Abstract
We have developed an efficient strategy to a skeletally diverse chemical library, which entailed a sequence of enyne cycloisomerization, [4 + 2] cycloaddition, alkene dihydroxylation, and diol carbamylation. Using this approach, only 16 readily available building blocks were needed to produce a representative 191-member library, which displayed broad distribution of molecular shapes and excellent physicochemical properties. This library further enabled identification of a small molecule, which effectively suppressed glycolytic production of ATP and lactate in CHO-K1 cell line, representing a potential lead for the development of a new class of glycolytic inhibitors.
Keywords: diversity-oriented synthesis, glycolysis, skeletal diversity
Structurally diverse collections of small molecules provide a validated source of chemical probes for basic and translational biomedical research (1, 2). Variation of the scaffold architecture of such compound libraries is particularly desirable to enable identification of new bioactive chemical probes with higher probability and greater efficiency. High-throughput synthesis of skeletally diverse small-molecule libraries represents one of the most challenging aspects of diversity-oriented synthesis and requires identification of efficient reaction sequences that can rapidly convert a small subset of readily available compounds to a large number of skeletally diverse chemical entities for subsequent biomedical applications (3).
Transition metal-catalyzed cycloisomerization of enynes represents a powerful method for structural diversification (4–6). Our group previously demonstrated that various reaction topologies could be controlled by a proper choice of the transition metal catalyst, as well as the functionalization of the starting enyne (7–10). Significant advances in this area can now enable incorporation of such transformations into synthetic strategies for the assembly of skeletally diverse chemical libraries (11–18). However, several challenges remain to be addressed to facilitate access to high-diversity chemical libraries. Typically, multiple cycloisomerization precursors are manually assembled to yield different skeletal frameworks upon their cycloisomerizations. A smaller number of building blocks would minimize this laborious process and increase efficiency. Another limitation is the difficulty of subsequent diversification of cycloisomerization products, which is complicated by the lack of common functional groups and variable chemical reactivity of such compounds. Ideally, the cycloisomerization should provide access to products containing the same functional group to enable the next diversity-generating step, which should yield another common functional group. If such common and reactive functional groups are efficiently produced at every stage of the synthesis, this synthetic pathway can readily provide access to a structurally diverse library starting with only a small set of building blocks.
We describe the development of a unique approach, which harnesses the diversity-generating power of several transformations, including transition metal-catalyzed 1,6-enyne cycloisomerization, [4 + 2] cycloaddition, Os-catalyzed dihydroxylation and Sn-catalyzed carbamylation. This synthetic sequence is designed to efficiently create and rapidly process common functional groups, which are generated at each stage of the assembly process, to enable both skeletal and structural diversification. We were able to employ only 16 simple building blocks to assemble a representative library of 191 skeletally diverse, diastereomerically pure compounds with broad distribution of molecular shapes as determined by the principal moment-of-inertia (PMI) computational analysis. Subjection of this library to a cellular screen monitoring ATP production in CHO-K1 cells with impaired mitochondrial activity yielded a unique chemotype that effectively blocked glycolytic ATP synthesis, inhibited lactate production, and suppressed cellular proliferation of this cell line.
Results and Discussion
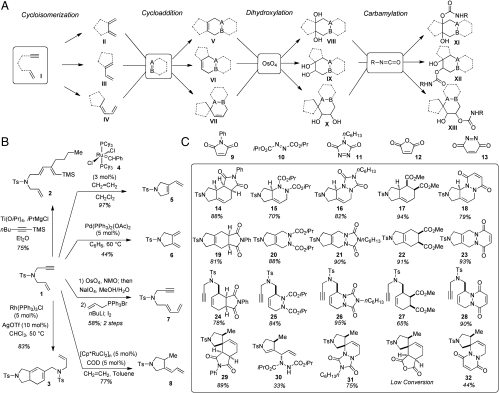
Our general synthetic strategy is depicted in Fig. 1A. The process begins with subjection of 1,6-enyne I to different cycloisomerization conditions to give diverse products II–IV, containing a common 1,3-diene subunit and setting the stage for a series of subsequent [4 + 2] cycloadditions. Each of the cycloadducts V–VII contains an alkene unit and provides an opportunity for a subsequent transformation; i.e., Os-catalyzed dihydroxylation to furnish a set of vicinal diols VIII–X. Each diol is further diversified by conversion into carbamates XI–XIII. This process would enable rapid conversion of a single 1,6-enyne I into a large number of diverse compounds by using only simple and common building blocks and reagents.
Fig. 1.
(A) Synthetic strategy for assembly of a skeletally diverse chemical library. The approach relies on the initial series of cycloisomerizations of acyclic enyne, followed by subsequent [4 + 2] cycloadditions and alkene diversifications via dihydroxylation and carbamylation. (B) Transformations of enyne 1 into a series of 1,3-dienes. (C) Structures and isolated yields of products of [4 + 2] cycloadditions of dienes 5, 6, 7, and 8 with dienophiles 9, 10, 11, 12, and 13.
We first examined a number of protocols for converting 1,6-enyne 1 into a series of structurally diverse 1,3-dienes (Fig. 1B). Enyne 1 can be efficiently produced by N-sulfonylation and propargylation of allyl amine (19). Subjection of enyne 1 to 1-trimethylsilyl-hexyne in the presence of Ti(Oi- Pr )4 and i-PrMgCl gave triene 2 (20). Whereas the level of chemoselectivity of this process was excellent, the main product 2 was produced as a 5∶1 mixture of inseparable regioisomeric 1,3-dienes. Treatment of enyne 1 with Rh(PPh3)3Cl and AgOTf resulted in cyclodimerization to give pyrroline-fused cyclohexadiene 3 (21). Treatment of 1,6-enyne 1 with the Grubbs catalyst 4 triggered enyne metathesis and afforded the desired 1,3-diene 5 (22). We found that higher efficiency was achieved when the reaction was conducted at ambient temperature in the presence of ethylene (23). Another 1,6-enyne cycloisomerization topology could be realized using of a catalytic amount Pd(PPh3)2(OAc)2 to give bis-methylene pyrrolidine 6 (24). Chemoselective Os-catalyzed dihydroxylation, followed by periodinate-induced oxidative cleavage of the resulting diol and subsequent Wittig olefination enabled conversion of enyne 1 to dieneyne 7. Moderate diastereoselectivity of the Wittig reaction was improved by treatment of the olefination product with a catalytic amount of iodine. Treatment of enyne 1 with catalytic amounts of pentamethyl cyclopentadienyl ruthenium dichloride and cyclooctadiene in the presence of ethylene produced exocyclic 1,3-diene 8 (25). Thus, six structurally diverse 1,3-dienes were readily prepared starting from a single acyclic enyne precursor 1.
The next stage of our investigation focused on subsequent diversification of initially produced 1,3-dienes via a series of [4 + 2] cycloadditions. We selected five symmetric dienophiles, including maleimide 9, azodicarboxylate 10, 1,2,4-triazoline-3,5-dione 11, maleic anhydride 12 and pyridazine-3,6-dione 13 (Fig. 1C). Diene 2, which was initially produced as a mixture of inseparable regioisomers, produced mixtures of the corresponding cycloadducts and was not used for subsequent reactions. Bicyclic diene 3 proved to be unreactive toward most of the dienophiles. The four remaining 1,3-dienes 5–8 successfully participated in a majority of the required [4 + 2] cycloadditions (Fig. 1C). Cycloaddition of diene 5 with maleimide 9 readily proceeded at 60 °C to give cycloadduct 14 as a single diastereomer. Similarly high efficiency was observed in cycloadditions of diene 5 with azodicarboxylate 10, and 1,2,4-triazoline-3,5-dione 11, to give the adducts 15, 16, respectively. Cycloaddition of maleic anhydride 12 with diene 5 proceeded with high diastereoselectivity. The initially produced adduct was subjected to methanolysis to give diester 17 in 94% yield. Reaction of 5 with pyridazine-3,6-dione 13, which was generated in situ by oxidation of the corresponding maleic hydrazide with Pb(OAc)4, efficiently delivered tricyclic cycloadduct 18. All five cycloadditions of diene 6 produced desired products 19–23 in high yields, under mild conditions. Methanolysis of the initially produced maleic anhydride adduct was employed to give diester 22. Tricyclic cycloadduct 23 was produced with higher efficiency using hypochlorite-induced generation of dienophile 13 (26). All five dienophiles successfully reacted with diene 7 to give the corresponding adducts 24–28 in 65–90% yield. Single diastereomers 24 and 27 were obtained in the case of maleimide and maleic anhydride cycloadditions; the latter reaction was again subjected to methanolysis. The reactivity of exocyclic diene 8 proved low presumably due to the difficulty of achieving the required s-cis- conformation. Indeed, reactions of diene 8 with 9 and 11 required activation with 4 M lithium bis-trifluoromethanesulfonimide in acetone (LTAC) (27) to produce cycloadducts 29 and 31, respectively. Reactions of 8 with maleic anhydride 12 proceeded with low efficiency despite significant optimization efforts. Treatment of diene 8 with azodicarboxylate 10 in the presence of LTAC resulted in formation of ene-reaction product 30. Thus, 19 alkenes were produced successfully with a considerable level of skeletal diversity ranging from carbocyclic or heterocyclic rings (i.e., 25, 27, and 30) to bicyclic compounds (i.e., 15, 22, and 28) and fused and spirotricyclic molecules (i.e., 29, 31, and 32).
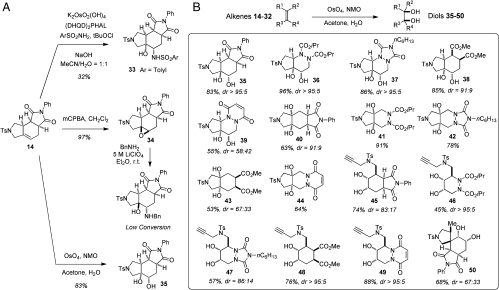
Our next objective was to identify an efficient and robust protocol, which would enable subsequent diversification of skeletally diverse alkenes 14–32 despite their variable level of chemical reactivity. Among various methods examined, three protocols proved to be particularly promising, which included dihydroxylation, aminohydroxylation and epoxidation (Fig. 2A). Treatment of alkene 14 with N-methylmorpholine N-oxide and catalytic amount of OsO4 in acetone/water successfully produced diol 35 in 83% yield as a single diastereomer, which was characterized by X-ray crystallography as an acetal. Subjection of alkene 14 to Os-catalyzed aminohydroxylation (28, 29) gave N-sulfonyl amino alcohol 33 in 32% yield as a single regio and diastereomeric product. Subsequent attempts to increase the efficiency of this process proved unsuccessful. Treatment of alkene 14 with metachloroperoxybenzoic acid (mCPBA) gave epoxide 34 as a single diastereomer, which was characterized by X-ray crystallography. The epoxide was produced from the more hindered concave face of 14 possibly due to the directing ability of the imide. However, opening of epoxide 34 with a range of nucleophiles, including primary amines under a variety of conditions proved unsuccessful. This exploratory effort established alkene Os-catalyzed dihydroxylation as the most reliable, efficient and robust method for further diversification. This method was next applied to all 19 cycloadducts 14–32 (Fig. 1C). We found that 16 reactions proceeded successfully to give the corresponding diols 35–50 (Fig. 2B). Generally, excellent distereoselectivity was observed with only 3 reactions proceeding with moderate diastreoselection (39, 43, and 50). However, in such cases, major products were obtained in diastereomerically pure form following simple chromatographic purification.
Fig. 2.
(A) Selected chemical transformations of alkene 14. Such reactions were designed to enable subsequent structural diversification of this representative cycloadduct. (B) Results of Os-catalyzed dihydroxylations of alkenes 14–32. Structures of major diastereomers of each product are shown, as well as isolated yields following by silica gel chromatographic purification. Diastereoselectivity of each transformation was determined by analysis of crude reaction mixtures by 1H NMR. Structures of 35, 36, 37, 38, 39, 42, 43, 49, and 50 were determined by X-ray crystallography of either the parent diol or the corresponding acetal (SI Appendix and Tables S1–S10).
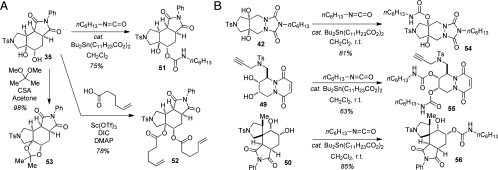
We next examined three alternative strategies for diol functionalization (Fig. 3A). Subjection of a representative substrate 35 to hexyl isocyanate in the presence of a catalytic amount of dibutyltin dilaurate afforded a 6∶1 mixture of regioisomeric carbamate products, with the major isomer 51 isolated in 75% yield. Treatment of diol 35 with 5-hexenoic acid in the presence of diisopropylcarbodiimide (DIC) and scandium triflate gave diester 52. Subjection of diol 35 to 2,2-dimethoxypropane in the presence of camphor sulfonic acid (CSA) afforded the corresponding acetal 53. Whereas all reactions showed promise, we felt that both the acetal and the ester functionalities in 52 and 53 may not be compatible with cell-based screening conditions. To avoid such complications, we selected the transformation of diols to the corresponding carbamates as the final diversification strategy. The carbamates would be expected to elicit greater chemical stability and higher resistance to the action of hydrolytic enzymes. This approach proved to be general for converting a series of representative diols 42, 49, and 50 into the corresponding carbamates 54, 55, and 56 (Fig. 3B). Such reactions could be readily performed on the desired scale to produce an average of 15 mg of final products following parallel liquid chromatography mass spectrometry (LCMS) purification.
Fig. 3.
(A) Representative transformations of diol 35. Such reactions were investigated to enable subsequent diversification of all the initially produced diols. (B) Carbamylation of a selected subset of diols using n-hexyl isocynate. Depending on the structure of the diol, either mono- or bis-carbamates were selectively produced.
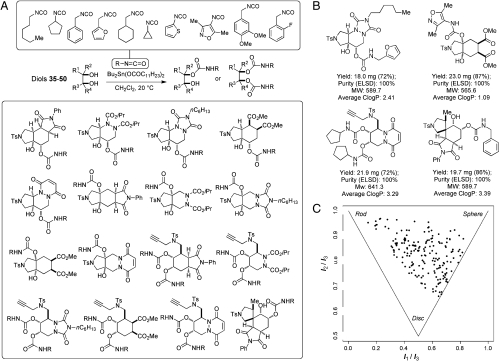
The final stage of the synthesis entailed parallel carbamylation of 16 diols with a set of reactive and structurally diverse isocyanates. We identified a representative set of commercially available 10 isocyanates (Fig. 4A) that would efficiently participate in this process and afford compounds with structurally diverse and favorable physicochemical properties (vide infra). We then employed dibutyltin dilaurate-based catalytic protocol for parallel diversification of 16 diols 35–50 to produce a collection of the corresponding carbamates (Fig. 4A). We determined that 159 of the 160 reactions proceeded successfully. Depending on the structure of each diol, either mono- or bis-carbamates were produced in high purity (typically greater than 90%) and 66% average yield following parallel preparative LCMS purification. Structures, yields, and selected physicochemical properties of four randomly selected carbamates are shown in Fig. 4B. This data illustrate the high efficiency of each final reaction, excellent purity, and favorable physicochemical properties of these compounds. This effort completed the assembly of a 191-compound library, which included 16 alkenes (Fig. 1C), 16 diols (Fig. 2B), and 159 carbamates (Fig. 4A).
Fig. 4.
(A) Carbamylation of diols 35–50. Reactions were performed on 18–20 mg scale to deliver final compounds, which were purified by preparative LCMS. (B) Structures, yields, purities, and selected physicochemical properties of four selected library members. (C) Molecular shape analysis using PMI computational method. The graph shows broad shape distribution of the 191-member library using a standard triangular plot.
Recent cheminformatics studies strongly suggest that the higher molecular weight of bioactive compounds can be tolerated provided that the lipophilicity and aqueous solubility are properly addressed (30). Such parameters can be estimated by calculating logarithmic value of n-octanol/water partition coefficient (LogP), which should ideally fall within a range of 0–5. We employed the XLogP3 program (31) to calculate lipophilicity distribution of our 191-member compound library. This effort revealed that LogPs of 90% of the library fell into the range of 0–6 with an average value of 2.64 (SI Appendix and Fig. S1A). The average molecular weight was determined to be 599 (SI Appendix and Fig. S1B). No decomposition of any compounds was observed in either neat form or when dissolved in methanol or dimethyl sulfoxide, which demonstrated their high chemical stability. We also assessed the overall diversity level of the library using a computational method based on the normalized PMI ratios (32) (Fig. 4C), which was recently employed by Schreiber, Clemons, and coworkers (15). We first derived three-dimensional structures for each compound using the OMEGA software (33). The three principal moments of inertia (I1, I2, and I3) were then calculated for each structure and sorted in ascending order (I1 < I2 < I3). The two characteristic normalized PMI ratios (I1/I3 and I2/I3) were then calculated for each compound and plotted against each other. Due to the intrinsic characteristics of the inertia tensor, all plotted values will fall within an isosceles triangle defined by the points (0,1), (0.5,0.5) and (1,1). The three points (0,1), (0.5,0.5) and (1,1) of the triangle also define the three extreme principal shapes of rod, disk, and sphere, respectively and intermediary shapes will fall at various locations within the triangle. Plotting the normalized PMI ratios using this scheme provides a descriptive visualization of the shape diversity of a compound library. Using this method, we analyzed the normalized PMI ratios for all individual compounds of our 191-member library using lowest energy conformers of each compound (Fig. 4C). This analysis revealed that the molecular shapes are evenly distributed within the intermediate region of the triangular plot, indicating that the library members elicit substantial shape diversity. We also randomly selected 300 diverse compounds from a commercial library subset from ZINC (ChemDiv, 60% Tanimoto cut-off) (34) and subjected them to the same analysis. The commercial library PMI ratio plot revealed a high concentration of points located toward the upper left of the triangular plot representing rod and rod-disk shape compounds (SI Appendix and Fig. S2). This analysis demonstrated unique structural features of our library, which was overall more spherically distributed in shape compared to a representative commercially available compound collection and consistent with presence of stereochemically rich, polycyclic structures.
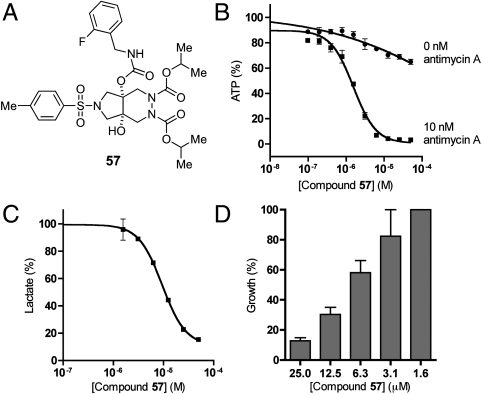
We next examined the ability of this skeletally diverse small-molecule library to enable identification of unique inhibitors of aerobic glycolysis. Such compounds would be useful not only as chemical probes of cellular energy metabolism but also as potential leads for development of drugs targeting upregulation of aerobic glycolysis in cancer (35). We employed our recently developed chemical genetic screen (36), which monitors glycolytic ATP production in cells with suppressed mitochondrial activity. This screen was performed by subjecting antimycin A-treated CHO-K1 cells to a newly synthesized 191-member library and measuring effects of each library member on ATP synthesis following 30 min of incubation. This effort identified compound 57 as the most potent inhibitor (Fig. 5A), which depleted ATP production with an IC50 of 2.2 μM in the presence of antimycin A while not substantially altering ATP levels in cells with normal mitochondrial activity (Fig. 5B). To validate suppression of glycolysis in CHO-K1 cells by 57, we examined the effects of this compound on lactate production, cell cycle progression, and cellular proliferation. Subjecting CHO-K1 cells to 57 resulted in dose-dependent suppression of lactate production (Fig. 5C) and growth inhibition (Fig. 5D). Both effects were fully consistent with impairment of glycolysis by 57 with similar potency to that observed in the primary ATP screen. Furthermore, exposure of CHO-K1 cells to 57 (16.7 μM) for 18 h resulted in a marked increase in the G0/G1 population to 54% from 44% in the control, and a decrease in the S cell population to 19% from 29% (SI Appendix and Fig. S3), which was again fully consistent with inhibition of glycolysis.
Fig. 5.
Effects of compound 57 on ATP synthesis, lactate production and cell proliferation. (A) Chemical structure of 57. (B) Inhibition of intracellular ATP level in CHO-K1 cells upon treatment with 57 in the presence or absence of antimycin A. (C) Inhibition of lactate production in CHO-K1 cells upon treatment of 57. (D) Effect of 57 on the growth of CHO-K1 cells. All values are presented as percentage of vehicle treated samples. Each value is the mean ± SEM of duplicate or triplicate values from a representative experiment.
In closing, we presented an efficient synthetic strategy for parallel assembly of a skeletally diverse chemical library starting from a small number of simple and readily available building blocks. This general approach required the availability of reactive fragments at every stage of the parallel assembly process and robust reactions for efficient functionalization of compounds with common functional groups but diverse reactivity profiles. This concept was validated by a production of a unique 191-member library with broad distribution of molecular shapes starting from only 16 simple building blocks. Subsequent cellular screen of this compound collection identified a unique small-molecule probe 57, which effectively suppressed glycolytic production of ATP and lactate in CHO-K1 cell line. Determination of the cellular mechanism of action of 57 and further optimization of its activity profile are in progress and will be reported in due course.
Materials and Methods
Cycloisomerizations of Enyne 1.
Six detailed experimental procedures for conversion of 1.6-enyne 1 to 1,3-dienes 2, 3, 5, 6, 7, and 8 are provided in the SI Appendix, as well as characterization of all unique compounds by 1H NMR, 13C NMR, and MS.
[4 + 2] Cycloadditions.
Detailed procedures for cycloadditions of dienes 5–8 with dienophiles 9–13 are provided in the SI Appendix. All unique compounds were fully characterized by 1H NMR, 13C NMR, and MS.
Alkene Dihydroxylation.
Experimental protocols for Os-catalyzed dihydroxylation of 16 alkenes 14–32 to give the corresponding diols 35–50 are provided in the SI Appendix. All unique compounds were fully characterized by 1H NMR, 13C NMR and MS. Relative stereochemistry was established at this stage for all products 35–50 either by X-ray crystallography or a combination of NMR spectroscopic techniques (SI Appendix and Tables S1–S10).
Diol Carbamylation.
A general protocol of conversion of 16 diols 35–50 to the corresponding 159 carbamates is described in the SI Appendix. Several representative carbamates were fully characterized by 1H NMR, 13C NMR, and MS. In addition, detailed analytical purity characterization of 32 randomly selected carbamates following their LCMS preparative purification is also provided in the SI Appendix.
Supplementary Material
Acknowledgments.
We thank Kevin Marin for LCMS purifications, Dr. Ian Steele for X-ray analysis, Marco Maggioni, and Dr. Weifan Zheng for help in generating 3D structures. This work was funded by the National Institutes of Health (Grant P50 GM086145) and the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015253108/-/DCSupplemental.
References
- 1.Stockwell BR. Chemical genetics: Ligand-based discovery of gene function. Nat Rev Genet. 2000;1:116–125. doi: 10.1038/35038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredel M, Jacoby E. Chemogenomics: An emerging strategy for rapid target and drug discovery. Nat Rev Genet. 2004;5:262–275. doi: 10.1038/nrg1317. [DOI] [PubMed] [Google Scholar]
- 3.Burke MD, Schreiber SL. A planning strategy for diversity-oriented synthesis. Angew Chem Int Edit. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 4.Aubert C, Buisine O, Malacria M. The behavior of 1,n-enynes in the presence of transition metals. Chem Rev. 2002;102:813–834. doi: 10.1021/cr980054f. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Sun J, Kozmin S. Gold and platinum catalysis of enyne cycloisomerization. Adv Synth Catal. 2006;348:2271–2296. [Google Scholar]
- 6.Michelet V, Toullec P, Genêt JP. Cycloisomerization of 1,n-enynes: challenging metal-catalyzed rearrangements and mechanistic insights. Angew Chem Int Edit. 2008;47:4268–4315. doi: 10.1002/anie.200701589. [DOI] [PubMed] [Google Scholar]
- 7.Schramm MP, Reddy DS, Kozmin SA. Siloxyalkyne-alkene metathesis: Rapid access to highly functionalized enones. Angew Chem Int Edit. 2001;40:4274–4277. doi: 10.1002/1521-3773(20011119)40:22<4274::AID-ANIE4274>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Kozmin SA. Gold-catalyzed cycloisomerizations of siloxy enynes to cyclohexadienes. J Am Chem Soc. 2004;126:11806–11807. doi: 10.1021/ja046112y. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Kozmin SA. Gold-catalyzed assembly of heterobicyclic systems. J Am Chem Soc. 2005;127:6962–6963. doi: 10.1021/ja051110e. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Conley MP, Zhang L, Kozmin SA. Gold and platinum-catalyzed cycloisomerizations of 1,5-enynes to cyclohexadienes with a broad alkyne scope. J Am Chem Soc. 2006;128:9705–9710. doi: 10.1021/ja063384n. [DOI] [PubMed] [Google Scholar]
- 11.Micalizio GC, Schreiber SL. A boronic ester annulation strategy for diversity-oriented organic synthesis. Angew Chem Int Edit. 2002;41:152–154. doi: 10.1002/1521-3773(20020104)41:1<152::aid-anie152>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel DA, Schroeder FC, Duvall JR, Schreiber SL. An oligomer-based approach to skeletal diversity in small molecule synthesis. J Am Chem Soc. 2006;128:14766–14767. doi: 10.1021/ja065724a. [DOI] [PubMed] [Google Scholar]
- 13.Kumagai N, Muncipinto G, Schreiber SL. Short synthesis of skeletally and stereochemically diverse small molecules by coupling petasis condensation reactions to cyclization reactions. Angew Chem Int Edit. 2006;45:3635–3638. doi: 10.1002/anie.200600497. [DOI] [PubMed] [Google Scholar]
- 14.Luo T, Schreiber SL. Gold(I)-catalyzed coupling reactions for the synthesis of diverse small molecules using the build/couple/pair strategy. J Am Chem Soc. 2009;131:5667–5674. doi: 10.1021/ja900414s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pizzirani D, Kaya T, Clemons PA, Schreiber SL. Stereochemical and skeletal diversity arising from amino propargylic alcohols. Org Lett. 2010;12:2822–2825. doi: 10.1021/ol100914b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brummond KM, Mitasev B. Allenes and transition metals: A diverging approach to heterocycles. Org Lett. 2004;6:2245–2248. doi: 10.1021/ol0492391. [DOI] [PubMed] [Google Scholar]
- 17.Brummond KM, Mao S, Shinde SN, Johnston PJ, Day BW. Design and synthesis of a library of tetracyclic hydroazuleneisoindoles. J Comb Chem. 2009;11:486–494. doi: 10.1021/cc900024p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morton D, Leach S, Cordier C, Warriner S, Nelson A. Synthesis of natural-product-like molecules with over eighty distinct scaffolds. Angew Chem Int Edit. 2009;48:104–109. doi: 10.1002/anie.200804486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel MC, Livinghouse T, Pagenkopf BL. The catalytic intramolecular Pauson–Khand reaction: 2,3,3α,4-tetrahydro-2-[(4methylbenzene)sulfonyl]cyclopenta[c]pyrrol-5(1H)-one[(cyclopenta[b]pyrrol-5(1H)-one, 2,3,3α,4-tetrahydro-1-[(4-methylphenyl)sulfonyl]-)] Org Synth. 2003;80:93–103. [Google Scholar]
- 20.Hamada T, Suzuki D, Urabe H, Sato F. Titanium alkoxide-based method for stereoselective synthesis of functionalized conjugated dienes. J Am Chem Soc. 1999;121:7342–7344. [Google Scholar]
- 21.Oh CH, Sung HR, Jung SH, Lim YM. Rhodium-catalyzed [2 + 2 + 2] cyclizations of 1,6-enynes. Tetrahedron Lett. 2001;42:5493–5495. [Google Scholar]
- 22.Trnka TM, Grubbs RH. The development of L2X2RuCHR olefin metathesis catalysts: An organometallic success story. Acc Chem Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 23.Mori M, Sakakibara N, Kinoshita A. Remarkable effect of ethylene gas in the intramolecular enyne metathesis of terminal alkynes. J Org Chem. 1998;63:6082–6083. doi: 10.1021/jo980896e. [DOI] [PubMed] [Google Scholar]
- 24.Trost BM, Chen SF. A synthesis of substituted pyrrolidines via a palladium(2+)-catalyzed cyclization. An unusual approach to a carbapenem. J Am Chem Soc. 1986;108:6053–6054. doi: 10.1021/ja00279a071. [DOI] [PubMed] [Google Scholar]
- 25.Mori M, Saito N, Tanaka D, Takimoto M, Sato Y. Novel alkenylative cyclization using a ruthenium catalyst. J Am Chem Soc. 2003;125:5606–5607. doi: 10.1021/ja029747a. [DOI] [PubMed] [Google Scholar]
- 26.Kealy TJ. The chemistry of diazaquinones. 3,6-pyridazinedione and 1,4-phthalazinedione. J Am Chem Soc. 1962;84:966–973. [Google Scholar]
- 27.Handy ST, Grieco PA, Mineur C, Ghosez L. Lithium trifluoromethanesulfonimide in acetone or diethyl ether as a safe alternative to lithium perchlorate in diethyl ether for effecting Diels–Alder reactions. Unexpected influence of the counterion on exo/endo selectivity. Synlett. 1995;1995:565–567. [Google Scholar]
- 28.Li G, Chang H-T, Sharpless KB. Catalytic asymmetric aminohydroxylation (AA) of olefins. Angew Chem Int Edit. 1996;35:451–454. [Google Scholar]
- 29.Bodkin JA, McLeod MD. The Sharpless asymmetric aminohydroxylation. J Chem Soc Perk T 1. 2002:2733–2746. [Google Scholar]
- 30.Ganesan A. The impact of natural products upon modern drug discovery. Curr Opin Chem Biol. 2008;12:306–317. doi: 10.1016/j.cbpa.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Cheng T, et al. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J Chem Inf Model. 2007;47:2140–2148. doi: 10.1021/ci700257y. [DOI] [PubMed] [Google Scholar]
- 32.Sauer WHB, Schwarz MK. Molecular shape diversity of combinatorial libraries: A prerequisite for broad bioactivity. J Chem Inf Comp Sci. 2003;43:987–1003. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
- 33.OEChem, version 1.3.4. Santa Fe, NM: OpenEye Scientific Software; 2005. available at www.eyesopen.com. [Google Scholar]
- 34.Irwin JJ, Shoichet BK. ZINC—A free database of commercially available compounds for virtual screening. J Chem Inf Model. 2005;45:177–182. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulanovskaya OA, Cui J, Kron SJ, Kozmin SA. A pairwise chemical genetic screen identifies new inhibitors of glucose transport. Chem Biol. 2011;18 doi: 10.1016/j.chembiol.2010.12.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.