Abstract
It has long been hypothesized that invasive pests may be facilitated by the evolutionary naïveté of their new hosts, but this prediction has never been examined in a phylogenetic framework. To address the hypothesis, we have been studying the invasive viburnum leaf beetle (Pyrrhalta viburni), which is decimating North American native species of Viburnum, a clade of worldwide importance as understory shrubs and ornamentals. In a phylogenetic field experiment using 16 species of Viburnum, we show that old-world Viburnum species that evolved in the presence of Pyrrhalta beetles mount a massive defensive wound response that crushes eggs of the pest insect; in contrast, naïve North American species that share no evolutionary history with Pyrrhalta beetles show a markedly lower response. This convergent continental difference in the defensive response of Viburnum spp. against insect oviposition contrasts with little difference in the quality of leaves for beetle larvae. Females show strong oviposition preferences that correspond with larval performance regardless of continental origin, which has facilitated colonization of susceptible North American species. Thus, although much attention has been paid to escape from enemies as a factor in the establishment and spread of nonnative organisms, the colonization of undefended resources seems to play a major role in the success of invasive species such as the viburnum leaf beetle.
Keywords: defense-free space, invasion ecology, phylogenetic ecology, preference–performance relationship
The establishment and spread of nonnative pests in novel environments has often been viewed as a result of escape from natural enemies present in the native range (1–3), and this top-down view is supported by the success of classical biological control programs against a number of invasive pests (4–6). Nonetheless, there is growing evidence that a lack of defenses in evolutionarily naïve hosts creates opportunities for exotic pests (7–10). For example, because herbivores are potent agents of natural selection for plant defense (11–14), evolutionarily naïve plants may be particularly susceptible to invasive pest insects. Previous observations of island floras have indicated low defense and high susceptibility of naïve plants (15, 16), but this pattern has not been rigorously examined in an evolutionary framework. In particular, this bottom-up notion emphasizes past evolutionary ecology of plant–herbivore interactions as being critical for community stability.
We tested the hypothesis that a lack of shared evolutionary history between plants and herbivores creates defense-free space, facilitating pest invasions. In a phylogenetically controlled field experiment with 16 species of North American and Eurasian Viburnum spp. (Adoxaceae, Dipsacales) (Fig. 1), we tested for the impact of plant continental origin, a proxy for shared evolutionary history with specific herbivores, on defense against the Eurasian Pyrrhalta viburni, an emerging invasive pest in North America. Theory predicts that plant defenses should be convergently gained in species that have long interacted with P. viburni or convergently lost in species that have ceased interacting with this or similar pests. We, thus, measure twig defense (a wound response that crushes P. viburni eggs) (Fig. 2), larval performance, and traits potentially associated with these measures of plant defense (twig diameter and leaf nitrogen content). Because the correspondence between insect preference and plant defense can have critical implications for invasion biology (17–19), oviposition preferences of P. viburni were also measured for the 16 Viburnum species used in the study.
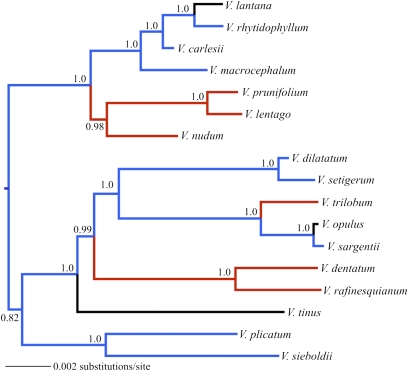
Fig. 1.
Phylogeny of 17 species of Viburnum pruned from a Bayesian consensus tree resulting from a broader analysis that included 90 species of Viburnum (Methods has details on phylogenetic analyses and pruning). Posterior probabilities are indicated at each node. Color of branches reflects the geographic origin of the species: blue, Asia; black, Europe; red, North America.
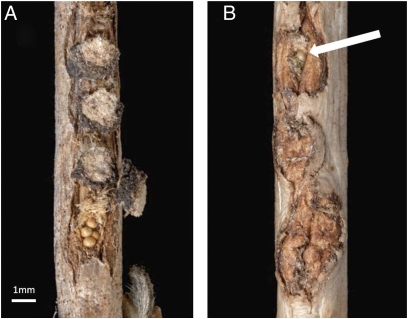
Fig. 2.
(A) P. viburni egg masses along a V. trilobum twig (egg cap removed from the bottom egg mass to show eggs). (B) Wound tissue produced by a V. trilobum twig in response to oviposition. The arrow indicates the sole intact egg remaining after production of wound tissue. (Photos by Kent Loeffler, Cornell University)
Biology of the Plants and Insects
Viburnum comprises a clade of ∼160 spp. of shrubs and small trees that are especially common in the Northern Hemisphere and that are of worldwide importance as ornamentals and understory shrubs. The initial diversification of Viburnum occurred in Asia, with movements to North America within five separate subclades and at several different times (20, 21). Among the herbivores of Viburnum, four closely related specialist leaf-feeding beetles (Pyrrhalta spp.) are endemic to areas of Eurasia but are absent from North America (22, 23). One of them, the viburnum leaf beetle P. viburni, whose native range extends from Europe to Central Asia, emerged as an outbreaking pest in northeastern North America in the 1970s (24, 25). P. viburni is univoltine and overwinters as eggs: eggs are laid in the summer in small clutches (8–12 eggs), deposited in round cavities excavated through the bark and into the pith of terminal twigs, and covered with a frass-like secretion (Fig. 2). At least three of four Pyrrhalta spp. that feed on Viburnum share this oviposition strategy, and they overlap with the Eurasian Viburnum species used in our study; three species, V. opulus, V. lantana, and V. tinus, are attacked specifically by P. viburni in its native range.
P. viburni oviposition is actively aggregative: females prefer twigs already infested by conspecifics for oviposition and preferentially lay eggs adjacent to existing egg masses (26). Consequently, in both the native and introduced range, egg masses are often found in clusters of up to dozens of egg masses along infested twigs (26). High egg mass densities cause direct twig mortality, and high egg survival, which is often the case in the introduced range, results in extensive defoliation first by the larvae and then by the adults. Repeated defoliations typically result in death of the entire shrub after 2–4 y (24).
The production of wound tissue in response to oviposition is a remarkably effective defense mechanism for several Viburnum species: undifferentiated plant tissue produced in the weeks after oviposition crushes or expels egg masses (Fig. 2). Previous work has shown that the extent of wound response predicts egg survivorship (y = −0.73x + 85.46, r2 = 0.52, P < 0.0001), with up to 88% egg mortality on twigs expressing a strong wound response (26). The extent of the wound response is typically positively correlated with twig diameter and negatively correlated with egg mass density (26).
We evaluated defense against P. viburni oviposition by measuring the production of wound tissue in response to low and high egg mass densities in a manipulative field experiment. To determine if the production of defensive wound tissue is mediated by specific insect-derived elicitors, an additional set of twigs of each species was artificially damaged to simulate Viburnum oviposition, and artificial wounds were treated with (i) water, (ii) jasmonic acid, or (iii) P. viburni egg mass extracts. To determine the correspondence between plant defenses against P. viburni adult oviposition and larval performance, we compared larval survivorship and female adult mass on each host species under field and laboratory conditions. Finally, oviposition preferences of P. viburni were evaluated by measuring natural levels of oviposition in a common garden with mature shrubs of the 16 Viburnum spp.
Results
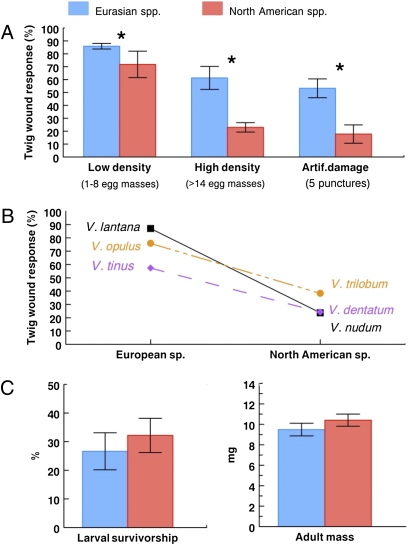
The twig wound response to oviposition was 16% lower on North American Viburnum spp. at low egg-mass density [phylogenetic generalized least squares (PGLS) analysis, n = 15 species, λ = 1, Likelihood Ratio (LR) = 4.92, P = 0.03] and 62% lower at high density (PGLS, n = 12, λ = 0, LR = 8.6, P = 0.003), showing that North American Viburnum spp. have a lower egg-crushing ability than their Eurasian congeners (Fig. 3A). At high egg densities (15 egg masses; i.e., 120 eggs per twig), which is the case of greatest concern in the introduced range, the difference in wound response between North American and Eurasian species would result in an estimated 67% higher larval density on North American species. Of the three European species that are specifically attacked by P. viburni (V. opulus, V. lantana, and V. tinus), their closest North American relatives (e.g., V. trilobum, V. nudum, and V. dentatum, respectively) showed a reduced wound response at high egg mass density (paired t test, n = 3, t = 4.73, P = 0.04) (Fig. 3B). Three Asian species (V. plicatum, V. rhytidophyllum, and V. sieboldii) could not be infested at high egg densities because of the reluctance of females to oviposit on these hosts.
Fig. 3.
(A) Plant defense (twig wound response) in response to beetle oviposition (low and high egg mass densities) and artificial damage on Viburnum spp. of different continental origin (summary of nine Eurasian and six North American species) from a field common garden (mean ± SEM). Asterisk indicates a significant difference after accounting for phylogenetic nonindependence (α = 0.05). (B) Specific comparison of the wound response at high egg mass density of the European species fed on by P. viburni in its native range and their close North American relatives. This result is robust to various species pairings (for example, if V. dentatum is substituted by V. rafinesquianum as the comparison species for V. tinus or if V. lentago or V. prunifolium is used as the comparison species for V. lantana) (Table S1). (C) Insect performance (average of laboratory and field values for survivorship and adult mass; mean ± SEM) on Viburnum spp. of different origin (10 Eurasian and 5 North American species) (Table S1).
The wound response to artificial damage was again 66% lower for North American than for Eurasian species (PGLS, n = 15, λ = 1, LR = 8.78, P = 0.003) (Fig. 3A), and there was a strong correlation between the response to high-density oviposition and artificial damage at the species level (PGLS, n = 12, λ = 0.46, LR = 13.96, P < 0.001). We found no evidence for wound response specificity, however, because the three treatments that we applied to artificial wounds did not differ in the defensive response (F3,227 = 0.57, P = 0.63). Twig diameter showed positive correlated evolution with the wound response to high egg-mass density (PGLS, n = 12, λ = 0, LR = 10.4, P = 0.001) and artificial wounds (PGLS, n = 15, λ = 1, LR = 7.48, P = 0.006) but less to the wound response at low egg-mass density (PGLS, n = 15, λ = 1, LR = 2.94, P = 0.09). Twig diameter did not significantly differ between North American and Eurasian Viburnum spp. (PGLS, n = 15, λ = 1, LR = 0.4, P = 0.53).
Insect performance did not differ among Viburnum hosts from Eurasia and North America (larval survivorship: PGLS, n = 15, λ = 1, LR = 0.5, P = 0.48; adult mass: PGLS, n = 13, λ = 1, LR = 0.22, P = 0.64), and P. viburni larvae were able to complete development on all six North American hosts (14–50% survivorship) (Table S1).
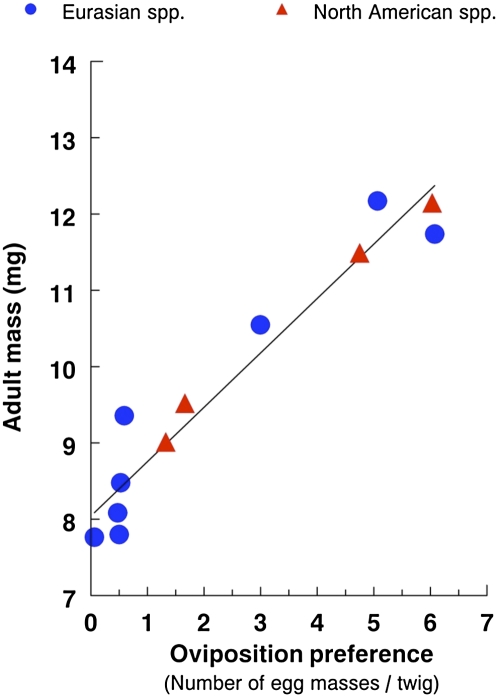
The number of egg masses laid by P. viburni females did not differ between North American (mean ± SEM egg masses across all twigs regardless of infestation status; 3.4 ± 1.2, n = 5) and Eurasian hosts (1.7 ± 0.7, n = 8) in the common garden (PGLS, n = 13, λ = 1, LR = 0.58, P = 0.45) (Table S1). However, there was striking correlated evolution between oviposition preferences of P. viburni females and insect performance (larval survivorship: PGLS, n = 13, λ = 0.44, LR = 6.46, P = 0.01; adult mass: PGLS, n = 12, λ = 0.32, LR = 27.26, P < 0.0001), regardless of host continental origin (Fig. 4). We found weak evidence for a positive correlation between leaf nitrogen content and oviposition preference (PGLS, n = 13, λ = 0.52, LR = 3.4, P = 0.06), and there was no relationship between nitrogen content and continental origin (PGLS, n = 16, λ = 1, LR = 0.04, P = 0.84). Finally, there was no relationship between oviposition preference and production of wound tissue (high egg density: PGLS, n = 10, λ = 0.63, LR = 0.14, P = 0.7) or twig diameter (PGLS, n = 13, λ = 1, LR = 0.06, P = 0.81).
Fig. 4.
Correlation between oviposition preference (average egg masses per twig for a random sample of field-collected twigs) and adult mass (mg) of P. viburni on Viburnum spp. of different origins (nine Eurasian and four North American species; n = 13). Oviposition data are from an untreated common garden; adult mass data are average values from laboratory and field experiments.
Discussion
From a macroevolutionary perspective, our data show that, in each of three distantly related Viburnum lineages that contain species in both Eurasia and North America, the North American species show significantly lower defenses against P. viburni compared with their Eurasian relatives. One possible explanation for this pattern is that movement into the New World occurred in lineages that already had experienced Pyrrhalta herbivory in the Old World. Under this scenario, movements to a Pyrrhalta-free continent were repeatedly associated with a reduction of defenses against these herbivores (i.e., reduced wound tissue and egg-crushing ability). The other possibility is that movement into the New World occurred before Pyrrhalta became associated with Old World Viburnum species: this scenario implies that stronger defense was independently derived in the Old World representatives of these lineages. Both scenarios imply a benefit of evolving enhanced egg-crushing ability when in the presence of specialist Pyrrhalta herbivores, but only the first one implies a cost of keeping these defenses in their absence. Twig diameter, a plant trait shown to be associated with wound response capacity, did not differ between hosts of different continental origins, supporting the notion that twig diameter per se may not be the only mechanism of Viburnum resistance to oviposition. In other words, the evolution of twig diameter alone, independent of herbivores, is not sufficient to explain the difference in plant defense against oviposition between New World and Old World Viburnum spp. In addition to wound response, the reluctance of P. viburni females to oviposit on some of the Asian hosts used in our study suggests that oviposition deterrence may complement egg-crushing ability in Eurasian viburnums.
Production of wound tissue is a slow process that usually takes a few weeks under field conditions. Because P. viburni eggs remain in the twigs for several months (and hatch the next spring), wound response constitutes an effective defense against this herbivore, but it is unlikely to impact other insects. Neither application of jasmonic acid nor egg-mass extracts on artificial wounds impacted wound tissue production in European or North American Viburnum spp. This suggests that, because the type of twig damage caused by oviposition is unique to Pyrrhalta herbivores, response to specific insect-derived elicitors may not be needed.
The ecological consequence of differentiation in defenses between Old World and New World Viburnum spp. has been dramatic with the introduction of P. viburni into North America. Suitable as food sources and poorly defended against oviposition, North American Viburnum spp. have provided a favorable environment for the establishment and spread of P. viburni. Furthermore, differences in defenses observed among North American species may reflect differences in vulnerability under natural conditions. Two of the most resistant North American species in our study, V. prunifolium and V. lentago (Table S1), are also the least defoliated under natural conditions in North America (27). Because P. viburni has relatively high fecundity (∼600 eggs/female) (28), high egg survivorship owing to the lack of wound responses to oviposition may have potentiated outbreaks of this invasive pest. Colonization of susceptible North American hosts is further facilitated by the oviposition preferences of females, seemingly unrelated to the evolutionary history of host association. The remarkable correlation between oviposition preferences and insect performance suggests that P. viburni oviposition choices are based on indicators of host quality and that these indicators are common to the range of Viburnum spp. that we tested. The absence of correlation between oviposition preferences and production of wound tissue indicates that plant defenses do not drive P. viburni oviposition preferences at the interspecific level.
Overall, North America constitutes a defense-free (or more accurately, defense-limited) novel environment for P. viburni, which likely contributed to its invasion success. Other factors, such as the apparent absence of coevolved natural enemies, may have also favored P. viburni invasion. However, exploration for natural enemies and potential biological control agents in Eurasia has been disappointing, with the most abundant natural enemy, an egg parasitoid, having minimal impact in natural populations (29). Thus, egg crushing may well be a critical mechanism of insect population control in the native range. In North America, adaptively choosy pests, together with the limited plant defense of their new Viburnum hosts, have resulted in a successful invasion by the viburnum leaf beetle. More generally, our study contributes to a growing body of evidence that a lack of shared evolutionary history between plants and enemies (competing plants, microbes, or herbivores) may result in devastation when enemies invade (7–10).
Methods
Plants, Insects, and Traits.
We used plant material from an untreated common garden located in Ithaca, NY. Most species (14 of 16) were represented by more than four mature shrubs. Insects were field-collected as eggs and reared under laboratory conditions [22 °C, light 15 h and dark 9 h (L15:D9) light regimen]. Defense against oviposition experiments were conducted on terminal twigs chosen within ±0.5 mm of the average diameter of each species calculated on the basis of a random sample of >20 twigs per species. Selected twigs received one of five treatments [low (1–8 egg masses) or high (>14 egg masses) egg density, artificial damage + water/jasmonic acid/egg cap extracts; five replicates per treatment per species]. Low and high egg densities were obtained by bagging 2–10 females and 1–5 males in fine mesh over twigs between July 27 and August 31. After the appropriate levels of eggs were reached, females were removed to avoid further oviposition.
Observations of natural egg clusters in Europe showed that native P. viburni oviposits a mean of 4.7 (range = 1–22) and 8.2 (range = 1–33) egg masses per infested twig in field populations of V. tinus and V. opulus, respectively. Similar observations for three susceptible North American Viburnum species (V. dentatum, V. rafinesquianum, and V. trilobum) documented mean infestation levels between 5 and 13 egg masses per twig, with a range of up to 60 egg masses. Thus, our experimental treatments of low and high egg-mass density per twig were well within the range of attack in native and introduced populations.
Data for V. tinus were collected during a field observational study conducted in the Mediterranean area (Montpellier, France): production of wound tissue on twigs naturally infested with high densities of egg masses (n = 6) was estimated at the end of the growing season.
Artificial damage treatments were implemented by inflicting five punctures approximating the dimensions of an egg mass to the bark and pith of noninfested twigs with sterilized scissors. Water, jasmonic acid (0.5 mM), or egg cap extract (a mixture of five egg caps per 1 mL water) was then applied to the fresh punctures using a soft, fine brush, and twigs were bagged to prevent oviposition. Bags were removed on October 15, and production of wound tissue was visually estimated as the percentage of crushed or expelled eggs. For artificially damaged twigs, percentage wound response was gauged for each puncture from 0% (no wound tissue) to 100% (wound tissue completely closing the puncture) and averaged for each twig.
Larval performance experiments on each species were conducted under both laboratory and field conditions from May 7 to July 15. In the laboratory, newly hatched larvae were placed in groups of 5 or 20 with young shoots of each Viburnum species tested (five replicates per density per species) in cylindrical plastic containers with a vented lid and were monitored daily for survivorship, and plant material was replaced as needed. In the field, one or three egg masses were placed on terminal twigs of each Viburnum species (five replicates per density per species) in fine-mesh nets enclosing the twigs. For both settings, a thin layer of potting mix was added to the container or bag after larvae reached final larval instar as pupation substrate. Newly emerged adults were sexed and weighed in the 24 h after emergence. Percentage survivorship was calculated as the number of adults emerged divided by the number of larvae initially placed in the container or bag (assuming an average of eight eggs per egg mass and 100% larval emergence). Data presented are the species means of the laboratory and field trials.
Natural levels of oviposition were measured as the average number of eggs per twig for a random sample of terminal twigs collected for each species at the end of the growing season (>35 twigs per species).
Percentage nitrogen in leaf tissue was measured in a sample of five field-collected leaves per species in May using a combustion elemental analyzer.
Phylogenetic Analysis.
A phylogeny of 90 Viburnum species was reconstructed from 9,552 bp [8,910 chloroplast and 642 Internal Transcribed Spacer region (ITS)]. Coding regions of the chloroplast included matK, ndhF, and rbcL, and noncoding regions of the chloroplast included petB-petD, psbA-trnH, rpl32-trnL, trnC-ycf6, trnK, and trnS-trnG. Bayesian analyses were conducted using MrBayes v3.1.2 (30) with three partitions (chloroplast coding regions, chloroplast noncoding regions, and ITS), each analyzed under a GTR+I+G model of sequence evolution with all parameters unlinked. We used 12 chains that ran for 30 million generations, sampling the posterior distribution every 1,000 generations. We determined that the analysis had reached stationarity when there was sufficient evidence of chain swapping, the split frequencies were less than 0.01, and by visual inspection of parameter plots in Tracer 1.5 (31). Using the plots of likelihood and model parameters, we determined an appropriate burn-in to remove before summarizing model parameters and building a 50% majority rule consensus tree. The Bayesian consensus tree was pruned (retaining branch lengths) to include only the 17 taxa examined in this study using TreeEdit v1.0a10 (32) (hereafter pruned tree). Additionally, we reanalyzed the 9,552-bp dataset with only the 17 taxa of interest and V. clemensae for rooting purposes (hereafter, 17 taxa tree). Analysis conditions were the same as described above, except that we used two partitions instead of three partitions, with one partition comprising all of the chloroplast data and the second comprising ITS.
Comparative phylogenetic analyses were conducted with the continuous module of Mark Pagel's BayesTraits (http://www.evolution.rdg.ac.uk/BayesTraits.html) (33) using the phylogeny in Fig. 1. For each analysis, we first assessed phylogenetic signal (λ) using maximum likelihood and tested it against a value of one (Brownian motion) or zero (no phylogenetic signal). We used λ = 1 when the maximum likelihood estimated value was statistically indistinguishable from one (using likelihood ratio tests). We otherwise used the estimated value. Results were qualitatively identical when we used the pruned tree, the 17 taxa tree, and 1,000 Bayesian trees.
Supplementary Material
Acknowledgments
Much of the work presented here benefited from a Viburnum common garden established in 1999 by Paul Weston. N. Becker, A. Hastings, T. Ramsey, L. Schunk, and E. Woods helped with laboratory and field work, J. Thaler provided jasmonic acid, and Dan Herms, Sergio Rasmann, Sharon Strauss, and M. G. Weber provided comments on the manuscript. Viburnum phylogenetic studies were supported by National Science Foundation Grant IOS-0842800 (to M.J.D.). This study was supported by US National Science Foundation Grant DEB-0950231 (to A.A.A.) and Federal Formula Funds allocated by the Cornell University Agricultural Experiment Station (to A.A.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102891108/-/DCSupplemental.
References
- 1.Elton CS. The Ecology of Invasions by Animals and Plants. London: Methuen; 1958. [Google Scholar]
- 2.Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Stiling P. Testing the enemy release hypothesis: A review and meta-analysis. Biol Invasions. 2006;8:1535–1545. [Google Scholar]
- 4.Roland J, Embree DG. Biological-control of the winter moth. Annu Rev Entomol. 1995;40:475–492. [Google Scholar]
- 5.Hajek AE, Elkinton JS, Witcosky JJ. Introduction and spread of the fungal pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) along the leading edge of gypsy moth (Lepidoptera: Lymantriidae) spread. Environ Entomol. 1996;25:1235–1247. [Google Scholar]
- 6.Caltagirone LE. Landmark examples in classical biological control. Annu Rev Entomol. 1981;26:213–232. [Google Scholar]
- 7.Parker JD, Burkepile DE, Hay ME. Opposing effects of native and exotic herbivores on plant invasions. Science. 2006;311:1459–1461. doi: 10.1126/science.1121407. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi K, Herms D. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions. 2010;12:389–405. [Google Scholar]
- 9.Raupp MJ, Shrewsbury PM, Herms DA. Ecology of herbivorous arthropods in urban landscapes. Annu Rev Entomol. 2010;55:19–38. doi: 10.1146/annurev-ento-112408-085351. [DOI] [PubMed] [Google Scholar]
- 10.Callaway RM, Ridenour WM. Novel weapons: Invasive success and the evolution of increased competitive ability. Front Ecol Environ. 2004;2:436–443. [Google Scholar]
- 11.Zangerl AR, Berenbaum MR. Increase in toxicity of an invasive weed after reassociation with its coevolved herbivore. Proc Natl Acad Sci USA. 2005;102:15529–15532. doi: 10.1073/pnas.0507805102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Futuyma DJ, Agrawal AA. Macroevolution and the biological diversity of plants and herbivores. Proc Natl Acad Sci USA. 2009;106:18054–18061. doi: 10.1073/pnas.0904106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siepielski AM, Benkman CW. Convergent patterns in the selection mosaic for two north American bird-dispersed pines. Ecol Monogr. 2007;77:203–220. [Google Scholar]
- 14.Stenberg JA, Witzell J, Ericson L. Tall herb herbivory resistance reflects historic exposure to leaf beetles in a boreal archipelago age-gradient. Oecologia. 2006;148:414–425. doi: 10.1007/s00442-006-0390-7. [DOI] [PubMed] [Google Scholar]
- 15.Bowen L, Vuren DV. Insular endemic plants lack defenses against herbivores. Conserv Biol. 1997;11:1249–1254. [Google Scholar]
- 16.Janzen DH. Behavior of Hymenaea courbaril when its predispersal seed predator is absent. Science. 1975;189:145–147. doi: 10.1126/science.189.4197.145. [DOI] [PubMed] [Google Scholar]
- 17.Solarz SL, Newman RM. Variation in hostplant preference and performance by the milfoil weevil, Euhrychiopsis lecontei Dietz, exposed to native and exotic watermilfoils. Oecologia. 2001;126:66–75. doi: 10.1007/s004420000484. [DOI] [PubMed] [Google Scholar]
- 18.Morewood WD, Neiner PR, McNeil JR, Sellmer JC, Hoover K. Oviposition preference and larval—performance of Anoplophora glabripennis (Coleoptera: Cerambycidae) in four eastern North American hardwood tree species. Environ Entomol. 2003;32:1028–1034. [Google Scholar]
- 19.DiTommaso A, Losey JE. Oviposition preference and larval performance of monarch butterflies (Danaus plexippus) on two invasive swallow-wort species. Entomol Exp Appl. 2003;108:205–209. [Google Scholar]
- 20.Winkworth RC, Donoghue MJ. Viburnum phylogeny based on combined molecular data: Implications for taxonomy and biogeography. Am J Bot. 2005;92:653–666. doi: 10.3732/ajb.92.4.653. [DOI] [PubMed] [Google Scholar]
- 21.Clement WL, Donoghue MJ. Dissolution of Viburnum section Megalotinus (Adoxaceae) of Southeast Asia and its implications for morphological evolution and biogeography. Int J Plant Sci. 2011 in press. [Google Scholar]
- 22.Park JY, Lee JE. A taxonomic study on the larvae of the genus Pyrrhalta Joannis (Coleoptera: Chrysomelidae: Galerucinae) from Korea. Entomol Res. 2004;34:229–234. [Google Scholar]
- 23.Satoh A. Within-plant distribution of the eggs and larvae of two congeneric chrysomelid beetles on the same host plant. Entomol Sci. 2002;5:171–177. [Google Scholar]
- 24.Weston PA, Desurmont G, Hoebeke ER. Viburnum leaf beetle: Biology, invasion history in North America, and management options. American Entomologist. 2007;53:96–101. [Google Scholar]
- 25.Majka CG, LeSage L. Introduced leaf beetles of the maritime provinces, 3: The viburnum leaf beetle, Pyrrhalta viburni (Paykull) (Coleoptera: Chrysomelidae) Proc Entomol Soc Wash. 2007;109:454–462. [Google Scholar]
- 26.Desurmont GA, Weston PA. Aggregative oviposition of viburnum leaf beetle overcomes plant defenses. Ecol Entomol. 2011 in press. [Google Scholar]
- 27.Weston PA. Viburnum Leaf Beetle Citizen Science Susceptibility to Infestation. 2006. Available at http://www.hort.cornell.edu/vlb/suscept.html. Accessed November 1, 2010.
- 28.Weston PA, Diaz MD, Desurmont GA. Ovipositional biology of Viburnum leaf beetle, Pyrrhalta viburni (Coleoptera: Chrysomelidae) Environ Entomol. 2008;37:520–524. doi: 10.1603/0046-225x(2008)37[520:obovlb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Desurmont GA. Ithaca, NY: Cornell University; 2009. Oviposition of viburnum leaf beetle [Pyrrhalta viburni (Paykull)]: From ecology to biological control of an emerging landscape pestPhD thesis. [Google Scholar]
- 30.Huelsenbeck JP. MRBAYES: Bayesian inference of phylogeny. Biometrics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 31.Rambaut A. Tracer v1.5. 2009. Available at http://beast.bio.ed.ac.uk/software/tracer. Accessed November 1, 2010.
- 32.Rambaut A. TreeEdit v1.0a10. 2001. Available at http://tree.bio.ed.ac.uk/software/treeedit. Accessed November 1, 2010.
- 33.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.