Abstract
We summarize the literature on rates of multiple paternity and sire numbers per clutch in viviparous fishes vs. mammals, two vertebrate groups in which pregnancy is common but entails very different numbers of embryos (for species surveyed, piscine broods averaged >10-fold larger than mammalian litters). As deduced from genetic parentage analyses, multiple mating by the pregnant sex proved to be common in assayed species but averaged significantly higher in fish than mammals. However, within either of these groups we found no significant correlations between brood size and genetically deduced incidence of multiple mating by females. Overall, these findings offer little support for the hypothesis that clutch size in pregnant species predicts the outcome of selection for multiple mating by brooders. Instead, whatever factors promote multiple mating by members of the gestating sex seem to do so in surprisingly similar ways in live-bearing vertebrates otherwise as different as fish and mammals. Similar conclusions emerged when we extended the survey to viviparous amphibians and reptiles. One notion consistent with these empirical observations is that although several fitness benefits probably accrue from multiple mating, logistical constraints on mate-encounter rates routinely truncate multiple mating far below levels that otherwise could be accommodated, especially in species with larger broods. We develop this concept into a “logistical constraint hypothesis” that may help to explain these mating outcomes in viviparous vertebrates. Under the logistical constraint hypothesis, propensities for multiple mating in each species register a balance between near-universal fitness benefits from multiple mating and species-idiosyncratic logistical limits on polygamy.
Keywords: mating systems, fecundity, genetic parentage analysis, sexual selection
Pregnancy is a reproductive burden normally borne by females, ultimately because of anisogamy [the difference in size and mobility between male and female gametes (1, 2)]. In other words, because females in sexual species produce the costlier and less mobile class of gametes, the female sex is evolutionarily predisposed to be the sex that gestates its progeny internally. Indeed, pregnancy or embryonic brooding by females has evolved independently on numerous occasions, such that many taxonomic families of fishes, reptiles, and amphibians (as well as most mammals and many invertebrate taxa) include viviparous (live-bearing) representatives. In contrast, male-pregnancy is exceedingly rare in the biological world, being displayed by only a few organisms (notably pipefishes and seahorses, Syngnathidae) that thus offer model systems for counterpoint evolutionary studies of reproductive modes, sexual selection, sex roles, and sexual dimorphism (3–12). All forms of pregnancy offer extreme examples of sex-biased parental investment (13), which ever since Bateman (14) has shared center stage with sex-biased sexual selection in research on animal mating systems (15–19).
In effect, female-pregnancy merely amplifies what anisogamy had started by making females and their ova even more of a limiting resource in reproduction. Thus, anisogamy set the evolutionary stage by making females intrinsically far more fecundity-limited than males, and pregnancy heightens this sexual difference by placing further constraints on how many progeny a female can bear. In turn, these inherent fertility differences between the sexes routinely translate into steeper sexual-selection gradients (14) for males than for females, meaning that males in many species tend to profit more than females from having multiple sexual partners. Because a male's genetic fitness can increase greatly with mate count—whereas a female's reproductive success is limited mostly by her fecundity, regardless of mate number—males in many species are under stronger selection pressure than females to seek multiple sexual partners.
To explore possible relationships between mating behaviors and alternative forms of pregnancy, in a companion study (20) we compared rates of multiple mating by members of the pregnant sex in fish species with: (i) internal female-pregnancy, (ii) internal male-pregnancy, and (iii) external male-pregnancy (nest-tending by males). In conducting that literature review, we took advantage of the fact that multiple successful mating by the pregnant parent is relatively straightforward to detect in nature via molecular parentage analyses because each resulting brood of half-sibling embryos is physically associated with its pregnant sire or pregnant dam. (In contrast, documenting the frequency of multiple mating by members of the nonpregnant sex is far more problematic because any such individual might have parented additional broods that were not included in the genetic assays.) Several results from our earlier study were consistent with a “fecundity-limitation hypothesis” (FLH) stating that pregnancy truncates individual fecundity in ways that should impact selective pressures on each individual's proclivity to seek multiple mates. For example, rates of multiple mating by bourgeois (“shopkeeper”) males in nest-tending fish species proved to be higher than in viviparous fish species, consistent with the thesis that a nest offers far more brood space than does an internal brood pouch and thereby can pay greater absolute fitness dividends for nest-tenders who have multiple mates. On the other hand, any empirical support for the FLH was tempered by the realization that all of the predicted mating-pattern trends that we uncovered in our comparisons of male-pregnant vs. female-pregnant fishes were statistically mild at best, rather than highly significant.
Here we extend this type of comparative analysis by reviewing the literature on genetic parentage for female-pregnant mammals (as well as viviparous reptiles and amphibians) and comparing results to those for the pregnant fishes. Our interest in such comparisons relates mostly to the fact that mammalian pregnancies typically involve only a few progeny each, whereas a pregnant fish normally carries dozens to hundreds or sometimes even more embryos. Of course, fishes and mammals differ in countless biological characteristics that theoretically might influence rates of multiple mating, so any observed difference in mating proclivities between these two groups could have little or nothing to do with brood size per se. On the other hand, if pregnant fishes and mammals prove not to differ dramatically in their genetically deduced rates or patterns of polygamy, then we can at least conclude that brood size itself probably has not been a primary selective factor in the evolution of multiple mating in viviparous vertebrates. In other words, if huge numerical disparities in brood size have not translated during evolution into very different proclivities for multiple mating by live-bearing fishes vs. mammals or other viviparous vertebrates, then we may be forced to explore avenues other than the FLH for any empirical mating patterns that might be unveiled.
Another general category of explanations for multiple mating by pregnant males or by pregnant females mostly sidesteps the fecundity issue per se and focuses instead on other potential fitness benefits from having multiple sexual partners. Regardless of its number of mates, each male or female in any sexual species presumably can enhance its genetic fitness by choosing one or more mates of the highest possible genetic quality. Thus, for example, despite any inherent fecundity limitations imposed by pregnancy, any sire in a male-pregnant species or any dam in a female-pregnant species might be under selection to seek multiple mates for any of the following reasons: more “nuptial gifts,” fertilization insurance against the risk that a mate is sterile, higher genetic diversity among the half-sibling progeny with its brood (21, 22), better chances of finding a genetically compatible mate, or more opportunities to imbue at least some of the offspring with “good genes” for viability (23, 24). Of course, multiple mating by either sex can incur appreciable fitness costs as well, such as the time and energy required to secure mates and the increased chance of contracting a sexually transmitted disease.
Results
Incidences of Multiple Mating by the Pregnant Sex.
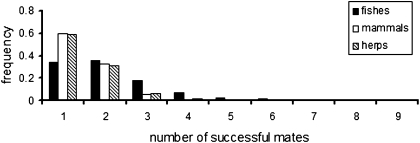
Our literature review uncovered genetic estimates of mate numbers and rates of multiple mating by members of the pregnant sex in a total of: (i) 533 internal pregnancies (broods) in 18 viviparous or ovoviviparous fish species (table 1 in ref. 20); (ii) 1,930 broods in 49 mammalian species (Table S1); and (iii) 362 broods in 29 live-bearing species of reptiles and amphibians (Table S2). In all of these vertebrate assemblages, multiple mating by members of the pregnant sex proved to be a rather common phenomenon. Thus, in mammals approximately 40% of surveyed broods had multiple sires and the pregnant females averaged about 1.5 mates per litter; in the fish, approximately 66% of pregnancies evidenced polygamy and the gestating sex averaged 2.2 mates per brood; and, finally, in the viviparous reptiles and amphibians (hereafter referred to as “herps”), approximately 41% of assayed broods had multiple sires and a typical pregnant female had mated successfully with about 1.5 males. The record for the highest number of genetically deduced mates in the entire study was nine sires [for one brood in the female-pregnant fish species Poecilia reticulata (25)].
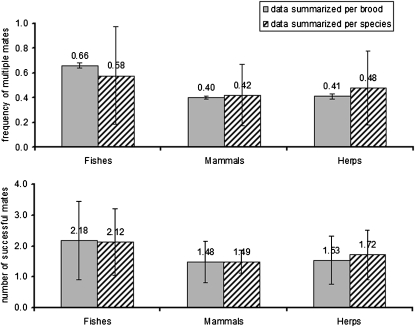
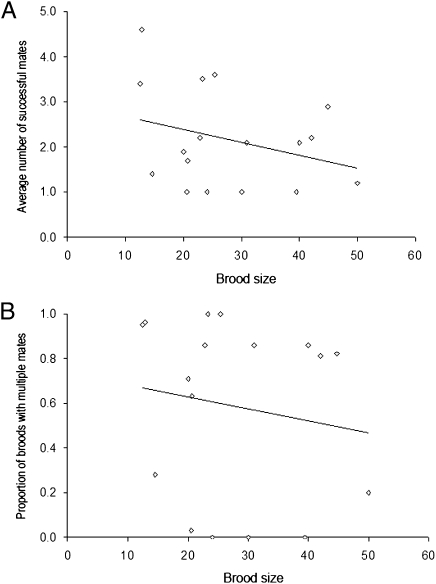
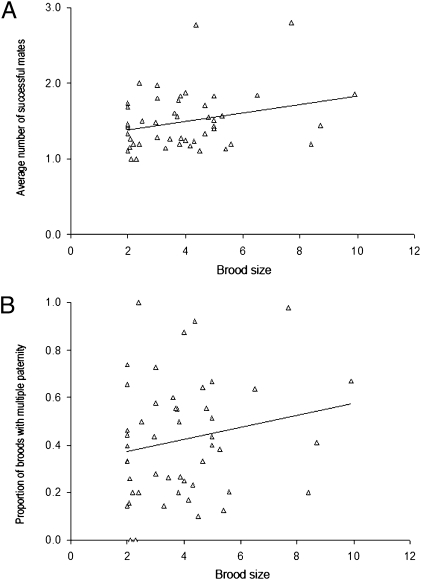
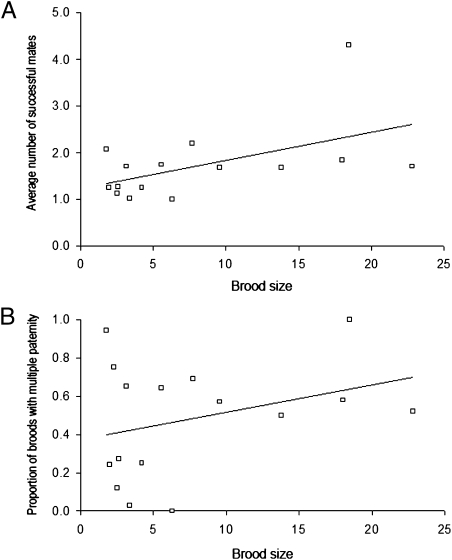
For the three vertebrate groups (fishes, mammals, and herps) examined in the current study, polygamy rates are summarized and compared in Figs. 1 and 2. Regardless of whether the data were analyzed on a per brood basis (which gives equal statistical weight to each brood) or on a per species basis (which gives equal weight to each species), pregnant fish showed significantly higher mean incidences of multiple mating than did their mammalian counterparts (Table 1). This result is generally consistent with the FLH (20), which posits that physical constraints on brood size in pregnant species help to predict how many successful mates a gravid individual is likely to obtain. On the other hand, within the surveyed live-bearing fishes we observed no significant positive correlations between brood size and the incidence of multiple mating (Fig. 3); and within the mammals such correlations were at best only mildly significant (Fig. 4). Thus, these within-group findings imply that mating proclivities in members of the pregnant sex probably have been impacted by evolutionary factors in addition to the brood-size constraints per se that are the explicit focus of the FLH (see below).
Fig. 1.
Frequency distributions of piscine, mammalian, and herpetological broods in which pregnant individuals had the indicated numbers of mates, as deduced from genetic parentage analyses.
Fig. 2.
Frequencies of multiple mating and the estimated mate numbers in 1,930 broods of 49 mammal species, 533 broods of 18 viviparous fish species, and 362 broods of 29 herpetological species. Note only herpetological species with four or more clutches analyzed were included in the per species analyses.
Table 1.
Comparisons of brood size and multiple-mating parameters (two-tailed t tests assuming unequal variances, as calculated in Microsoft Excel) for fishes, mammals, and herps
| Comparison | t Stat | df | P |
| Number of successful mates based on per brood data | |||
| Fishes vs. mammals | 12.20 | 613 | 0.00 |
| Fishes vs. herps | 9.45 | 881 | 0.00 |
| Herps vs. mammals | 1.22 | 467 | 0.22 |
| Number of successful mates based on per species data* | |||
| Fishes vs. mammals | 2.46 | 19 | 0.02 |
| Fishes vs. herps | 1.24 | 31 | 0.22 |
| Herps vs. mammals | 1.06 | 16 | 0.30 |
| Frequency of successful mates based on per species data | |||
| Fishes vs. mammals | 1.62 | 22 | 0.11 |
| Fishes vs. herps | 0.80 | 32 | 0.42 |
| Herps vs. mammals | 0.76 | 22 | 0.46 |
| Brood size | |||
| Fishes vs. mammals | 8.50 | 16 | 0.00 |
| Fishes vs. herps | 6.12 | 26 | 0.00 |
| Herps vs. mammals | 2.22 | 16 | 0.04 |
*Only herpetological species with four or more clutches analyzed were included in the per species analyses.
Fig. 3.
Empirical correlations between brood size and (A) the average number of mates per brood (r = 0.31, P = 0.23) and (B) the proportion of broods with multiple mates (r = 0.16, P = 0.54) in 17 viviparous fish species.
Fig. 4.
Empirical correlations between brood size and (A) the average number of mates per brood (r = 0.27, P = 0.053) and (B) the proportion of broods with multiple mates (r = 0.19, P = 0.19) in 49 viviparous mammalian species.
Quite similar patterns and conclusions emerged when we also consider the findings for viviparous reptiles and amphibians (Figs. 1 and 2). Thus, although mean brood size in the surveyed herps was intermediate and differed significantly from those of both mammals and fishes, the herps did not differ from either fishes or mammals with respect to the frequency of successful multiple mating by the pregnant parent (Table 1). However, generally consistent with the FLH, the assayed vivparous herps did exhibit a significant positive correlation between brood size and the incidence of multiple mating by the pregnant sex (Fig. 5).
Fig. 5.
Empirical correlations between brood size and (A) the average number of mates per brood (r = 0.52, P = 0.04) in 15 viviparous herpetological species and (B) the proportion of broods with multiple mates (r = 0.32, P = 0.23) in 16 viviparous herpetological species.
Discussion
Even among closely related viviparous species, brood sizes sometimes differ by orders-of-magnitude. For example, in some live-bearing fish in the Bathytidae, a pregnant dam carries only about a dozen embryos, whereas other species in the same taxonomic family may give birth to 10,000 or more offspring per pregnancy. However, relatively little is known about either the etiologies or the evolutionary ramifications of such dramatic differences in brood size (fecundity) in viviparous species. Thus, as phrased by Wourms (26), “The relationship between fecundity and reproductive strategies needs to be re-examined when we are in possession of a greater comparative knowledge…” Here we have initiated such analyses by compiling and comparing information on brood size vis-à-vis patterns of genetic parentage in three major groups of viviparous vertebrates with grossly different brood sizes and fecundity potentials during a typical pregnancy.
In any viviparous species, the phenomenon of pregnancy is a double-edged evolutionary sword that presumably enhances survival rates for embryos, even when otherwise placing both physical and energetic constraints on how many offspring a pregnant individual and its mate or mates can produce during each reproductive episode. The physical ceiling reflects the fact that internal brood space is finite in any pregnant individual and the energetic ceiling reflects the fact that a gestating parent typically supplies nutrients or other resources to its brooded young. In theory, viviparity should be selectively favored over oviparity when the net fitness gains from pregnancy are positive (i.e., when the fitness benefits of internal gestation outweigh the costs). Here we have compared genetically deduced rates of multiple mating by members of the pregnant sex in major vertebrate groups in which viviparity is common yet brood sizes typically differ dramatically.
Because of a distinction between the social mating system and the realized or genetic mating system of a species (12), multiple paternity in the brood carried by a pregnant female (or multiple maternity in the brood carried by a pregnant male) is not necessarily the same thing as multiple mating by the brooding sex. However, genetic mating systems—the focus of the present report—should be especially germane for deciphering any selection pressures that may have impacted the comparative evolution of polygamous mating behaviors in viviparous species.
Brood Sizes and Multiple Mating: The Theory.
Based in part on the logic introduced by Bateman (14), we take it almost as a given that members of the nonpregnant sex (usually males) tend to evolve behavioral dispositions to seek copulation with members of the pregnant sex (usually females). In other words, because males tend to be fitness-rewarded for impregnating multiple females, whereas gravid females normally cannot expect comparable gains in fitness from having multiple mates, males in nearly all species should tend to be eager maters, whereas females typically are the more reticent sex with respect to seeking multiple sex partners. In viviparous species, the pregnancy phenomenon presumably amplifies these tendencies because pregnant females in effect become even more of a limiting reproductive resource than would be true based on anisogamy alone.
The primary question addressed in the present study is whether female reticence for multiple mating is reduced (or male eagerness for multiple mating is increased) in viviparous species with large broods. There are several theoretical reasons to suspect that larger broods might indeed tend to have more sires than smaller broods. First, several studies, including those on fish (e.g., refs. 27, 28), have shown that increased numbers of mates result in increased reproductive success. Second, larger broods obviously provide more “statistical room” for multiple paternity, all else being equal. For example, any mammalian pregnancy with just two gestating offspring could have two sires at most, whereas a piscine brood with 50 gestating progeny could in principle have dozens of sires. Third, any indirect benefits that a female might receive by taking multiple mates (such as higher genetic diversity within her brood or the possibility of better paternal genes in her offspring) might be amplified by the additional sires that a large brood could accommodate. Fourth, the same kind of argument could be made for any direct benefits (such as nuptial gifts) that a female might receive from taking multiple mates. Finally, because of the greater potential fitness payoffs, males too might have much higher incentives to mate preferentially with females who can carry larger broods. For all these reasons, it would not be too surprising if suitable genetic parentage analyses revealed that large-brood pregnancies average far more sires per litter than do small-brood pregnancies.
Brood Sizes and Multiple Mating: The Data.
Here we have reviewed the genetic literature on biological parentage in viviparous vertebrates and thereby estimated mate numbers and frequencies of multiple mating by pregnant individuals in each of two comparative contexts: (i) among live-bearing fish species (which typically have large broods) vs. mammals (which typically have small broods) vs. herps (which have highly variable but often intermediate brood sizes); and (ii) within a diversity of piscine, mammalian, and herpetological species that collectively display a wide range of litter sizes. From these two respective vantages, our analyses of the available data seem at face value to yield potentially opposing outcomes. On the one hand, the surveyed viviparous fishes did indeed prove to have significantly higher incidences of multiple mating than did the pregnant mammals, as generally predicted under the FLH. In addition, herpetological species with larger broods tended to have higher incidences of multiple paternity than did those with smaller broods. On the other hand, the same genetic data revealed no significant correlations between brood size and the incidences of multiple mating within either the surveyed viviparous fishes or mammals, a result that does not square so easily with the FLH alone. Thus, in the next section we advance a more comprehensive hypothesis in an attempt to accommodate these seemingly conflicting lines of empirical evidence about mating proclivities in viviparous vertebrates.
Logistical Constraint Hypothesis.
From our current and previous (20) reviews of the literature on genetic parentage within broods of viviparous vertebrates, what emerges overall is a need to reconcile two seemingly conflicting observations: (i) in both the fish and mammals (as well as herps), members of the pregnant sex routinely have multiple successful mates; and (ii) notwithstanding the fact that piscine pregnancies often outpace mammalian pregnancies with respect to incidences of multiple mating, within neither group does there appear to be any significant correlation between brood size and multiple paternity within the gestated broods of female-pregnant species.
To accommodate all of these empirical trends and reconcile their apparent contradictions, we hereby introduce a more encompassing “logistical constraint hypothesis” (LCH) to rationalize why multiple-mating is common in most viviparous species and yet mate numbers remain much lower than they could be given the high fecundities of large-brood species (notably the fishes). Under the LCH, we propose that in every vertebrate species some balance arises between two powerful but opposing evolutionary considerations: (i) near-universal fitness benefits of one sort or another that can come from multiple mating and (ii) purely logistical constraints on mate acquisition. For members of the pregnant sex, the fitness benefits from polygamy are likely to include fertilization insurance, nuptial gifts, higher genetic diversity or better genes within the brood, and other direct benefits or indirect genetic benefits at least some of which may apply to nearly all viviparous species. The logistical constraints are also universal (albeit species-specific in magnitude) and include such obvious factors as the finite time and effort that any individual can devote to finding and courting mates. Such logistical constraints are likely to vary according to population density, length of the breeding season, the intensity of competition for mates and for fertilization per se (including postcopulatory sperm competition and female sperm choice in many species), predation intensity, and countless other such factors in each species’ natural history and biology. What emerges from this selective balance, we propose, is a near-universal tendency in viviparous species for multiple mating, albeit tempered by what is logistically feasible within each species.
The LCH (alone or in conjunction with the FLH) makes several predictions that will be interesting to test in future research. For example, the highest incidences of multiple mating (and multiple paternity) within a brood are likely to be uncovered in large-clutch species for which the logistical hurdles of mating are exceptionally low (as might be true, for example, in species that are abundant on spawning grounds, spend little time in courtship, or otherwise experience many mating opportunities). Second, even for species with huge broods, polygamy may be uncommon if opportunities for multiple mating are severely limited (as might be true, for example, in rare or sparsely distributed species or those with elaborate courtship displays). Third, the basic tenet of the LCH is likely to apply not only to viviparous vertebrates but also to nonviviparous taxa, including species with external fertilization or external brood care. All of these and similar predictions of the LCH should be empirically testable via parentage analyses of appropriate taxa.
Materials and Methods
Our literature review, conducted in the summer and fall of 2010, sought to identify all substantive articles that have reported rates of multiple paternity within the broods of viviparous fishes and mammals, as estimated from genetic parentage analyses using highly polymorphic molecular markers (typically microsatellite loci). For each article, we summarized reported information on brood size, the proportion of broods with multiple sires (or multiple dams in the case of male-pregnant species), and successful numbers of mates for the pregnant sex. We also likewise summarized the available genetic literature for viviparous reptiles and amphibians (referred to as “herps”). Different authors sometimes used slightly different procedures of parentage analysis (29) to estimate the incidence of multiple mating by the gestating sex, but in all cases we accepted the authors’ published estimates of mate numbers and rates of multiple paternity, without further statistical adjustments of their raw genetic data.
All of our statistical summaries of these datasets were performed in Microsoft Excel. For each relevant mating-system parameter (such as brood size and number of sires per brood), we calculated means and SEs and also performed regression analyses between the variables. ANOVA analyses and F tests were used to address statistical significance of regression analyses; t tests were carried out to assess whether the various vertebrate groups differed in relevant parameters.
Supplementary Material
Acknowledgments
We thank Rosemary Bryne, Andrei Tatarenkov, Louis Bernatchez, and Giacomo Bernardi for helpful comments on the manuscript. This work was supported by funds from the University of California at Irvine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103329108/-/DCSupplemental.
References
- 1.Parker GA, Baker RR, Smith VGF. The origin and evolution of gamete dimorphism and the male-female phenomenon. J Theor Biol. 1972;36:529–553. doi: 10.1016/0022-5193(72)90007-0. [DOI] [PubMed] [Google Scholar]
- 2.Avise JC. Hermaphroditism: The Biology, Ecology, and Evolution of Dual Sexuality. New York: Columbia University Press; 2011. [Google Scholar]
- 3.Williams GC. Adaptation and Natural Selection. Princeton, NJ: Princeton University Press; 1966. [Google Scholar]
- 4.Jones AG, Avise JC. Mating systems and sexual selection in male-pregnant pipefishes and seahorses: Insights from microsatellite-based studies of maternity. J Hered. 2001;92:150–158. doi: 10.1093/jhered/92.2.150. [DOI] [PubMed] [Google Scholar]
- 5.Berglund A, Rosenqvist G, Svennson I. Reversed sex roles and parental energy investment in zygotes of two pipefish (Syngnathidae) species. Mar Ecol Prog Ser. 1986;29:209–215. [Google Scholar]
- 6.Rosenqvist G. Sex role reversal in a pipefish. Mar Freshwat Behav Physiol. 1993;23:219–230. [Google Scholar]
- 7.Jones AG, Rosenqvist G, Berglund A, Arnold SJ, Avise JC. The Bateman gradient and the cause of sexual selection in a sex-role-reversed pipefish. Proc Biol Sci. 2000;267:677–680. doi: 10.1098/rspb.2000.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones AG, Avise JC. Microsatellite analysis of maternity and the mating system in the Gulf pipefish Syngnathus scovelli, a species with male pregnancy and sex-role reversal. Mol Ecol. 1997;6:203–213. doi: 10.1046/j.1365-294x.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones AG, Avise JC. Polygynandry in the duskey pipefish Syngnathus floridae revealed by microsatellite DNA markers. Evolution. 1997;51:1611–1622. doi: 10.1111/j.1558-5646.1997.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones AG, Kvarnemo C, Moore GI, Simmons LW, Avise JC. Microsatellite evidence for monogamy and sex-biased recombination in the Western Australian seahorse Hippocampus angustus. Mol Ecol. 1998;7:1497–1505. doi: 10.1046/j.1365-294x.1998.00481.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones AG, Rosenqvist G, Berglund A, Avise JC. The genetic mating system of a sex-role-reversed pipefish (Syngnathus typhle): A molecular inquiry. Behav Ecol Sociobiol. 1999;46:357–365. [Google Scholar]
- 12.Avise JC, Jones AG, Walker D, DeWoody JA. Genetic mating systems and reproductive natural histories of fishes: Lessons for ecology and evolution. Annu Rev Genet. 2002;36:19–45. doi: 10.1146/annurev.genet.36.030602.090831. [DOI] [PubMed] [Google Scholar]
- 13.Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man, 1871–1971. Chicago: Aldine; 1972. pp. 136–179. [Google Scholar]
- 14.Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 15.Wade MJ, Arnold SJ. The intensity of sexual selection in relation to male sexual behavior, female choice and sperm precedence. Anim Behav. 1980;28:446–461. [Google Scholar]
- 16.Wade MJ, Shuster SM. Bateman (1948): Pioneer in the measurement of sexual selection. Heredity. 2010;105:507–508. doi: 10.1038/hdy.2010.8. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SJ, Duvall D. Animal mating systems: A synthesis based on selection theory. Am Nat. 1994;143:317–348. [Google Scholar]
- 18.Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- 19.Jones AG. On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution. 2009;63:1673–1684. doi: 10.1111/j.1558-5646.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 20.Avise JC, Liu JX. Multiple mating and its relationship to alternative modes of gestation in male-pregnant versus female-pregnant fish species. Proc Natl Acad Sci USA. 2010;107:18915–18920. doi: 10.1073/pnas.1013786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasui Y. The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol Evol. 1998;13:246–250. doi: 10.1016/s0169-5347(98)01383-4. [DOI] [PubMed] [Google Scholar]
- 22.Yasui Y. Female multiple mating as a genetic bet-hedging strategy when mate choice criteria are unreliable. Ecol Res. 2001;16:605–616. [Google Scholar]
- 23.Møller AP, Alatalo RV. Good-genes effects in sexual selection. Proc Biol Sci. 1999;266:85–91. [Google Scholar]
- 24.Byers JA, Waits L. Good genes sexual selection in nature. Proc Natl Acad Sci USA. 2006;103:16343–16345. doi: 10.1073/pnas.0608184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neff BD, Pitcher TE, Ramnarine IW. Inter-population variation in multiple paternity and reproductive skew in the guppy. Mol Ecol. 2008;17:2975–2984. doi: 10.1111/j.1365-294X.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 26.Wourms JP. Viviparity: The maternal-fetal relationship in fishes. Am Zool. 1981;21:473–515. [Google Scholar]
- 27.Serbezov D, Bernatchez L, Olsen EM, Vøllestad LA. Mating patterns and determinants of individual reproductive success in brown trout (Salmo trutta) revealed by parentage analysis of an entire stream living population. Mol Ecol. 2010;19:3193–3205. doi: 10.1111/j.1365-294X.2010.04744.x. [DOI] [PubMed] [Google Scholar]
- 28.Garant D, Dodson JJ, Bernatchez L. Ecological determinants and temporal stability of the within-river population structure in Atlantic salmon (Salmo salar L.) Mol Ecol. 2000;9:615–628. doi: 10.1046/j.1365-294x.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 29.Jones AG, Ardren WR. Methods of parentage analysis in natural populations. Mol Ecol. 2003;12:2511–2523. doi: 10.1046/j.1365-294x.2003.01928.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.