Abstract
Human aging is accompanied by dramatic changes in daily sleep–wake behavior: Activity shifts to an earlier phase, and the consolidation of sleep and wake is disturbed. Although this daily circadian rhythm is brain-controlled, its mechanism is encoded by cell-autonomous circadian clocks functioning in nearly every cell of the body. In fact, human clock properties measured in peripheral cells such as fibroblasts closely mimic those measured physiologically and behaviorally in the same subjects. To understand better the molecular mechanisms by which human aging affects circadian clocks, we characterized the clock properties of fibroblasts cultivated from dermal biopsies of young and older subjects. Fibroblast period length, amplitude, and phase were identical in the two groups even though behavior was not, thereby suggesting that basic clock properties of peripheral cells do not change during aging. Interestingly, measurement of the same cells in the presence of human serum from older donors shortened period length and advanced the phase of cellular circadian rhythms compared with treatment with serum from young subjects, indicating that a circulating factor might alter human chronotype. Further experiments demonstrated that this effect is caused by a thermolabile factor present in serum of older individuals. Thus, even though the molecular machinery of peripheral circadian clocks does not change with age, some age-related circadian dysfunction observed in vivo might be of hormonal origin and therefore might be pharmacologically remediable.
Keywords: chronobiology, peripheral oscillators, human behavior
Circadian clocks possess an endogenous periodicity of about 24 h and play a key role in physiological adaptation to the solar day for all living organisms, from cyanobacteria and fungi (1) to insects (2) and mammals (3). Circadian clocks influence nearly all aspects of physiology and behavior, including sleep–wake cycles, body temperature, and the function of many organs (3). During normal aging, clock function is attenuated, with consequences both for health and quality of life. Older individuals have an earlier phase of everyday activity compared with the young (4). Not only is the consolidation of sleep and wake dramatically reduced (5, 6), but overall circadian amplitude of hormones and body temperature are lower (7, 8), and many aging-associated sleep–wake pathologies have been reported (9–11). As a result, one in five healthy older individuals reports taking sleep medications regularly (9). In cases of pathological aging, chronobiological disturbance is even more acute: Huntington disease, Parkinson disease, and Alzheimer's disease are all associated with profound alterations in sleeping patterns (10–12). These effects of aging on circadian rhythms—diminished circadian amplitude, earlier phase, shorter circadian period, and desynchronization of rhythms in peripheral organs—have been observed widely in several species of mammals (7, 13, 14). Paradoxically, however, even though the behavioral phase is earlier in aged humans, multiple studies conclude that the free-running period remains unchanged (15–18). Thus, changes in phase have been ascribed to alterations in overall circadian amplitude and changed sleep architecture. However, the nature and mechanism of these changes remains entirely unknown.
Mammalian circadian clocks are organized in a hierarchical fashion: The suprachiasmatic nuclei (SCN) of the anterior hypothalamus serve as a master clock, receiving light signals from the external environment via the retina and retino-hypothalamic tract and elaborating these stimuli into signals that are sent all over the body to synchronize clocks in peripheral organs (3). Interestingly, the clock mechanism itself is cell-autonomous and involves interlocked feedback loops of transcription and translation. These loops are encoded by dedicated clock genes: For example, in one loop the heterodimer formed by the two transcription factors CLOCK and BMAL1 binds cis-acting E-box sequences present in Per and Cry gene promoters to activate their transcription. Subsequently, PER and CRY protein complexes inhibit the activity of CLOCK–BMAL1. As a consequence, Cry and Per mRNAs decrease in concentration, and a new cycle can start (19).
At a cellular level, the SCN and peripheral oscillators share the same molecular mechanism (20). Thus, cellular reporters composed of clock gene promoters driving expression of luciferase or GFP have proven to be very useful tools for the study of circadian rhythms in the SCN as well as in peripheral oscillators (21, 22). Using such reporters, we have shown previously that many differences in human circadian behavior also can be seen at a molecular level in peripheral cells. For example, the cellular clocks of early chronotypes (i.e., “larks”) have shorter circadian periods than those of later chronotypes (“owls”) (23), and circadian period length in vitro is proportional to physiological period in vivo (24). Under entrained conditions in which cellular clocks are constrained to 24 h via an entrainment protocol that mimics diurnal variations in mammalian body temperature (25), fibroblasts show the early or late circadian phases of their owners (23).
In principle, alterations in circadian behavior caused by aging could arise by a variety of mechanisms. Changing neural networks might perturb sleep–wake timing or alter the communication between the SCN clock and other brain regions. Hormonal signals critical for maintaining physiological homeostasis might be perturbed. On a cellular level, molecular changes associated with aging (e.g., oxidative damage, telomere attrition) might alter basic clock function. In this paper we have addressed the effects of aging on molecular circadian clock properties using a fibroblast-based assay. Our results are consistent with the hypothesis that the molecular machinery of circadian rhythms in peripheral oscillators is not altered by age but that molecules present in serum might be responsible for some of the circadian changes that occur in the elderly.
Results
Aging Changes Human Circadian Behavior in Vivo but Does Not Alter Fibroblast Circadian Clocks in Vitro.
To try to understand the molecular changes that might underlie modifications in daily behavior in elderly individuals, we characterized the circadian rhythms of dermal skin fibroblasts obtained from young and older donors. Subjects were recruited based on age but also were asked to give information about daytime preference (their preferred waking time and bedtime both on workdays and during leisure) by completing the Munich Chronotype Questionnaire (MCTQ) (26). The 18 young and 18 older sex-matched subjects participating in our study are summarized in Table S1 and are described individually in Table S2.
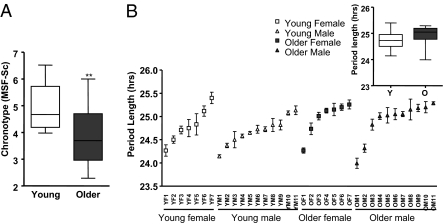
From the completed MCTQ, older subjects in our study displayed a significantly earlier sleep phase compared with young subjects (Fig.1A; unpaired t test; P < 0.01). This difference reflected well the epidemiological trend that is observed in the general population, e.g., as reported by Roenneberg and colleagues (27). To characterize possible cellular origins of these differences, two 2-mm dermal punch biopsies were taken from every subject. Primary fibroblast cultures were isolated from the biopsies and infected with a lentivirus that harbored a circadian reporter construct (the Bmal1 promoter driving expression of the firefly luciferase gene (28)). Circadian clocks in infected fibroblast cultures were synchronized with dexamethasone (29), and circadian bioluminescence corresponding to Bmal1 promoter activity was measured for at least 5 d under constant conditions in a cell-culture incubator. The circadian oscillations from fibroblasts from young and elderly subjects then were examined systematically for differences in period length, amplitude, and phase. It had been shown previously that chronotype correlates negatively with period length in vivo (30) and in vitro (23). Hence, if the origins of aging-related differences were cell intrinsic, we hoped to see correlations between clock properties in vitro and subject age.
Fig. 1.
Influence of age on period length and chronotype. (A) Chronotype of young and old subjects, as measured by the MCTQ. The y axis shows subject MSF-Sc. This statistic (the output of the MCTQ) is widely used as a reliable measure of human chronotype (27). Dataset variation is shown as a standard boxplot (n = 18; unpaired t test; **P < 0.01). (B) Period length of the primary fibroblasts of each subject participating in this study. For ease of display, data are sorted on the basis of the period length. Data are mean of six independent measurements of the period length for every subject ± SEM. (Inset) Population average of period lengths of skin fibroblasts from young (Y) and older (O) subjects, shown as a standard boxplot. No statistical difference was observed (n = 18; unpaired t test; P > 0.05).
The period length for each individual is shown in Fig. 1B. As we have reported for other subject populations (28), fibroblast period differed significantly among different individuals but not between different biopsies from the same individual (Fig. S1A). No differences were observed between the groups (Fig. 1B Inset; unpaired t test; P > 0.05; and Table S1). Additionally, no correlation was seen between period length and MCTQ sleep phase in either older or younger subjects (Fig. S1B; linear regression: P > 0.05). [Previous studies showing correlations between questionnaire-based sleep–wake behavior and period length were based on comparisons of extreme early vs. late chronotypes (23, 30).]
In addition to period length, other clock properties include amplitude (the difference between peak and nadir expression values) and phase (the relative timing of each cycle relative to a periodic entraining stimulus). We also studied the circadian amplitude of the oscillations that we observed in vitro. No correlation with aging was observed, nor did amplitude correlate with fibroblast cell passage number (i.e., a longer or shorter time in cultivation) (Fig. S2 A–C; unpaired t test; P > 0.05).
Theoretically and biologically, period and phase are tightly coupled: A longer period leads to a later phase. Under certain circumstances, amplitude and phase also are coupled, with lower amplitude leading to earlier phase (23). Nevertheless, phase differences also can be driven by rhythmic inputs from outside the circadian oscillator (e.g., by the timing of light to the retina in mammals). To measure circadian phase in fibroblasts in vitro, we entrained fibroblast clocks to a 24-h daily cycle using periodic oscillations of incubator temperature between 34 and 37 °C. After 6 d, fibroblast daily rhythms entrained well to these cycles in both young and old subjects regardless of their period lengths. On day 7, we measured the phase of reporter gene expression relative to the temperature cycle. An earlier phase was not observed in older vs. younger subjects (Fig. S2D; unpaired t test; P > 0.05), confirming the lack of effect of subject age on period and amplitude that we already had observed. In total, none of the physiological signs of human circadian aging could be detected or duplicated in cultured fibroblasts from elder subjects.
Human Sera Influence Fibroblast Circadian Period Length and Phase.
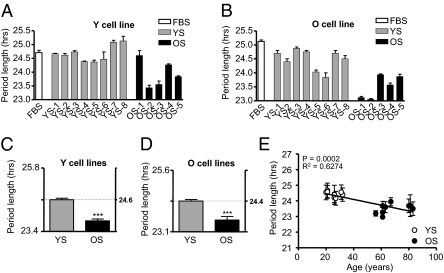
Even if the peripheral cells of elderly subjects do not differ from younger subjects in their chronobiological properties, it is well documented that the milieu in which these cells are found undergoes dramatic changes as individuals age (7, 31), and peripheral organs certainly show altered function with aging (7, 13, 14, 17, 32), If cellular circadian properties per se do not change with aging, we reasoned that age-related circadian alterations might be provoked by a circulating factor. To test this possibility, we replaced the normal standardized FBS used in our cell cultures with human serum harvested from donors of different ages. The circadian rhythms of four young (Y) and two old (O) cell lines were measured in the presence of eight different media containing human serum from young (YS) male donors and five different media containing human serum from three older male donors and two postmenopausal female donors (OS). Data regarding these blood donors are listed in Table S3. Fig. 2 A and B shows representative data from one Y and one O fibroblast cell line tested with all sera. (The complete data set is shown in Fig S3.) Fig. 2 C and D shows averages from all cell lines and all sera collectively. Irrespective of whether the treated fibroblasts were from young or older subjects, cells measured in serum from older donors had a significantly shorter circadian period than cells in serum from young donors (Fig. 2 C and D; unpaired t test; PY < 0.001; PO < 0.001). In the presence of YS, cells from young and older subjects showed a period length of 24.61 ± 0.18 h and 24.35 ± 0.18 h, respectively, whereas in the presence of OS cells from young and older subjects showed a period length of 23.79 ± 0.16 h and 23.60 ± 0.33 h, respectively. Analyzed individually, period measurements with different sera showed a strong correlation between donor age and period length (Fig. 2E; P < 0.0001; R2 = 0.91).
Fig. 2.
Length of circadian period of skin fibroblasts treated with media containing human serum. (A) Lengths of circadian period in one representative cell line taken from a young subject (Y) measured in medium containing FBS (white) and media containing human serum from eight young (YS; gray) and five older (OS; black) donors. Bars represent the mean of three independent measurements ± SEM. (B) Equivalent measurements from a representative cell line from an older subject (O). (C) Bar graph showing the average differences in period length in four Y cell lines treated with YS and OS. Results are expressed as average ± SEM. (D) Equivalent measurements for two O cell lines. In both cases, treatment with YS gave a highly different period length (unpaired t test; ***P < 0.001 compared with the treatment with OS in Y cell lines). (E) Average period length across experiments using human serum, as a function of the age of the serum donor (linear regression: P < 0.0002; r2 = 0.6274).
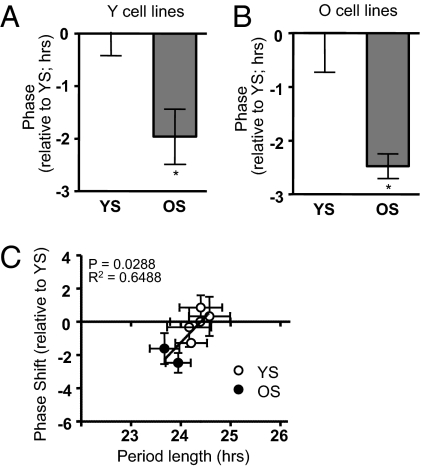
Because changes in period are coupled to changes in phase (as described above), to verify these results we wanted to ensure that the dramatic changes in circadian period that we observed were mirrored by corresponding changes in phase. Using the temperature entrainment paradigm described earlier to entrain clocks in all cells to rhythms of 24 h, we measured the circadian phase after temperature entrainment of two Y cell lines and two O cell lines in the presence of two YS and two OS. Serum from older subjects indeed phase-advanced the circadian rhythms of cells from both young (Fig. 3A) and older (Fig. 3B) subjects, compared with the same cells in YS (unpaired t test; P < 0.05), and there was a strong correlation between period length and phase shifting in individual sera (Fig. 3C; P = 0.0265; R2 = 0.66). Therefore, the shortened period observed with sera from older individuals manifested as earlier phase under entrained conditions, at least in cells.
Fig. 3.
Circadian phase of fibroblasts in the presence of serum from young and older subjects. (A) Phase of cell lines from young subjects (Y) was determined after temperature entrainment in the presence of serum from younger subjects (YS) or from older subjects (OS). Results are expressed as phase difference (in h) between the two treatments. Each bar results from the average of two different cell lines, each treated with two different sera, ± SEM. (B) Equivalent data using cell lines from older subjects. In both cases, there was a significant advance in the phase of cells treated with OS compared with the same cell lines treated with YS (unpaired t test; *P < 0.05). (C) Correlation between phase-shift differences seen in this figure and differences in period length seen in Fig. 2. Phase shifts are plotted relative to the average phase for all sera from young subjects (linear regression: P = 0.0265; r2 = 0.6488).
Influence of Human Serum on Period Length Is Caused by Heat-Sensitive Substances in Sera from Older Subjects.
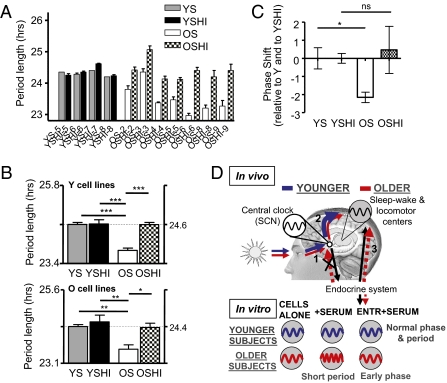
Our results suggested that one or more substances in human serum can recapitulate at a cellular level the differences in circadian phase seen between younger and older subjects. In principle, such effects could arise from substances either in YS or in OS. To investigate further the nature of the substance(s) responsible for the aging effects, we attempted to heat-inactivate four YS (YSHI) and four OS (OSHI). In this way the secondary structure of proteins and unstable metabolites would be destroyed, and a first indicator of molecular identity would be given. The circadian period length from four cell lines (two Y and two O) were analyzed in the presence of medium containing each of these sera. Individual values for each serum in a single cell line are shown in Fig. 4A. (The complete data set is shown in Fig S4.) Average values for cell lines from younger and older individuals are shown separately in Fig. 4B. Heat treatment had no effect on YS: Measurements from cells with YSHI were not different from measurements with YS [period Y = 24.61 ± 0.18 h (with YS) vs. 24.63 ± 0.27 h (with YSHI); period O = 24.35 ± 0.18 (with YS) vs. 24.50 ± 0.45 (with YSHI); PY > 0.05; PO > 0.05]. Nevertheless, measurements with OSHI were significantly longer than with OS and were not significantly different from those with YS [period Y = 23.79 ± 0.16 (with OS) vs. 24.60 ± 0.14 (with OSHI); period O = 23.60 ± 0.33 (with OS) vs. 24.32 ± 0.28 (with OSHI); one-way ANOVA Tukey's multiple comparison test: PY < 0.001; PO < 0.01]. Thus, the longer periods observed with young sera were rescued by the heat inactivation of OS, providing strong evidence for heat-sensitive circulating factors present in serum from older individuals that can shorten period. In support of this hypothesis, iterative dilution of OS with FBS results in a progressive loss of this period-shortening effect (Fig S5A).
Fig. 4.
Length of the circadian period of skin fibroblasts treated with media containing normal or heat-inactivated human serum. For ease of analysis, data for normal serum are replotted from Fig. 2. (A) Length of circadian period obtained from one representative cell line from a young subject (Y) measured in media containing normal (YS or OS) or heat-inactivated (YSHI or OSHI) human serum from four young and seven older donors. Every bar shows the mean of three independent measurements ± SEM. (B) (Upper) Graph showing the average period length from Y cell lines treated with normal and heat-inactivated serum from both young and old subjects. Each bar represents the average of two different cell lines, each treated with four different sera, ± SE. (Lower) Equivalent graph for cell lines from older subjects. In both cases, YSHI did not modify the period length compared with YS (one-way ANOVA Tukey's multiple comparison test: P > 0.05), whereas OSHI increased the period length compared with OS (one-way ANOVA Tukey's multiple comparison test: *P < 0.05; **P < 0.01; ***P < 0.001) to a length equivalent to that obtained with YS (one-way ANOVA Tukey's multiple comparison test: P > 0.05). (C) Under temperature-entrained conditions, comparison of phase shifts obtained with untreated and heat-inactivated sera tested on two cell lines from young subjects and two from older subjects. Results are expressed relative to phase obtained with young serum. No differences were observed in phase among YS, YSHI, and OSHI (one-way ANOVA Tukey's multiple comparison test: P > 0.05), but OS resulted in significantly earlier phase (one-way ANOVA Tukey's multiple comparison test: *P < 0.05). (D) Model demonstrating why older subjects show altered circadian behavior. Blue, younger subjects. Red, older subjects. (1) In elderly individuals, lower light levels caused by less light exposure and changed lens properties reduce the ability of light to entrain the central clock in SCN. (2) A weakened circadian drive from the SCN results in fragmented sleep–wake cycles, which in turn affect self-selected light preferences. (3) Altered hormonal balance in elderly individuals changes cellular clock properties and shifts phase earlier at sleep–wake centers but cannot entrain the SCN. In cells, only intrinsic and hormonal influences are operative, resulting in shorter period and earlier phase in the presence of serum from older individuals.
If the changes in period that we observe with OS are responsible for changes in phase, then it would be likely that heat-inactivated OS also should no longer produce phase changes. We tested this hypothesis by examining phase in a temperature entrainment protocol with two sera each from old and young donors, tested on two cell lines each from old and young subjects. As expected, heat treatment abolished the earlier phase produced by serum from older individuals (Fig. 4C).
Theoretically, either a diurnal factor or one constantly present could achieve the effects that we observe. Multiple phase-shifting activities are known to be present in serum, no matter what time of day it is drawn (29). Two obvious labile circadian candidates would be melatonin and cortisol, hormones widely used as circadian markers and known to phase-shift the circadian clock. Melatonin would be barely present in serum at these times, although cortisol would be abundant. We tested the levels of both these substances explicitly in our sera: They were not significantly different in young and older blood donors at the time that these sera were taken (ca. 2:00 PM) (Fig. S5 B and C; unpaired t test; P > 0.05). Because a trend toward elevated cortisol was visible in older subjects, we tested sera supplemented with different amounts of cortisol to see whether this hormone could cause the changes we observe. Because fibroblasts treated with different amounts of cortisol at levels found in the blood of these donors did not show differences in period length compared with untreated cells, we conclude that cortisol is not the factor responsible for our effects (Fig. S5D).
Discussion
In this study, we have shown that skin fibroblasts taken from young and older subjects do not differ in their circadian properties per se, but incubation of both cells in serum from older subjects results in a shortening of circadian period and a shift to earlier phase compared with incubation with serum from younger subjects. Moreover, the effects that we observe are caused by a thermolabile activity in serum from older subjects. Our results suggest that hormonal changes can alter cellular clocks, and these changes in turn might underlie the differences in circadian behavior caused by aging.
Various theories have been proposed to explain a shift toward earlier behavior in elderly individuals. According to one hypothesis, fragmentation of the sleep–wake cycle coupled with increased daytime napping results in less nighttime sleep and a shift to an earlier activity phase (6, 33). In this scenario, changes in sleep structure alter self-selected timing of light, and thereby circadian phase. Such changes in sleep structure might be caused by age-related reduction in the evening circadian signal that opposes homeostatic sleep pressure (15, 34). According to another theory, changes in eye physiology with age (e.g., lens yellowing and senile miosis or cataracts) and changes in behavior (less time in direct sunlight outdoors) are thought to reduce the entraining effects of solar light, exacerbating a circadian entrainment problem in elderly individuals (35). Recent studies corroborate these hypotheses and demonstrate clearly that the circadian system in the elderly is less sensitive to light (36, 37).
The results that we present provide an additional cellular explanation for the shift toward earlier chronotype based on changes in hormone balance in elderly individuals. The putative causal factor could be constantly present in serum and use asymmetry in phase-response curves to shift overall phase. In this case, we would expect that the magnitude of the factor would be different in older and younger individuals at all times of day. Alternatively, if the factor were rhythmically secreted, it could have the same amplitude but a different phase profile in older and younger individuals. In this case, the difference in abundance that we saw by sampling at a single time would reflect different circadian dynamics of the putative clock-modifying substance in older and younger individuals.
Nevertheless, there is one obvious problem with this idea: Excellent studies suggest that the physiological period of human beings does not change with age (15, 16, 38). If a circulating hormone were shortening period, then why does free-running physiological period remain unchanged? We propose that such a factor might act upon non-SCN regions of the human brain and periphery but not upon the SCN itself. This hypothesis would explain our data and also would explain nicely why the phase of sleep–wake timing is shifted earlier relative to the timing of the endogenous circadian clock even in “forced desynchrony” laboratory studies. In rodents, brain structures other than the SCN (e.g., hippocampus, thalamus, and cortex) actually show an entrainment reminiscent of peripheral cells: They have a clock phase 4 h later than the SCN, and they are entrained to feeding and temperature-entrainment signals, whereas the SCN is not (25, 39). Other types of decoupling of locomotor activity rhythms from SCN clock phase (e.g., by methamphetamine in rats or by darkness in certain mouse strains) also are accompanied by alterations in the phase of clock gene expression in non-SCN brain areas (40, 41).
Overall, compelling evidence exists for all three models, and it is likely that each could contribute to the dramatic changes in behavior seen in elderly individuals (a model is shown in Fig. 4D). Our findings open the possibility that circadian difficulties associated with aging might be hormonally driven in part and therefore might be pharmacologically treatable without recourse to potentially addictive sleep aids. If so, this approach would represent a major benefit to health.
Materials and Methods
Subject Recruitment Criteria and Chronotype Determination.
Eighteen healthy young (age 21–30 y) and 18 healthy older subjects (age 60–88 y) were chosen for participation based on age alone and were asked to fill out the MCTQ. MSF-Sc was calculated from this questionnaire and used as a measure of chronotype (26). Subject statistics are shown in Table S1. Prior ethical consent for the use of human skin tissues was given by the Ethical Committee of Basel, and informed written consent for participation in this study was obtained from all human subjects.
Tissue Isolation, Fibroblast Culture, and Viral Infection.
Two cylindrical cutaneous biopsies (2 mm diameter) were taken from the buttocks of each recruited healthy subjects. Fibroblasts were isolated from biopsies by 4-h digestion of tissue in DMEM/1% penicillin streptomycin (Sigma)/1% Glutamax (Sigma) (DMEMc)/20% FBS (Sigma)/87.5 ng/mL Liberase (Roche), and cultured in DMEMc/20% FBS. Confluent cells were infected using Bmal1::luciferase lentivirus. Transfected cells were positively selected 3 d after infection (28).
Harvesting of Sera.
At 2:00 PM, 45 mL of blood were collected from eight healthy young male (age 25.5 ± 4.6 y) and five healthy older (three male and two postmenopausal female; age 74.4 ± 9.8 y) subjects in clot-activator vacutainers (BD Vacutainer System). Whole blood was incubated 30 min at room temperature and then centrifuged 10 min at 2,000 × g. Serum was harvested and stored at −20 °C. When specified, human serum was heat-inactivated by treatment for 30 min at 56 °C.
Synchronization and Measurement of Circadian Period and Phase.
Five days or more after human fibroblast infection, circadian rhythms were synchronized by 100 nM dexamethasone (Sigma) in DMEMc + 20% FBS (42). DMEMc without phenol red was supplemented with 0.1 mM luciferin (Molecular Probes) to obtain the counting medium (CM), and light output was measured in homemade light-tight atmosphere-controlled boxes for at least 5 d. To measure the fibroblast basal circadian rhythms, CM was supplemented with 10% FBS; to determine the influence of human serum on circadian period length, CM was supplemented with 10% human serum; to determine the influence of cortisol on period length, CM was supplemented with 10% FBS or 10% FBS and 25 ng/mL cortisol (Sigma) or 10% FBS and 75 ng/mL cortisol; to determine the influence of heat-inactivated human serum on period length, CM was supplemented with 10% heat-inactivated human serum. For phase determination experiments, cells in luciferin-supplemented medium were synchronized by incubation for 6 d in a temperature-controlled incubator under a 16 h/8 h 35 °C/37 °C daily temperature cycle. On day 7, cells were transferred to the Lumicycle device at 37 °C, and bioluminescence was measured for an additional 16 h. Cellular phase was determined by measuring the time of the transcriptional maximum of reporter gene expression in the smoothed and normalized dataset during this interval (23).
Melatonin Determination in Sera.
A direct double-antibody RIA was used for the melatonin assay, validated by gas chromatography-mass spectroscopy (Buehlmann Laboratories). The minimum detectable dose of melatonin (analytical sensitivity) was determined to be 0.2 pg/mL The functional least-detectable dose using the <20% coefficient of interassay variation criterion was <0.65 pg/mL Melatonin concentrations in the sera were expressed as pg/mL of serum.
Cortisol Determination in Sera.
Quantification of cortisol in sera was performed using a Cortisol ELISA Kit (R&D) following the manufacturer's instruction. Cortisol concentration in the sera was expressed as ng/mL of serum, and the assays were performed in duplicate.
Statistical Methods.
Details of statistical methods are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Robert Dallmann for critical reading of this manuscript. This project was supported by Grant E.U. #LSHM-CT-2006-018741 from EUCLOCK, Grants #310000-122572 and #31003-113874 from the Swiss National Science Foundation, and by grants from the Désireé et Niels Yde Foundation and from the Fonds der Freiwilligen Akademischen Gesellschaft Basel.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008882108/-/DCSupplemental.
References
- 1.Brunner M, Schafmeier T. Transcriptional and post-transcriptional regulation of the circadian clock of cyanobacteria and Neurospora. Genes Dev. 2006;20:1061–1074. doi: 10.1101/gad.1410406. [DOI] [PubMed] [Google Scholar]
- 2.Rosato E, Tauber E, Kyriacou CP. Molecular genetics of the fruit-fly circadian clock. Eur J Hum Genet. 2006;14:729–738. doi: 10.1038/sj.ejhg.5201547. [DOI] [PubMed] [Google Scholar]
- 3.Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: From gene expression to physiology. Chromosoma. 2004;113:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- 4.Roenneberg T, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Renfrew JW, Pettigrew KD, Rapoport SI. Motor activity and sleep duration as a function of age in healthy men. Physiol Behav. 1987;41:627–634. doi: 10.1016/0031-9384(87)90321-0. [DOI] [PubMed] [Google Scholar]
- 6.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: Impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–577. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari E, et al. Age-related changes of the hypothalamic-pituitary-adrenal axis: Pathophysiological correlates. Eur J Endocrinol. 2001;144:319–329. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- 8.Weinert D. Circadian temperature variation and ageing. Ageing Res Rev. 2010;9(1):51–60. doi: 10.1016/j.arr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Englert S, Linden M. Differences in self-reported sleep complaints in elderly persons living in the community who do or do not take sleep medication. J Clin Psychiatry. 1998;59:137–144. doi: 10.4088/jcp.v59n0310. quiz 145. [DOI] [PubMed] [Google Scholar]
- 10.Morton AJ, et al. Disintegration of the sleep-wake cycle and circadian timing in Huntington's disease. J Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer's disease. Sleep Med. 2007;8:623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Willis GL. Parkinson's disease as a neuroendocrine disorder of circadian function: Dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci. 2008;19:245–316. doi: 10.1515/revneuro.2008.19.4-5.245. [DOI] [PubMed] [Google Scholar]
- 13.Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008;29:471–477. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki S, et al. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 17.Duffy JF, et al. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- 18.Yoon IY, et al. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 19.Ripperger J, Brown SA. In: The Circadian Clock. Albrecht U, editor. Vol 12. New York: Springer; 2009. pp. 37–78. [Google Scholar]
- 20.Yagita K, Tamanini F, van Der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- 21.Lowrey PL, et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo SH, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SA, et al. Molecular insights into human daily behavior. Proc Natl Acad Sci USA. 2008;105:1602–1607. doi: 10.1073/pnas.0707772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagani L, et al. The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS ONE. 2010;5:e13376. doi: 10.1371/journal.pone.0013376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574–1583. doi: 10.1016/s0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- 26.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 27.Roenneberg T, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Brown SA, et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005;3:e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol. 2000;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- 30.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 31.Van Cauter E, Plat L, Leproult R, Copinschi G. Alterations of circadian rhythmicity and sleep in aging: Endocrine consequences. Horm Res. 1998;49:147–152. doi: 10.1159/000023162. [DOI] [PubMed] [Google Scholar]
- 32.Dori D, et al. Chrono-neuroendocrinological aspects of physiological aging and senile dementia. Chronobiologia. 1994;21:121–126. [PubMed] [Google Scholar]
- 33.Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med. 1999;31:130–140. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- 34.Cajochen C, Münch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23:461–474. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- 35.Charman WN. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol Opt. 2003;23:181–187. doi: 10.1046/j.1475-1313.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 36.Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24:73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- 38.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–120. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 39.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abe H, Honma S, Namihira M, Masubuchi S, Honma K. Behavioural rhythm splitting in the CS mouse is related to clock gene expression outside the suprachiasmatic nucleus. Eur J Neurosci. 2001;14:1121–1128. doi: 10.1046/j.0953-816x.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- 41.Masubuchi S, et al. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12:4206–4214. [PubMed] [Google Scholar]
- 42.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.