ser-9, a putative cyclin-dependent kinase 5 (CDK5) target. Accordingly, CDK5 activity is decreased, and its cellular activator p35, strikingly absent in the affected brain areas. As opposed to tau, p35 is an excellent PP2AT61δ substrate. Our data imply a nonredundant function for PR61/B’δ in phospho-tau homeostasis via an unexpected spatially restricted mechanism preventing p35 hyperphosphorylation and its subsequent degradation.
Keywords: brain stem, knockout mouse, AT8, AT180, transgenic
Reversible protein phosphorylation, spatio-temporally controlled by kinases and phosphatases, is a key mechanism regulating a plethora of physiological processes. Protein phosphatase 2A (PP2A) is a major Ser/Thr phosphatase broadly implicated in cellular signaling (1, 2), and its deregulation results in pathologies such as cancer (2, 3) and Alzheimer’s disease (AD) (4). Gene knockout (KO) of the most abundant isoform of the catalytic subunit (PP2ACα) in mice is embryonically lethal (5), underscoring the broad physiological importance of PP2A in mammals.
The underlying basis of this functional diversity is formed by PP2A’s complex structure and regulation. PP2A enzymes are heterotrimers (PP2AT) consisting of a core dimer (PP2AD) encompassing a structural A and a catalytic C subunit that is associated with one regulatory B-type subunit. The B-type subunits critically control PP2A activity by regulating substrate selectivity and/or directing enzyme localization within cells (1–3). Four families of B-type subunits have been identified: PR55/B (B55), PR61/B’ (B56), PR72/B” and striatins/SG2NA/B”’. Within each family, distinct (alternatively spliced) genes encode various structurally related isoforms, which—combined with two isoforms of both A and C—results in a variety of holoenzymes, all theoretically exhibiting distinct functions (1–3). However, despite substantial efforts, specific holoenzymes involved in a particular physiological process often remain elusive, and it is far from clear whether related subunits may perform redundant functions.
In the current study, we generated KO mice deficient for the B-type subunit PR61/B’δ (also called B56δ). PR61/B’δ represents the longest isoform (74 kDa) of the PR61/B’ class (6, 7) and is ubiquitously expressed, with highest abundance in brain and embryo (7). In contrast to PP2ACα KO mice, mice lacking PR61/B’δ are viable without an obvious phenotype early in life. However, because of the high expression in brain in wild type (WT), a neural phenotype could be expected in the KO, and this was further analyzed. In addition to some general functional redundancies, our findings demonstrate an indirect and spatially restricted role for PP2AT61δ in tau phosphorylation homeostasis, implying PP2A B-type subunits exert specific nonredundant functions in vivo.
Results
PR61/B’δ-Null Mice are Viable and Fertile.
Mice lacking the PR61/B’δ gene (Ppp2r5d) were generated by homologous recombination (Fig. S1). Loss of protein expression was confirmed with a specific PR61/B’δ antibody (7) in liver, brain, and heart lysates (Fig. S1). Despite high PR61/B’δ levels during embryogenesis in WT (7), PR61/B’δ-/- mice turned out to be viable. Breeding of PR61/B’δ+/- mice resulted in Mendelian ratios of progeny without sexual bias, and litter sizes of KOxKO and WTxWT matings were comparable (7.55 vs 6.83 pups/litter, p < 0.05). Placental sections of pregnant females from KOxKO matings showed normal decidualization and implantation with differentiated trophoblasts (Fig. S2). No significant changes were observed in expression of PR61/B’α/γ2/γ3/ϵ, PR55/Bα, PR130/B”α1, A or C subunits in total extracts of KO liver, heart, and brain at three months (Fig. S3).
Localization of PR61/B’δ in Neural Tissues and PC-12 Cells.
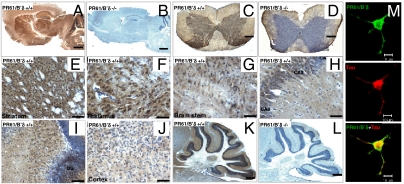
Immunohistochemical (IHC) stainings with the PR61/B’δ antibody (7) revealed neuronal, cytoplasmic expression in most brain areas (Fig. 1A) with highest expression in striatum (Fig. 1E) and thalamus (Fig. 1F), intermediate expression in brain stem (Fig. 1G), CA1, CA2, CA3 regions (Fig. 1H) and dentate gyrus of hippocampus (Fig. 1I), and weak expression in cortex (Fig. 1J) and cerebellum (Fig. 1K). These patterns generally match well with previous in situ hybridization data (7). No staining was detected in corresponding KO brain areas (Fig. 1B and 1L), further demonstrating antibody specificity. Expression of PR61/B’δ was also seen in spinal cord (Fig. 1C) and again absent in the KO (Fig. 1D). Immunofluorescence (IF) demonstrated PR61/B’δ localization in cytoplasm, nucleus (weak), and dendrites of neuronally differentiated PC-12 cells, along partial colocalization with F-actin, microtubuli, and tau (Fig. 1M).
Fig. 1.
PR61/B’δ localization in brain, spinal cord, and PC-12 cells. Immunohistochemistry staining (3-step procedure) of PR61/B’δ in WT brain (A) and spinal cord (C). Brain (B) and spinal cord (D) of PR61/B’δ KO mice are shown as control. Detailed expression of PR61/B’δ in WT striatum (E), thalamus (F), brain stem (G), CA1, 2 and 3 regions (H) and dentate gyrus (DG) of hippocampus (I), cortex (J), and cerebellum (K). KO cerebellum is shown as control (L). Nuclei are counterstained with haematoxylin (blue). Scale bars: 1 mm (A–D, K, and L); 50 μm (E–J). (M) Immunofluorescence showing colocalization of PR61/B’δ (green) and tau (red) in NGF-differentiated PC12 cells (Scale bar: 10 μm).
Progressive Tau (Hyper)Phosphorylation in Brain and Spinal Cord of PR61/B’δ KO Mice.
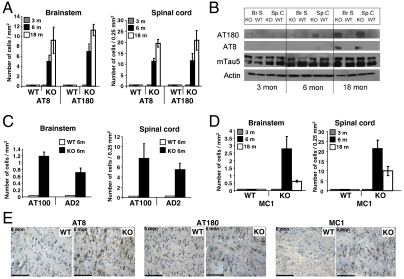
Tau binds and stabilizes microtubules depending on its phosphorylation state (8). Because PP2A is a well-established tau phosphatase in vivo (4), capable of dephosphorylating specific sites in vitro and in situ (9–12), we monitored tau phosphorylation in PR61/B’δ KO mice using phosphorylation-sensitive monoclonal antibodies AT8 (Ser202/Thr205), AT180 (Thr231), AT100 (Thr212/Ser214), and AD2 (Ser396/Ser404). These immunostainings indicated phosphorylation at AT8 and AT180 epitopes in brain stem and dorsal horn of the cervical spinal cord of six-month-old KO mice, whereas no or very little immunoreactivity was detected in age-matched WT controls (Fig. 2A and Fig. S4). In younger mice ( three months) no enhanced tau phosphorylation was observed (Fig. 2A and Fig. S4). Because it is known from transgenic models tau phosphorylation increases with age (13), we also performed IHC studies with 18-month-old mice. Aging did not only correlate with increased tau hyperphosphorylation in brainstem and spinal cord (Fig. 2A and Fig. S4), it also resulted in a broader distribution of this phenotype as these mice also displayed weak tau phosphorylation in subiculum, lateral dentate cerebellar nucleus, and cortex (very weak). Western blotting confirmed increased AT8/AT180 immunoreactivity in brain stem and spinal cord of 18-month-old KO mice, while total tau levels did not significantly change with age (Fig. 2B). Staining with AT100, recognizing a phospho-epitope preceding neurofibrillary tangle (NFT) formation (14), was positive in brain stem and spinal cord of six-month-old KO mice (Fig. 2C and Fig. S4) but did not increase with age. Similar observations were made for AD2, recognizing phospho-Ser396/Ser404 (Fig. 2C and Fig. S4). AT100 and AD2 Western blots were negative. In addition, cytoplasmic MC1 staining, recognizing a conformational tau epitope found in AD (15), was detected again in brain stem and spinal cord of six-month-old KO mice, while it decreased at 18 months (Fig. 2D and Fig. S4) and was absent in WT mice. Because tau conformation defined by MC1 highlights transition from soluble to filamentous tau (13, 15), these data indicate that depending on age, tau is in a hyperphosphorylated (AT8, AT180 and less AT100, AD2), structurally different (MC1) state in the KO. Despite these indications, tau did not aggregate into filaments or tangles in older mice because CongoRed/X34 and Bielschowsky staining did not reveal an associated NFT pathology (Fig. S4). TUNEL staining did not indicate any apoptotic cell death (Fig. S4). NFT absence might be explained by physiological clearance of MC1-positive tau by the protective chaperone-tau processing pathway (16). Chaperones HSP70 and HSP90 are indeed significantly elevated in brain stem and spinal cord of six-month-old KO mice as compared to WT, while this is not the case in older mice (Fig. S5).
Fig. 2.
Age-related tau hyperphosphorylation and misfolding in brain stem and spinal cord of PR61/B’δ KO mice. (A) Immunohistochemistry (IHC) with AT8 (Ser202/Thr205) and AT180 (Thr231) showing progressive tau phosphorylation in brain stem (left) and dorsal horn of the cervical spinal cord (right) of PR61/B’δ KO mice. (B) Western blot of brain stem and spinal cord lysates (n = 5) developed with the indicated antibodies. (C) IHC with AT100 (Ser212/Ser214) and AD2 (Ser396/Ser404) at six months in brainstem (left) and spinal cord (right). (D) IHC with MC1 (recognizing misfolded tau) in KO and age-matched WT brain stem (left) and spinal cord (right). Only cytoplasmic tau stainings were quantified (A, C, and D) with Leica QWin V2.8 software. (E) Representative IHC images from brainstem at six months (for complete overview, see Fig. S4). Scale bars: 100 μM.
Phenotypes of Tau-Overexpressing PR61/B’δ KO (TKO) Mice.
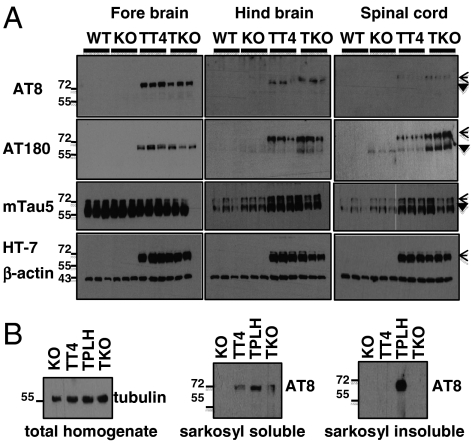
We crossed PR61/B’δ KO with hTau4 (TT4+/-) mice (17), mildly expressing WT four-repeat human tau in all neurons (TKO mice) (Fig. S6). In contrast to mutant tau transgenic mice (13, 14), TT4+/- mice do not show NFT pathology but a milder phenotype, characterized by progressive tau phosphorylation at specific pathological epitopes throughout the entire brain (17). Thus, we could ask whether and where deletion of PR61/B’δ in this model would be able to aggravate the TT4+/- phenotype and potentially lead to NFT. AT8 and AT180 Western blots showed again increased tau phosphorylation specifically in hindbrain and spinal cord lysates of 10-month-old TKO mice (Fig. 3A), thus confirming that even in the presence of overexpressed tau, PR61/B’δ absence fails to result in a forebrain tau pathology. Likewise, NFT are still absent in TKO mice as witnessed by CongoRed/X34 and absence of AT8 immunoreactivity in the sarkosyl-insoluble fraction of total TKO brain lysates (Fig. 3B). The latter was only detected in Tau-P301L (TPLH) transgenic mice, as previously reported (13) (Fig. 3B).
Fig. 3.
Tau phosphorylation in TKO mice. (A) Forebrain, hindbrain (brain stem/cerebellum), and spinal cord extracts of three independent WT, KO, TT4, and TKO mice (10 months) subjected to Western blotting with the indicated antibodies. Open arrowheads: transgenic human tau; filled arrowheads: endogenous tau. (B) Western blots with the indicated antibodies of total brain lysates and sarkosyl (in)soluble fractions of these lysates from KO, TT4, TKO, and TPLH mice.
Behavioral Testing of PR61/B’δ KO and TKO Mice.
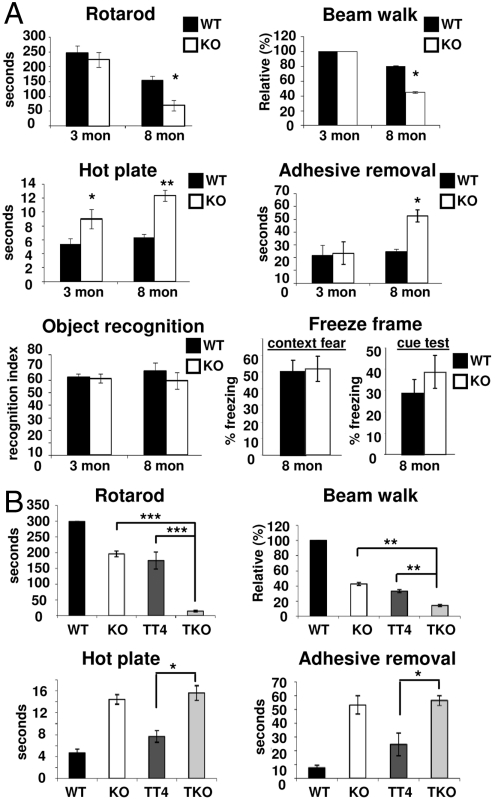
We examined three- and eight-month-old KO mice, alongside 10-month-old TKO mice for (sensori)motor and cognitive deficits. Motor coordination, evaluated by beam walk and rotarod tests, exhibited poor performance for eight-month-old KO mice in comparison with three-month-old KO and age-matched WT mice (Fig. 4A), correlating well with the age-dependent occurrence of tauopathy. This was further confirmed in TKO mice, where these defects were more than additively affected compared to the single KO or TT4+/- background (Fig. 4B). Average reaction time on the hot plate was significantly delayed in both KO age groups compared to WT controls (Fig. 4A), indicative of decreased nociception independently of the tau phenotype. This was confirmed in TKO mice, where no additional defects were observed compared to KO mice (Fig. 4B). In adhesive removal tests, only eight-month-old KO mice took more time to remove the adhesive sticker than WT mice (Fig. 4A), but this was not further affected in TKO mice (Fig. 4B). No significant differences between KO or WT animals could be observed in freeze frame and object recognition tests (Fig. 4A).
Fig. 4.
Behavior tests in age- and gender-matched WT, KO, TT4, and TKO mice. (A) In three- or eight-month-old PR61/B’δ KO mice compared to WT (n = 6 for each condition). (B) In 10-month-old TKO mice compared to TT4, KO, and WT (n = 6 for each condition). Statistical significance was evaluated with Student’s t-test (* : p < 0.05; ** : p < 0.01; *** : p < 0.001).
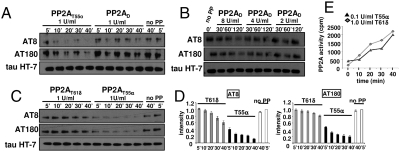
Poor Direct Tau Dephosphorylation by PP2AT61δ.
Although it is generally believed that PP2AT55 is the likely in vivo tau phosphatase (11, 12), the simplest explanation for increased AT8/AT180 phosphorylation in the KO would be lack of direct dephosphorylation by PP2AT61δ. To test this, we exploited tau heat stability to isolate AT8/AT180-positive tau from TPLH mice (13) in the absence of phosphatase inhibitors. Phosphatase assays with de novo purified PP2AT55 and PP2AD (18) on this substrate confirmed that PP2AT55 is by far the better S/T-P site phosphatase (19) (Fig. 5A), even if eightfold more PP2AD is used (Fig. 5B). In addition, by using carefully equilibrated amounts of PP2AT55α and PP2AT61δ retrieved from COS7 cells expressing PR55/Bα and PR61/B’δ GST fusion proteins (20), PP2AT55α proved at least 15-fold better in dephosphorylating AT8 and AT180 than PP2AT61δ (Fig. 5 C and D). We confirmed this with recombinant [32P]-tau in vitro labeled with CDK2/cyclinA at AT8 and AT180 epitopes (21). In this case PP2AT55α was ±eightfold better than PP2AT61δ (Fig. 5E) as 10-fold less units of PP2AT55α dephosphorylate tau with almost equal velocity. Thus, in the presence of PP2AT55, the absence of PP2AT61δ in vivo is unlikely to cause tau phosphorylation by lack of direct dephosphorylation.
Fig. 5.
In vitro tau dephosphorylation with various PP2A holoenzymes. (A) Equilibrated amounts of de novo purified PP2AD and PP2AT55 were incubated with phospho-tau isolated from TPLH mice for the indicated times. Dephosphorylation was monitored with AT8 and AT180 antibodies. (B) Same experiment with different amounts of PP2AD (units indicated). (C) Same experiment with PP2AT55α and PP2AT61δ isolated from GST-PR55α and GST-PR61δ expressing COS7 cells. Normalized quantifications in D. (E) [32P]-tau, in vitro labeled by CDK2/cyclinA was incubated with the indicated amounts of PP2AT61δ or PP2AT55α. At several time points aliquots were taken and TCA soluble [32P] measured.
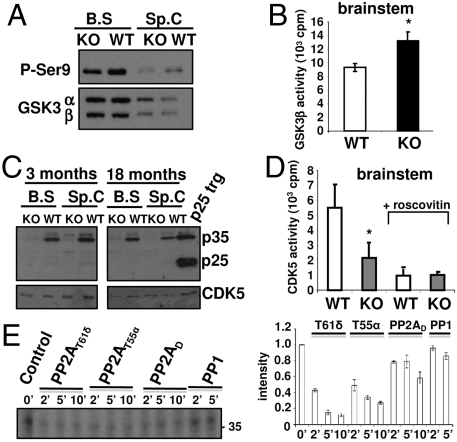
Increased GSK3β Activity in PR61/B’δ KO Mice.
Because many kinases are regulated by PP2A (2), we wondered whether tau phosphorylation might be indirectly the consequence of an effect of PP2AT61δ on the major in vivo tau AT8/AT180 kinase, GSK3β (8, 22). We observed decreased phosphorylation of the inhibitory GSK3β Ser9 site in KO brain stem/spinal cord, without a change in total GSK3β levels (Fig. 6A). Consequently, GSK3β activity measured with a specifically primed peptide (23) in GSK3β immunoprecipitates (IPs) from brain stem extracts, showed a 30% increase in the KO (Fig. 6B), providing a reasonable explanation for the observed tau hyperphosphorylation. While GSK3β was activated, another potential tau kinase, p42/p44 ERK, did not show any significant activity change in the affected KO brain regions (Fig. S7).
Fig. 6.
GSK3β and CDK5 status in PR61/B’δ KO brainstem and spinal cord. (A) Western blot of brain stem and spinal cord extracts of 18-month-old mice (n = 5) showing decreased GSK3β Ser9 phosphorylation in KO mice. (B) Increased activity in GSK3β IPs from brain stem extracts of 18-month-old KO mice (n = 5) measured with phospho-glycogen synthase peptide 2 substrate. (C) Western blot showing highly reduced levels of p35 in brain stem and spinal cord of three- and 18-month-old KO mice. Positive control: brain extract of p25 transgenic (trg) mice (42). (D) Decreased activity in CDK5 IPs from brain stem extracts of six-month-old KO mice (n = 5) measured with Histone IIA as substrate and roscovitin (10 μM) as specificity control. (E) [32P]-p35, in vitro labeled by CDK2/cyclinA was incubated for the indicated times with PP2AT61δ, PP2AT55α, PP2AD, or PP1 (all 1 U/ml). Dephosphorylation was monitored by autoradiography following SDS/PAGE and quantified (averages of two independent experiments).
Decreased CDK5 Activity in PR61/B’δ KO Mice.
Because decreased phosphorylation of GSK3β Ser9 was observed in the KO, this site cannot be the direct target of PP2AT61δ. In the absence of an effect on Akt phosphorylation (Fig. S7), we analyzed activity of another proposed upstream GSK3β Ser9 kinase, the brain-enriched CDK5 (8, 24, 25). While total levels of CDK5 were similar in brain stem and spinal cord of WT and KO mice (Fig. 6C), less than 50% of roscovitin-sensitive histone kinase activity could be measured in CDK5 IPs from KO vs. WT brain stem (Fig. 6D). Surprisingly, this was due to nearly complete absence of the CDK5 activator p35 (as well as its calpain-generated fragment p25) (Fig. 6C). Notably, p35 levels and GSK3β Ser9 phosphorylation were normal in lysates of KO cortex and cerebellum (Fig. S8). This was further confirmed by p35 IHC (Fig. S8). p35 changes are probably due to increased protein degradation, because no quantitative differences in CDK5, p35, or p39 mRNA levels were found by quantitative PCR. Interestingly, previous studies suggested p35 phosphorylation by CDK5 or other kinases regulates its proteasomal degradation, and this is stimulated by okadaic acid (OA), a general PP2A inhibitor (26–28). To test whether PP2AT61δ might act as a p35 phosphatase, we subjected in vitro CDK2/cyclinA-phosphorylated p35, expressed and purified from bacteria, to dephosphorylation with equal amounts (1 U/ml) of several OA-sensitive phosphatases (Fig. 6E). In contrast to CDK2/cyclinA-phosphorylated tau (Fig. 5), p35 was a good in vitro PP2AT61δ substrate as this holoenzyme dephosphorylated p35 with at least equal or even better velocity than PP2AT55, and was significantly better than PP2AD (Fig. 6E). PP1 hardly dephosphorylated p35 at all (Fig. 6E), confirming the OA effect on p35 stability (27, 28) is due to inhibition of a PP2A phosphatase.
Spatio-Temporal Changes in PR55/Bα and PR61/B’δ Expression in WT and KO Brain.
To further underscore the model emerging from our biochemical data, we wondered whether tauopathy might be correlating with spatio-temporal changes in PP2A subunit expression, focused on PR61/B’δ and PR55/Bα. We found higher absolute levels of PR61/B’δ in WT cortex vs. WT brain stem of young mice and decreased expression upon aging in the cortex but not in brain stem (Fig. S9). In contrast, we found higher PR55/Bα expression in cortex upon aging but not in brain stem (Fig. S9). Finally, PR55/Bα expression in a given brain area and age group did not significantly differ between WT and KO (Fig. S9), suggesting no additional aberrancies due to PR61/B’δ absence.
Discussion
In this study, we present the murine KO of PR61/B’δ, a member of PR61/B’, the largest PP2A B-type subunit family with multiple roles in cellular signaling (2, 29, 30). Despite high embryonic PR61/B’δ expression in WT mice (7), PR61/B’δ KO mice are viable and fertile, with normal placental development and trophoblast differentiation. Because the reported PP2AT61δ function in dephosphorylation of developmental transcription factor HAND1 specifically is suppressed during trophoblast differentiation (31), this finding was thus not so surprising. Specifically PR61/B’δ also dephosphorylates the Cdc25 Thr138 site to control mitosis (32), but how this might occur in the KO remains unclear given no overt growth abnormalities were observed. Notably, a functional compensation exists for this dephosphorylation as overexpression of Wee-1 (the Cdc25 opposing kinase) was observed in PR61δ KO MEFs (33). Recently, a role was identified specifically for PR61/B’δ in mast cell degranulation (34), but if and how this is affected in the KO remains to be defined.
Given high expression of PR61/B’δ in brain (7), we focused on neural phenotypes in the KO and found evidence for tauopathy, characterized by tau hyperphosphorylation at distinct pathological sites (AT8, AT180, AT100, and AD2) and an altered, pretangle conformation defined by MC1. Remarkably, this tauopathy is spatio-temporally restricted: It localizes in brain stem and the dorsal horn of the cervical spinal cord and becomes evident at six months, subsequently spreading to subiculum, cerebellum, and cortex in 18-month-old mice. The MC1 epitope, highlighting tau misfolding, is one of the earliest pathological alterations in AD tau and absent in normal brain (35). Despite MC1-positivity, we did not observe any NFT pathology, further underscoring that tau hyperphosphorylation and/or misfolding do not necessarily result in NFT formation (36). Our observation of an earlier (six months) increase in HSP90/70 expression in the KO supports the hypothesis that these chaperones may be involved in clearance of MC1-positive tau at that age, thus possibly preventing NFT in older mice, as reported by others (16).
The restricted distribution of tau phosphorylation in the KO, further substantiated by the very same spatial pattern of increased tau phosphorylation in TKO mice, is intriguing given that PR61/B’δ is broadly expressed throughout WT brain (Fig. 1). One might explain this by functional redundancy in other brain regions by other PR61/B’ and possibly PR55/B subunits, most of which are present in brain (7, 37, 38). We did however not notice any significant compensatory changes in expression of several B-type subunits in total brain extracts of young KO mice (three months), nor in PR55/Bα expression in cortex or brainstem of three- or 20-month-old KO mice. Thus potential redundancies in nonaffected KO brain regions might occur by other mechanisms (through other PP2A subunits, other Ser/Thr phosphatases, or even through protein kinases) or are not exemplified by compensatory expression but occur at a functional level. Although it is hardly possible in a biological model as complex as a whole organism to obtain a comprehensive picture of all such possible redundancies, our current and previously published (33) data confirm the existence of general functional compensations. Nonetheless, the pathology in brain stem/spinal cord of aged mice also favors the view of nonredundant functions of PR61/B’δ. Our data indicate preferential rather than exclusive dephosphorylation of substrates by PP2AT61δ, exemplified by our biochemical assays in which PP2AT61δ proved only slightly more efficient than PP2AT55α in dephosphorylating p35 and further underscored by a redundant role of PR61/B’δ in neurotrophin receptor signaling (39). Only if redundant mechanisms entirely or partially fail, are absent, or spatially restricted, a phenotype such as phosphorylated, pretangle tau may appear. Obviously, such observations are difficult to make or reproduce in vitro and thus highlight the importance of in vivo models to understand a protein’s true physiological role.
One of the affected pathways in the KO unexpectedly involves CDK5/p35 signaling. We indeed observed a dramatic decrease in p35, specifically in the affected brain regions, probably via increased proteasomal degradation, although we cannot exclude translation is also affected. Previous studies have suggested phosphorylation regulates p35 degradation and this is stimulated by OA (26–28). Our results would fit into this concept, identifying PP2AT61δ as a major p35 phosphatase in brain stem/spinal cord: In the absence of PR61/B’δ and presence of normal PR55/Bα levels (Fig. S9), dephosphorylation is hampered resulting in increased p35 degradation. This is sustained by our in vitro assays, showing that in contrast to tau, which is severalfold better dephosphorylated by PP2AT55, p35 is a relatively much better PP2AT61δ substrate. Although CDK5 has been repeatedly reported as a tau kinase, CDK5 deregulation resulting in NFT in vivo is rather linked to pathological generation of p25, a calpain-dependent proteolytic p35 fragment (40) that is absent in the affected KO brain regions. Decreased CDK5 activity in the KO is in any case inconsistent with CDK5 directly phosphorylating the pathological tau epitopes but concurs with transgenic mice in which overexpression of CDK5 and/or p25 does not lead to AT8/AT180 hyperphosphorylation (41, 42). Similarly, CDK5 KO mice die perinatally with increased phosphorylation of neurofilaments in brain stem (43). These observations add to the controversy whether CDK5, activated by p35, can act as a physiological tau kinase in vivo (8) and rather support CDK5 is a “negative tau kinase” indirectly affecting tau phosphorylation through control of GSK3β (8, 24, 25, 41). Our results would sustain this, because we observed decreased GSK3β Ser9 phosphorylation and subsequent kinase activation, reminiscent of the overt GSK3β activation in p35 KO mice (44). As GSK3β is an established tau AT8/AT180 kinase in vivo (8, 22, 41, 45, 46) this elevated GSK3β activity is the likely cause of tau hyperphosphorylation in the KO. Exactly how CDK5 promotes GSK3β Ser9 phosphorylation remains elusive and may be achieved through direct as well as indirect mechanisms—for instance by controlling PP1 activity (24, 47). By no means, however, do our data support that PP2AT61δ is a GSK3β Ser9 phosphatase, opposing recent findings in a genomic poorly characterized gene trap model (48). Whether PR61/B’δ-mediated control of p35 stability has wider implications on other CDK5 substrates, such as the predicted PP2AT61δ substrate DARPP-32 (49, 50) awaits further exploration. Likewise, at least one behavioral defect—the increased latency on a hot plate—might be related to the role of CDK5 in nociception (51). Our other behavioral studies provided clear support for impairment of some (sensori)motor and coordination functions in the KO (rotarod/beamwalk). In these cases a causal relationship with tauopathy is suggested by the age-dependency of the defects in the KO and the more than additive defects in TKO mice. Such a relationship would also imply that tauopathy can result in motoric defects in the absence of NFT or neurodegeneration—for instance by affecting proper neuronal function. Finally, cognitive functions in the KO appeared normal, consistent with absence of tauopathy in areas such as hippocampus.
Several reports have highlighted PP2A impairment is clinically relevant in AD. In addition to decreased enzyme activity, mRNA levels of several PP2A subunits are significantly decreased in AD brain (52, 53). In transgenic mice expressing a dominant-negative PP2AC mutant (L199P), tau is hyperphosphorylated at AT8 and Ser422 (54). AT8 phosphorylation also occurred in mice expressing PP2AC L309A (55), specifically deficient in PR55/B recruitment (1). Although it is generally believed and confirmed here that PP2AT55 is the major tau phosphatase (9–12, 56) and PP2AT55α protein levels are reduced in AD brain (53), PR61/B’ expression can be equally well affected (52), so far with unclear consequences. Based on the present study it is tempting to speculate that specifically PR61/B’δ may be involved early in the disease etiology, not necessarily by a direct mechanism, but by maintaining a proper kinase-phosphatase balance.
Materials and Methods
Protocols for the generation of PR61/B’δ KO and TKO mice and for behavior tests can be found in SI Materials and Methods. Tissue extracts and sarkosyl (in)soluble tau were prepared as described (13). Procedures for brain/spinal cord collection, IHC and CongoRed/X34 staining were as described (13, 42). Primary antibodies can be found in Table S1. PC12 IF and protocols for kinase and phosphatase assays are given in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We highly appreciate technical help of M. Veeckmans and R. Verbiest. We acknowledge B. Hemmings, S. Dilworth, P. Davis, and J. Vandenheede for antibodies; M. Beullens for PP1; and A. Goris for help with qPCR. C.L. received a fellowship from the Flemish Agency for Innovation by Science and Technology. This work was supported by the Flemish government (GOA/2008/16), Belgian Science Policy (IAP P6/28), Research Foundation–Flanders (G.0529.06), and National Health and Medical Research Council Australia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018777108/-/DCSupplemental.
References
- 1.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: In cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Janssens V, Goris J, Van Hoof C. PP2A: The expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Tian Q, Wang J. Role of serine/threonine protein phosphatase in Alzheimer’s disease. Neurosignals. 2002;11:262–269. doi: 10.1159/000067425. [DOI] [PubMed] [Google Scholar]
- 5.Götz J, Probst A, Ehler E, Hemmings B, Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proc Natl Acad Sci USA. 1998;95:12370–12375. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- 7.Martens E, et al. Genomic organisation, chromosomal localisation tissue distribution and developmental regulation of the PR61/B’ regulatory subunits of protein phosphatase 2A in mice. J Mol Biol. 2004;336:971–986. doi: 10.1016/j.jmb.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 9.Goedert M, Cohen ES, Jakes R, Cohen P. p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer’s disease. FEBS Lett. 1992;312:95–99. doi: 10.1016/0014-5793(92)81418-l. [DOI] [PubMed] [Google Scholar]
- 10.Drewes G, et al. Dephosphorylation of tau protein and Alzheimer paired helical filaments by calcineurin and phosphatase-2A. FEBS Lett. 1993;336:425–432. doi: 10.1016/0014-5793(93)80850-t. [DOI] [PubMed] [Google Scholar]
- 11.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 12.Sontag E, et al. Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J Biol Chem. 1999;274:25490–25498. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- 13.Terwel D, et al. Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. J Biol Chem. 2005;280:3963–3973. doi: 10.1074/jbc.M409876200. [DOI] [PubMed] [Google Scholar]
- 14.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 15.Jicha GA, Bowser R, Kazam IG, Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res. 1997;48:128–132. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Dickey CA, et al. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. FASEB J. 2006;20:753–755. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- 17.Spittaels K, et al. Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am J Pathol. 1999;155:2153–2165. doi: 10.1016/S0002-9440(10)65533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waelkens E, Goris J, Merlevede W. Purification and properties of polycation-stimulated phosphorylase phosphatases from rabbit skeletal muscle. J Biol Chem. 1987;262:1049–1059. [PubMed] [Google Scholar]
- 19.Agostinis P, Derua R, Sarno S, Goris J, Merlevede W. Specificity of the polycation-stimulated (type-2A) and ATP,Mg-dependent (type-1) protein phosphatases toward substrates phosphorylated by p34cdc2 kinase. Eur J Biochem. 1992;205:241–248. doi: 10.1111/j.1432-1033.1992.tb16774.x. [DOI] [PubMed] [Google Scholar]
- 20.Longin S, et al. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic subunit. J Biol Chem. 2007;282:26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- 21.Amniai L, et al. Alzheimer disease specific phosphoepitopes of Tau interfere with assembly of tubulin but not binding to microtubules. FASEB J. 2009;23:1146–1152. doi: 10.1096/fj.08-121590. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Paudel HK. Glycogen synthase kinase 3beta phosphorylates Alzheimer’s disease-specific Ser396 of microtubule-associated protein tau by a sequential mechanism. Biochemistry. 2006;45:3125–3133. doi: 10.1021/bi051634r. [DOI] [PubMed] [Google Scholar]
- 23.Van Lint J, Khandelwal RL, Merlevede W, Vandenheede JR. A specific immunoprecipitation assay for the protein kinase FA/glycogen synthase kinase 3. Anal Biochem. 1993;208:132–137. doi: 10.1006/abio.1993.1018. [DOI] [PubMed] [Google Scholar]
- 24.Morfini G, et al. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen Y, et al. Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing. J Neurosci. 2008;28:2624–2632. doi: 10.1523/JNEUROSCI.5245-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:24057–24064. doi: 10.1074/jbc.273.37.24057. [DOI] [PubMed] [Google Scholar]
- 27.Saito T, et al. Okadaic acid-stimulated degradation of p35, an activator of CDK5, by proteasome in cultured neurons. Biochem Biophys Res Commun. 1998;252:775–778. doi: 10.1006/bbrc.1998.9739. [DOI] [PubMed] [Google Scholar]
- 28.Kerokoski P, Suuronen T, Salminen A, Soininen H, Pirttila T. Influence of phosphorylation of p35, an activator of cyclin-dependent kinase 5 (cdk5), on the proteolysis of p35. Mol Brain Res. 2002;106:50–56. doi: 10.1016/s0169-328x(02)00409-6. [DOI] [PubMed] [Google Scholar]
- 29.Yan L, et al. PP2A T61 epsilon is an inhibitor of MAP4K3 in nutrient signaling to mTOR. Mol Cell. 2010;37:633–642. doi: 10.1016/j.molcel.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Phiel C. Functions of B56-containing PP2As in major developmental and cancer signaling pathways. Life Sci. 2010;87:659–666. doi: 10.1016/j.lfs.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Firulli BA, et al. PKA, PKC, and the protein phosphatase 2A influence HAND factor function: A mechanism for tissue-specific transcriptional regulation. Mol Cell. 2003;12:1225–1237. doi: 10.1016/s1097-2765(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 32.Margolis SS, et al. Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell. 2006;127:759–773. doi: 10.1016/j.cell.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forester CM, Maddox J, Louis JV, Goris J, Virshup DM. Control of mitotic exit by PP2A regulation of Cdc25C and Cdk1. Proc Natl Acad Sci USA. 2007;104:19867–19872. doi: 10.1073/pnas.0709879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kranias G, et al. Protein phosphatase 2A carboxymethylation and regulatory B subunits differentially regulate mast cell degranulation. Cell Signal. 2010;22:1882–1890. doi: 10.1016/j.cellsig.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging. 2000;21:719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 36.Götz J, et al. Animal models reveal role for tau phosphorylation in human disease. Biochim Biophys Acta. 2010;1802:860–871. doi: 10.1016/j.bbadis.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Strack S, Zaucha JA, Ebner FF, Colbran RJ, Wadzinski BE. Brain protein phosphatase 2A: Developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998;392:515–527. [PubMed] [Google Scholar]
- 38.Schmidt K, et al. Diversity, developmental regulation and distribution of murine PR55/B subunits of protein phosphatase 2A. Eur J Neurosci. 2002;16:2039–2048. doi: 10.1046/j.1460-9568.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Kanegan MJ, Strack S. The protein phosphatase 2A regulatory subunits B’beta and B’delta mediate sustained TrkA neurotrophin receptor autophosphorylation and neuronal differentiation. Mol Cell Biol. 2009;29:662–674. doi: 10.1128/MCB.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MS, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 41.Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- 42.Muyllaert D, et al. Neurodegeneration and neuroinflammation in cdk5/p25-inducible mice: A model for hippocampal sclerosis and neocortical degeneration. Am J Pathol. 2008;172:470–485. doi: 10.2353/ajpath.2008.070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hallows JL, Chen K, DePinho RA, Vincent I. Decreased cyclin-dependent kinase 5 (cdk5) activity is accompanied by redistribution of cdk5 and cytoskeletal proteins and increased cytoskeletal protein phosphorylation in p35 null mice. J Neurosci. 2003;23:10633–10644. doi: 10.1523/JNEUROSCI.23-33-10633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spittaels K, et al. Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J Biol Chem. 2000;275:41340–41349. doi: 10.1074/jbc.M006219200. [DOI] [PubMed] [Google Scholar]
- 46.Lucas JJ, et al. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen C, et al. Regulation of protein phosphatase inhibitor-1 by cyclin-dependent kinase 5. J Biol Chem. 2007;282:16511–16520. doi: 10.1074/jbc.M701046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapfhamer D, et al. Protein Phosphatase 2a and glycogen synthase kinase 3 signaling modulate prepulse inhibition of the acoustic startle response by altering cortical M-Type potassium channel activity. J Neurosci. 2010;30:8830–8840. doi: 10.1523/JNEUROSCI.1292-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahn JH, et al. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci USA. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stipanovich A, et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pareek TK, et al. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc Natl Acad Sci USA. 2006;103:791–796. doi: 10.1073/pnas.0510405103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 53.Sontag E, et al. Altered expression levels of the protein phosphatase 2A ABαC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 54.Kins S, et al. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001;276:38193–38200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- 55.Schild A, Ittner LM, Götz J. Altered phosphorylation of cytoskeletal proteins in mutant protein phosphatase 2A transgenic mice. Biochem Biophys Res Commun. 2006;343:1171–1178. doi: 10.1016/j.bbrc.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 56.Xu Y, Chen Y, Zhang P, Jeffrey PD, Shi Y. Structure of a protein phosphatase 2A holoenzyme: Insights into B55-mediated Tau dephosphorylation. Mol Cell. 2008;31:873–885. doi: 10.1016/j.molcel.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.