Abstract
The endothelium plays a critical role in promoting inflammation in cardiovascular disease and other chronic inflammatory conditions, and many small-molecule screens have sought to identify agents that prevent endothelial cell activation. Conversely, an augmented immune response can be protective against microbial pathogens and in cancer immunotherapy. Yet, small-molecule screens to identify agents that induce endothelial cell activation have not been reported. In this regard, a bioassay was developed that identifies activated endothelium by its capacity to trigger macrophage inflammatory protein 1 beta from primary monocytes. Subsequently, a 642-compound library of 39 distinctive scaffolds generated by a diversity-oriented synthesis based on the nucleophilic phosphine catalysis was screened for small molecules that activated the endothelium. Among the active compounds identified, the major classes were synthesized through the sequence of phosphine-catalyzed annulation, Tebbe reaction, Diels–Alder reaction, and in some cases, hydrolysis. Ninety-six analogs of one particular class of compounds, octahydro-1,6-naphthyridin-4-ones, were efficiently prepared by a solid-phase split-and-pool technique and by solution phase analog synthesis. Structure-function analysis combined with transcriptional profiling of active and inactive octahydro-1,6-naphthyridin-4-one analogs identified inflammatory gene networks induced exclusively by the active compound. The identification of a family of chemical probes that augment innate immunity through endothelial cell activation provides a framework for understanding gene networks involved in endothelial inflammation as well as the development of novel endothelium-driven immunotherapeutic agents.
Keywords: solid-phase library synthesis, immune activator, medicinal chemistry, nucleophilic phosphine-catalyzed annulations, structure-activity relationship analysis
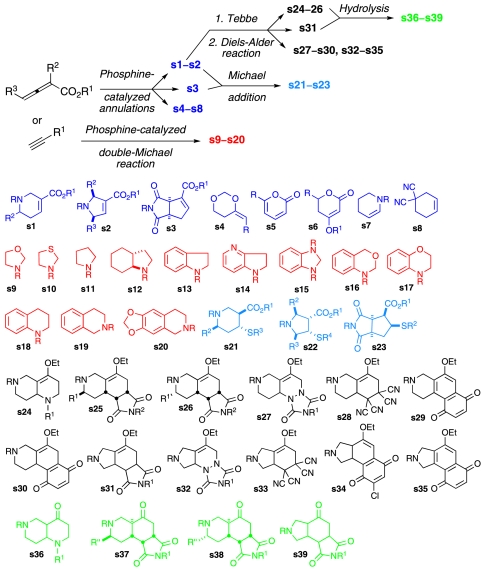
The synthesis and use of bioactive small molecules to gain insight into biological systems is a major facet of modern chemical biology (1) and several hypotheses regarding the most effective ways to increase the probability of discovering chemical probes of desired activity have been put forth (2–4). In this context, combinatorial chemistry emerged as the fastest way to generate a large number of candidate compounds (5, 6). However, early practices of large library synthesis and screening unveiled that the large number alone is not sufficient for increased hit rates and the structural diversity within the library may be important. The idea of generating a structurally diverse collection of compounds within a streamlined sequence of reactions has been elegantly formulated in the algorithms of diversity-oriented synthesis (DOS) (7–10). In this vein, a series of nucleophilic phosphine catalysis reactions has been developed, resulting in the production of 20 distinctive carbo- and heterocyclic scaffolds (Scheme 1, s1–s20). The goal in developing previously undescribed reactions was to provide previously unexampled heterocyclic frameworks that deviate from the relatively limited pool of molecular motifs used by pharmaceutical companies. For allenes, one-step ring-forming reactions were carried out using either commercially available or otherwise readily available imines, maleimides, aldehydes, aziridines, and electron-deficient olefins, resulting in scaffolds s1–s8 (11–20). For electron-deficient acetylenes, a one-step double-Michael reaction with readily available dinucleophiles was effected by a diphenylphosphinopropane catalyst, providing heterocyclic frameworks s9–s20 (21, 22). The α,β-unsaturated ester functionality in tetrahydropyridine s1, dihydropyrrole s2, and bicyclic succinimide s3 was further utilized in a highly diastereoselective Michael addition of thiols to produce piperidine s21, pyrrolidine s22, and bicyclic succinimide s23 (23). The carbonyl group of the α,β-unsaturated ester was also methylenated using Tebbe reagent to provide alkoxy dienes, which upon exposure to dienophiles (imines, maleimides, triazolinediones, tetracyano ethylene, and benzoquinones) underwent diastereoselective Diels–Alder reaction and produced multicyclic compounds s24–s35 (24). A stereoselective hydrolysis of the enol ether group in the Diels–Alder adducts further produced multicyclic ketones s36–s39. The library of 642 compounds of 39 distinctive scaffolds was tested in several bioassays and resulted in the identification of geranylgeranyltransferase type I (GGTase I) inhibitors (25, 26), RabGGTase inhibitors (23), and antimigratory compounds (24). These outcomes powerfully demonstrate the premise behind DOS—the more structural diversity in the screening collection, the higher the probability of discovering small-molecule biomodulators. Therefore, the nucleophilic phosphine catalysis-based synthesis resulted in a compound library with rich structural diversity, well poised to probe critical biological questions.
Scheme 1.
Diversity-oriented synthesis based on nucleophilic phosphine catalysis. For clarity, detailed designation of stereochemistry and substituents are removed. For the detailed structural information, see the following references: For scaffold s1, refs. 11 and 12; s2, refs. 13 and 14; s4, ref. 15; s5, ref. 16; s6, refs. 17 and 18; s7, ref. 19; s8, ref. 20; s9–s12, ref. 21; s13–s20, ref. 22; s21–s23, ref. 23; s24–s39, ref. 24.
In order to most efficiently interrogate compound libraries, high-throughput screening systems to detect compounds with prespecified molecular targets have been developed. However, with few exceptions, a limitation of current screens is that they do not integrate the interactions that occur between heterogeneous cell types involved in disease pathogenesis (27, 28). We aimed to establish a biological platform amenable to high-throughput screening, but with sufficient complexity to identify molecular probes that resulted in (i) endothelial cell activation and (ii) subsequent endothelial cell (EC)-triggered induction of innate immune responses. Activation of the endothelium occurs when proinflammatory cytokines such as interferon gamma (IFNγ) induce the production of chemokines and expression of cell adhesion molecules on the endothelial surface. Consequently, leukocytes home to activated endothelium and transmigration ensues. Such activation of the endothelium is a physiologic and necessary process, desirable for eradicating infections and some cancers (29–31).
Despite the homeostatic benefits of endothelial cell activation in host defense, such activation also plays a central role in the pathogenesis of many chronic inflammatory disorders (32); this has resulted in chemical screens predominantly focused on the identification of compounds that decrease endothelial cell inflammation (33–37). In stark contrast, far less effort has been put forth to understand the underlying networks by which compounds may activate or inflame the endothelium. Yet, such insights may have important implications for understanding salutary and maladaptive aspects of inflammation at the vessel wall. Furthermore, given that an increasing number of drugs have been removed from the market as a result of cardiovascular complications (38–41), understanding how small molecules may promote inflammation in the vasculature is an important question. Herein, we report how a DOS library approach, applied to a unique biological platform, has led to the identification of a previously undescribed class of chemical probes that trigger human EC-driven activation of innate immunity, a biological process integral to both host defense and disease pathogenesis.
Results
Identification of Small Molecules That Activate Endothelium.
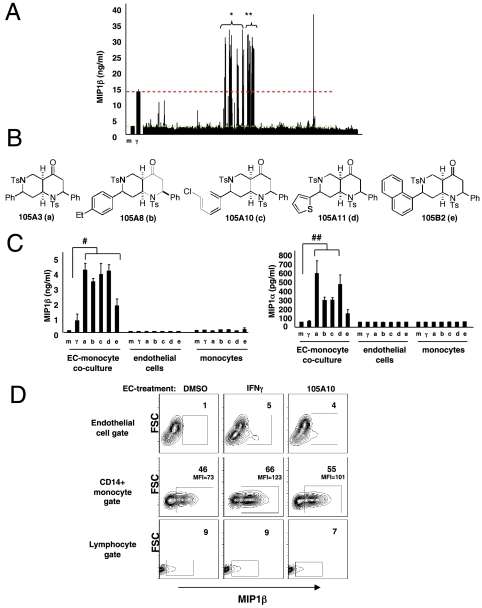
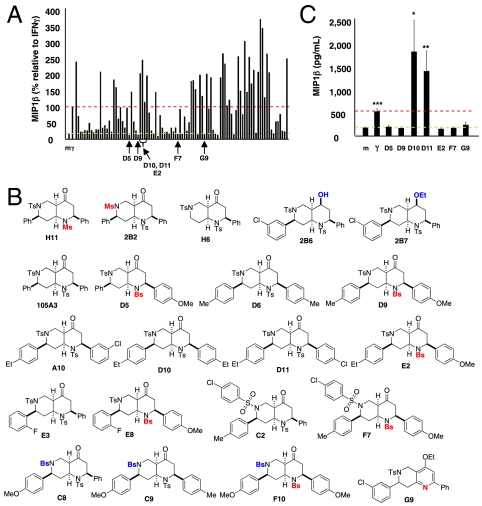
Activation of the endothelium is intricately linked to innate immunity. For example, ECs activated by IFNγ were shown to trigger the production of macrophage inflammatory protein 1 alpha (MIP1α) from primary human monocytes (42). Interestingly, neither EC nor monocytes treated with IFNγ produced this chemokine; coculture between the activated EC and monocytes was required. Therefore we hypothesized that compounds that “activate” the endothelium could be identified indirectly—endothelium encountering activating compounds would gain the capacity to trigger chemokine production by monocytes. To test this hypothesis, we designed an EC-monocyte coculture system amenable to high-throughput compound screening (Fig. S1A). In this platform, MIP1β production, rather than MIP1α or IL8, proved to be the most reliable marker of EC activation by IFNγ (Fig. S1B). Subsequently, EC were treated with DMSO (vehicle control), IFNγ (EC-activation control), and a collection of 642 compounds (final concentration, 10 μM) (Fig. S1C). Of the 642 compounds, 37 (5.8%) promoted MIP1β production (Fig. 1A). By contrast, IFNγ-treated EC inhibited IL-8 production from monocytes; likewise, every EC-activating compound also decreased IL8 production (Fig. S2A). MIP1α was not induced by IFN-treated EC; however, most compounds that induced MIP1β also induced MIP1α production (Fig. S2B).
Fig. 1.
Identification of small molecules that activate human endothelial cells. (A) IFNγ (10 ng/mL), DMSO controls (n = 60 total replicates), and 642 compounds (10 μM) were tested for their ability to promote MIP1β production. The library contained 37 compounds capable of inducing MIP1β at least to the level of IFNγ (red dashed line). * represents five distinct scaffolds with activity, whereas ** represents the two naphtyridine families. (B) Structures of five octahydronaphthyridinones selected for further study. (C) Confirmation of a subset of active compounds: Coculture is required for MIP1β production. Data represents mean ± SEM of triplicate wells from one of two comparable experiments. # p values for MIP1β: (a) 105A3 = 0.008, (b) A8 = 0.003, (c) A10 = 0.026, (d) A11 = 0.007, and (e) B2 = 0.046. ## p values for MIP1α: (a) 105A3 = 0.042, (b) A8 = 0.016, (c) A10 = 0.009, (d) A11 = 0.041, and (e) B2 = 0.13. (D) Activated endothelium triggers MIP1β production from CD14+ monocytes. Percent positive MIP1β cells and mean fluorescence intensity (MFI) of MIP1β+ cells are shown.
Intriguingly, most EC-activating compounds (35 out of 37; Fig. S3) possess the scaffolds derived from the sequence of phosphine-catalyzed annulation, Tebbe methylenation, Diels–Alder reaction, and sometimes hydrolysis (scaffolds s24–s27, s31, s32, and s36 in Scheme 1). As reported earlier (24), tetrahydropyridine s1 and pyrrolidine s2 were formed through the phopshine-catalyzed [4 + 2] and [3 + 2] annulation reaction between allenoates and imines (11–14) and converted into the corresponding ethoxy dienes via Tebbe reaction. The subsequent Diels–Alder reaction of the dienes with maleimides, N-phenyl triazolinedione, and N-sulfonamido arylimines provided multicyclic enol ethers s24, s25/s26, s27, s31, s32, each of which provided 9, 5, 5, 3, and 8 hits out of 10, 12, 5, 9, and 10 compounds, respectively, in confirmatory experiments (Fig. S4). The enol ether adducts s24, s25/s26, and s31 were stereoselectively hydrolyzed into the corresponding ketones s36, s37/s38, and s39. Although ketones s37/s38 and s39 did not provide any EC-activating compounds, all 10 of the octahydro-1,6-naphthyridin-4-ones were active. The octahydro-1,6-naphthyridine framework was particularly interesting in that it exhibited the highest hit rate, and the solid-phase split-and-pool synthesis of the octahydro-1,6-naphthyridin-4-one analogs should be possible by using resin-bound allenoates (vide infra).
With the anticipation of the analog synthesis, five octahydro-1,6-naphthyridin-4-ones were selected for further validation (Fig. 1B). Direct treatment of independent EC or peripheral blood mononuclear cell (PBMC) with these activating compounds did not result in significant production of MIP1β or MIP1α; as with IFNγ, coculture of activated EC and PBMC was required for chemokine induction (Fig. 1C). Intracellular flow cytometry of cocultures revealed that when PBMCs are added to ECs activated either by IFNγ or an octahydro-1,6-naphthyridin-4-one 105A10, the CD14+ monocytes, rather than ECs or lymphocytes, are the dominant producers of MIP1β (Fig. 1D). This underscores the finding that a screen testing isolated endothelial cells or monocytes would have missed this particular class of EC activators. Therefore, like IFNγ, this unique family of compounds mediates EC-triggered induction of innate immune activation.
Synthesis of Naphthyridinone Analogs.
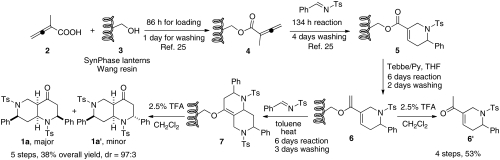
The discovery of promising chemical probes capable of promoting a robust innate immune response through the activation of the endothelium warranted the development of efficient and rapid synthesis of analogs, allowing for determination of structure-activity relationship (SAR). We envisioned a short, modular synthetic route using SynPhase lanterns as the solid support (Scheme 2). Establishment of the solid-phase synthesis began with the coupling of the Wang resin 3 with 2-methyl-2,3-butadienoic acid (2) and the subsequent phosphine-catalyzed [4 + 2] annulation of resin-bound allenoate 4 with N-tosylbenzaldimine (25). The resin-bound tetrahydropyridine 5 was treated with Tebbe reagent and anhydrous pyridine in THF and converted into the dienol ether 6. To test the efficiency of the Tebbe reaction, the enol ether 6 was cleaved off the resin using TFA (2.5%) in dichloromethane (DCM). Because there are very few successful examples of Tebbe reaction in the solid phase (43, 44), we were pleased to find the methylenation of the polymer-supported α,β-unsaturated enoate 5 proceeded smoothly in good yield; enone 6′ was obtained in 53% overall yield over four steps and excellent purity (> 95%, 1H NMR). The subsequent endo-selective Diels–Alder reaction with N-tosylbenzaldimine in toluene at 80 °C gave octahydro-1,6-naphthyridine 7, which was hydrolyzed off the resin using 2.5% TFA in DCM to provide octahydro-1,6-naphthyridin-4-ones 1a and 1a′ in 38% overall yield with high diastereoselectivity (diastereoisomeric ratio = 97∶3) after chromatographic purification. The structures of both compounds 1a and 1a′ were unequivocally established through X-ray crystallography. X-ray crystallography revealed that the tetrahydropyridine (11) and the octahydronaphthyridine (24) featured antirelationships between their C7-phenyl and N6-tosyl groups. Interestingly, the imine dienophile approached the diene 6 from the opposite face of the N6-tosyl group. It is also noteworthy that the hydrolysis of the enol ether produced octahydronaphthyridinone 1a and 1a′ featuring a cis-fused [4.4.0] bicyclic framework.
Scheme 2.
Solid-phase synthesis of octahydro-1,6-naphthyridin-4-ones.
Having successfully established the solid-phase reaction conditions, next we prepared the N-sulfonylimine building blocks. According to the result of phosphine-catalyzed [4 + 2] annulation of resin-bound allenoates from our previous work (25), we chose 10 N sulfonylimines, which provided excellent reaction yields (87–108%) and purities (80–99%) (Fig. S5). It is noteworthy that the strategy of our combinatorial library construction is very efficient in terms of building blocks because the N sulfonylimines were used in both phosphine-catalyzed [4 + 2] annulation and the Diels–Alder reaction. With the building blocks in hand, the library synthesis commenced. The individual lanterns were tagged with colored spindles and cogs to encode the imine building blocks of the [4 + 2] annulation used for each lantern. Because the Diels–Alder reaction was the last split step of the synthesis, tagging for the imine building blocks of the Diels–Alder reaction was not necessary. By using the tagging and split-and-pool combinatorial techniques, 2-methyl-2,3-butadienoic acid and 10 N-sulfonylimine building blocks resulted in the preparation of 100 (1 × 10 × 10) octahydro-1,6-naphthyridin-4-one analogs. The overall yields are up to 39% in five steps and the purities of the final products are up to 99% after the prep HPLC purification.
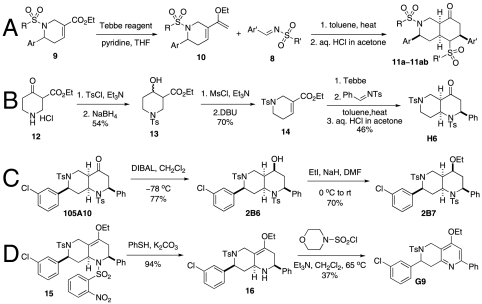
At the same time, additional octahydro-1,6-naphthyridine analogs with different functional groups were synthesized in the solution phase (Scheme 3) for the further SAR analysis. The octahydro-1,6-naphthyridin-4-one analogs 11a–11ab with different substituents, Ar, R, Ar′, and R′, were synthesized according to procedures reported previously (Scheme 3A) (24). For the preparation of an octahydronaphthyridinone without the C7 substituent, a previously undescribed sequence of reactions was designed (Scheme 3B). The commercially available ethyl 4-piperidone-3-carboxylate hydrochloride (12) was protected with p-toluenesulfonyl (tosyl, TS) group and the ketone was chemoselectively reduced with sodium borohydride (NaBH4). Methanesulfonyl(mesyl)ation of the resulting alcohol and β-elimination of the mesylate provided tetrahydropyridine 14. The Tebbe methylenation, Diels–Alder reaction with N-tosylbenzaldimine, and acid hydrolysis gave the desired product H6. To probe the importance of the C4-carbonyl group, the corresponding alcohol and its ethyl ether were also prepared (Scheme 3C). The diisobutylaluminium hydride reduction of compound 105A10 gave the alcohol 2B6, which in turn was O-alkylated to the ethyl ether 2B7. Naphthyridinone without the N1 substituent was also prepared (Scheme 3D). To prepare tetrahydronaphthyridine G9, octahydronaphthyridine 15 was subjected to the Fukuyama et al.’s denosylation condition (45) followed by morpholinesulfonyl chloride and triethylamine for aromatization (46).
Scheme 3.
Solution-phase synthesis of octahydro-1,6-naphthyridin-4-ones.
Structure-Activity Relationship.
With the synthesis of analogs from the solid phase and the solution phase completed (Fig. S6), the products were dissolved in DMSO and analyzed for EC activation. Because the original 10 naphthyridinones were all EC-activating, the hope was that the SAR studies would reveal structural elements that obliterate EC-activating effects and thereby pinpoint the crucial motifs for the biological activity. Indeed, the focused library of 96 analogs produced compounds with distinct capacities for EC activation (Fig. 2A), indicating some essential structural features for the biological activity of the octahydro-1,6-naphthyridin-4-ones. In particular, the aryl groups on the N1- and N6-arylsulfonly groups were indispensible; both N1-mesyl (Ms) and N6-mesyl naphthyridinone compounds (H11 and 2B2, Fig. 2B) lost their activity. Based on the fact that the N1-mesylated naphthyridinone was inactive, it was not surprising to find that the derivative G9 with the pyridine ring was inactive as well. On the other hand, removal of the C7-aryl group (analog H6) did not affect the naphthyridinone’s EC activation. As we discovered in the initial screening, both enol ether (s24 in Scheme 1) and ketone octahydronaphthyridines (s36 in Scheme 1) were active, indicating that ketone group is not essential for the activity. Although we speculated that the tetrasubstituted enol ether functionality is intact under physiological conditions based on the fact that ketones (s37–s39) of EC-activating enol ethers (s25, s26, and s31) were not active, we wanted to confirm that the naphthyridinones are by no means acting as covalent modifiers through Schiff base formation. Indeed, alcohol 2B6 and its ethyl ether 2B7 were active, confirming that the ketone group was less essential for naphthyridinone’s EC-activating effect. The solid-phase split-and-pool synthesis provided a large number of active and inactive analogs that contain various substituents around the benzene rings of N1 and N6 arylsulfonyl as well as C2- and C7-aryl groups. One subtle yet powerful observation was that naphthyridinones D5, D9, E8, F7, and F10 (except analog E2) bearing N1 benzenesulfonyl and C2-p-methoxyphenyl group did not exhibit EC activation, whereas various naphthyridinones with the same N6 and C7 substituents retained their activity (Fig. 2B). Based on its structure, analog E2, which appeared to have some activity in the initial test (Fig. 2A), was predicted to behave more like the inactive analogs; indeed, confirmatory studies revealed it was relatively inactive (Fig. 2C). Although some synergistic substituent effects obscured a clear-cut SAR, useful patterns emerged (Fig. 3). The predictive power of the SAR data illustrates the advantage of having a diverse and significant number of analogs, which was possible only because of the short and efficient synthetic route based on the solid-phase nucleophilic phosphine catalysis.
Fig. 2.
EC-activating effects of 96 naphthyridinones. (A) Data represent percent induction relative to IFNγ for each of the 96 analogs analyzed over two experiments. (B) Structures of selected active (H6, 2B6, 2B7, 105A3, D6, A10, D10, D11, E3, C2, C8, and C9) and inactive octahydro-1,6-naphthyridin-4-one analogs (H11, 2B2, D5, D9, E2, E8, F7, F10, and G9). (C) Validation of seven (active and inactive) analogs. Data represent the mean ± SEM from two independent experiments performed in triplicate wells (n = 6 wells per condition), * p value < 0.05, ** p value < 0.03, *** p value < 0.003.
Fig. 3.
SAR analysis of octahydro-1,6-naphthyridin-4-ones.
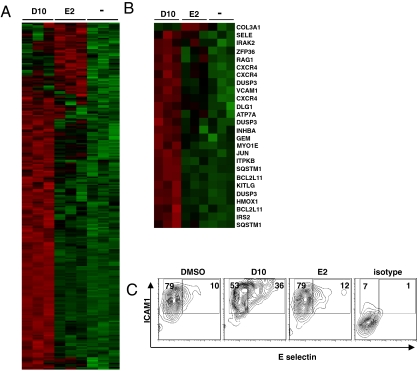
Based on the disparate biological activities of D10 and E2, we predicted that these two structurally similar molecules would induce distinct gene networks involved in EC activation. To test this idea, we performed transcriptome profiling in EC treated with active (D10) and inactive (E2) analogs. In comparison to vehicle control, the EC-activating analog D10 induced 201 gene probes (fold change > 1.25, p value < 0.05), whereas the inactive analog E2 activated only 49 gene probes (Fig. 4A). Remarkably, despite their structural similarity, the two analogs induced distinct sets of genes: Only two genes were shared in common between the two analogs. More specifically, genes involved in the regulation of immune processes were more frequently induced by D10 than E2 (Fig. 4B). The induced genes were additionally analyzed by ingenuity pathway analysis; in total, 32 canonical pathways were engaged by the active analog, and 10 of the top 20 canonical pathways are implicated in inflammation, including atherosclerosis signaling (p < 0.03), IL8 signaling (p < 0.006), and high mobility group box1 signaling (p < 0.004). By contrast, E2 did not activate any networks related to immune regulation. Of particular interest, E selectin was found to be uniquely induced by the active, but not by the inactive analog. This adhesion molecule plays a critical role in leukocyte rolling and firm adhesion to activated endothelium and is implicated in chronic inflammatory diseases (47, 48). To confirm this finding at the protein level, EC were treated with active or inactive analogs and E-selectin expression was measured by flow cytometric analysis. In comparison to vehicle control, only the active analog D10 markedly enhanced E-selectin expression levels (Fig. 4C). Therefore, despite subtle structural changes, these two naphthyridinones display distinct effects on EC activation and disparate gene expression profiles. Together, this work demonstrates how a DOS approach can be combined with screening platforms of adequate complexity, along with transcriptome profiling, to provide a framework for understanding complex biological processes, such as EC-triggered innate immunity.
Fig. 4.
Gene expression profiling of endothelial cells treated with active and inactive analogs. (A) Microarray analysis comparing gene expression induction in EC treated with active analog (D10, 10 μM) and inactive analog (E2, 10 μM). (B) Subset of genes from A with known involvement in inflammation: relative expression by active and inactive analogs. (C) E-selectin induction by naphthyridinone analogs. Percentages for intercellular adhesion molecule (ICAM) and ICAM/E-selectin expressing cells are displayed.
Discussion
The endothelium is perpetually exposed to systemic chemical and mechanical stressors, such as infection, hypertension, smoking, and diabetes-cardiovascular risk factors known to cause endothelial inflammation (49, 50). This is intricately linked with the induction of chemokines and adhesion molecules by the endothelium, which culminates in transmigration of circulating monocytes, a process that may be protective in infection and some cancers, but detrimental in chronic inflammatory diseases, such as atherosclerosis. We screened a library of 642 carbo- and heterocycles of 39 distinctive scaffolds assembled through DOS based on the nucleophilic phosphine catalysis for their ability to activate human endothelial cells. Seven distinctive scaffolds of 35 compounds were identified by their capacity to bestow upon the endothelium the ability to trigger MIP1α and MIP1β production from previously quiescent monocytes. Taking advantage of the exceedingly simple assembly strategy for one specific scaffold, octahydro-1,6-naphthyridin-4-one, 96 analogs were prepared through solid-phase split-and-pool synthesis and solution phase medicinal chemistry. The diverse library revealed structural features indispensible for activation of the endothelium, simultaneously serving as tools to dissect molecular networks necessary to mediate EC-triggered activation of innate immune responses.
MIP-1 chemokines are known to play a key role in the chemotaxis of lymphocytes and monocytes, as well as protective roles in HIV pathogenesis (51) and cancer immunotherapy (52). Therefore, the identification of small molecules with potent capacity to activate the innate immune response through the endothelium raises the possibility of developing compounds for clinical scenarios in which augmented immunity may be desirable. Nevertheless, inflammation in the vessel wall can lead to endothelial dysfunction and myocardial infarction (49, 50). We demonstrate that structurally similar compounds can have disparate effects on triggering EC-mediated induction of innate immune responses. Therefore, among otherwise equal drug candidates, it may be preferable to select agents that do not have “off-target” EC activation, which may then mediate undesirable innate immune activation in the vascular wall. Indeed, the increasing number of drugs withdrawn from the market or failed in clinical trials due to cardiovascular complications (38–41) indicates the need for biological platforms that identify endothelial cell activation.
This work demonstrates that the integration of extremely efficient DOS strategy and a biological screening platform of adequate complexity can lead to deep insights into structure–function relationships during small-molecule testing and development. We identified a unique group of small molecules, octahydro-1,6-naphthyridin-4-ones, that activate the endothelium, which in turn trigger monocyte activation. Together, the work provides a unique conceptual framework for dissecting critical regulatory networks involved in EC activation by small molecules, as well as the possibility of augmenting innate immunity through endothelium-triggered immune responses.
Materials and Methods
Synthesis.
Detailed synthetic procedures and characterization of the compounds are available in SI Appendix.
Biology.
Information regarding cells, reagents, assays, flow cytometry, and microarray experiments is provided in SI Appendix.
Supplementary Material
Acknowledgments.
This research was supported by the US National Institutes of Health (Grants R01GM071779 and P41GM081282 to O.K.; Grant K088HL092290 to D.C.), and the Harold Amos Medical Faculty Development Program (to D.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Crystallographic data for 1a and 1a′ have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom, http://www.ccdc.cam.ac.uk/ (CSD reference nos. 767112 and 802608, respectively).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015254108/-/DCSupplemental.
References
- 1.Schreiber SL, Kapoor TM, Wess G. Chemical Biology: From Small Molecules to Systems Biology and Drug Design. Weinheim, Germany: Wiley-VCH; 2008. [Google Scholar]
- 2.Schreiber SL. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 4.Strausberg RL, Schreiber SL. From knowing to controlling: A path from genomics to drugs using small molecule probes. Science. 2003;300:294–295. doi: 10.1126/science.1083395. [DOI] [PubMed] [Google Scholar]
- 5.Dolle RE, et al. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2009. J Comb Chem. 2010;12:765–806. doi: 10.1021/cc100128w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terret NK. Combinatorial Chemistry. Oxford, UK: Oxford Univ Press; 1998. [Google Scholar]
- 7.Burke MD, Schreiber SL. A planning strategy for diversity-oriented synthesis. Angew Chem Int Edit. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen TE, Schreiber SL. Towards the optimal screening collection: A synthesis strategy. Angew Chem Int Edit. 2008;47:48–56. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spandl RJ, Bender A, Spring DR. Diversity-oriented synthesis; a spectrum of approaches and results. Org Biomol Chem. 2008;6:1149–1158. doi: 10.1039/b719372f. [DOI] [PubMed] [Google Scholar]
- 10.Galloway WRJD, Isidro-Llobet A, Spring DR. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat Commun. 2010;1:80–92. doi: 10.1038/ncomms1081. [DOI] [PubMed] [Google Scholar]
- 11.Zhu XF, Lan J, Kwon O. An expedient phosphine-catalyzed [4 + 2] annulation: Synthesis of highly functionalized tetrahydropyridines. J Am Chem Soc. 2003;125:4716–4717. doi: 10.1021/ja0344009. [DOI] [PubMed] [Google Scholar]
- 12.Lu K, Kwon O. Phosphine-catalyzed [4 + 2] annulation: Synthesis of ethyl 6-phenyl-1-tosyl-1,2,5,6-tetrahydropyridine-3-carboxylate. Org Synth. 2009;2009:212–224. doi: 10.1002/0471264229.os086.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Lu X. A novel [3 + 2] cycloaddition approach to nitrogen heterocycles via phosphine-catalyzed reactions of 2,3-butadienoates or 2-butynoates and dimethyl acetylenedicarboxylates with imines: A convenient synthesis of pentabromopseudilin. J Org Chem. 1998;63:5031–5041. [Google Scholar]
- 14.Zhu X, Henry CE, Kwon O. A highly diastereoselective synthesis of 3-carbethoxy-2,5-disubstituted-3-pyrrolines by phosphine catalysis. Tetrahedron. 2005;61:6276–6282. [Google Scholar]
- 15.Zhu XF, Henry CE, Wang J, Dudding T, Kwon O. Phosphine-catalyzed synthesis of 1,3-dioxan-4-ylidenes. Org Lett. 2005;7:1387–1390. doi: 10.1021/ol050203y. [DOI] [PubMed] [Google Scholar]
- 16.Zhu XF, Schaffner AP, Li RC, Kwon O. Phosphine-catalyzed synthesis of 6-substituted 2-pyrones: Manifestation of E/Z-isomerism in the zwitterionic intermediate. Org Lett. 2005;7:2977–2980. doi: 10.1021/ol050946j. [DOI] [PubMed] [Google Scholar]
- 17.Creech GS, Kwon O. Alcohol-assisted phosphine catalysis: One-step syntheses of dihydropyrones from aldehydes and allenoates. Org Lett. 2008;10:429–432. doi: 10.1021/ol702462w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creech GS, Zhu X, Fonovic B, Dudding T, Kwon O. Theory-guided design of bronsted acid-assisted phosphine catalysis: Synthesis of dihydropyrones from aldehydes and allenoates. Tetrahedron. 2008;64:6935–6942. doi: 10.1016/j.tet.2008.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Xu Q, Kwon O. Phosphine-promoted [3 + 3] annulations of aziridines with allenoates: Facile entry into highly functionalized tetrahydropyridines. J Am Chem Soc. 2009;131:6318–6319. doi: 10.1021/ja8097349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran YS, Kwon O. Phosphine-catalyzed [4 + 2] annulation: Synthesis of cyclohexenes. J Am Chem Soc. 2007;129:12632–12633. doi: 10.1021/ja0752181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sriramurthy V, Barcan GA, Kwon O. Bisphosphine-catalyzed mixed double-Michael reactions: Asymmetric synthesis of oxazolidines, thiazolidines, and pyrrolidines. J Am Chem Soc. 2007;129:12928–12929. doi: 10.1021/ja073754n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sriramurthy V, Kwon O. Diphosphine-catalyzed mixed double-Michael reaction: A unified synthesis of indolines, dihydropyrrolopyridines, benzimidazolines, tetrahydroquinolines, tetrahydroisoquinolines, dihydrobenzo-1,4-oxazines, and dihydrobenzo-3,1-oxazines. Org Lett. 2010;12:1084–1087. doi: 10.1021/ol100078w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe M, et al. Inhibitors of protein geranylgeranyltransferase I and Rab geranylgeranyltransferase identified from a library of allenoate-derived compounds. J Biol Chem. 2008;283:9571–9579. doi: 10.1074/jbc.M706229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, et al. Diversity through a branched reaction pathway: Generation of multicyclic scaffolds and identification of antimigratory agents. Chem-Eur J. 2011;17:649–654. doi: 10.1002/chem.201002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellano S, et al. Small-molecule inhibitors of protein geranylgeranyltransferase type I. J Am Chem Soc. 2007;129:5843–5845. doi: 10.1021/ja070274n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, et al. In vivo antitumor effect of a novel inhibitor of protein geranylgeranyltransferase I. Mol Cancer Ther. 2009;8:1218–1226. doi: 10.1158/1535-7163.MCT-08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butcher EC, Berg EL, Kunkel EJ. Systems biology in drug discovery. Nat Biotechnol. 2004;22:1253–1259. doi: 10.1038/nbt1017. [DOI] [PubMed] [Google Scholar]
- 28.Berg EL, Kunkel EJ, Hytopoulos E, Plavec I. Characterization of compound mechanisms and secondary activities by BioMAP analysis. J Pharmacol Toxicol. 2006;53:67–74. doi: 10.1016/j.vascn.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Nakamoto T, Inagawa H, Takagi K, Soma G. A new method of antitumor therapy with a high dose of TNF perfusion for unresectable liver tumors. Anticancer Res. 2000;20:4087–4096. [PubMed] [Google Scholar]
- 30.Sorensen EW, Gerber SA, Frelinger JG, Lord EM. IL-12 suppresses vascular endothelial growth factor receptor 3 expression on tumor vessels by two distinct IFN-gamma-dependent mechanisms. J Immunol. 2010;184:1858–1866. doi: 10.4049/jimmunol.0903210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller CH, Maher SG, Young HA. Clinical use of interferon-gamma. Ann NY Acad Sci. 2009;1182:69–79. doi: 10.1111/j.1749-6632.2009.05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghazizadeh R, Shimizu H, Tosa M, Ghazizadeh M. Pathogenic mechanisms shared between psoriasis and cardiovascular disease. Int J Med Sci. 2010;7:284–289. doi: 10.7150/ijms.7.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice JW, Davis JE, Crowl RM, Johnston PA. Development of a high volume screen to identify inhibitors of endothelial cell activation. Anal Biochem. 1996;241:254–259. doi: 10.1006/abio.1996.0407. [DOI] [PubMed] [Google Scholar]
- 34.Zerwes HG, Peter JC, Link M, Gubler H, Scheel G. A multiparameter screening assay to assess the cytokine-induced expression of endothelial cell adhesion molecules. Anal Biochem. 2002;304:166–173. doi: 10.1006/abio.2002.5626. [DOI] [PubMed] [Google Scholar]
- 35.Ren DC, Du GH, Zhang JT. High throughput screening for intercellular adhesion molecule-1 inhibitor. Yao Xue Xue Bao. 2003;38:405–408. [PubMed] [Google Scholar]
- 36.May MJ, Wheeler-Jones CP, Pearson JD. Effects of protein tyrosine kinase inhibitors on cytokine-induced adhesion molecule expression by human umbilical vein endothelial cells. Br J Pharmacol. 1996;118:1761–1771. doi: 10.1111/j.1476-5381.1996.tb15602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey MK, et al. Design, synthesis and anti-inflammatory evaluation of PEGylated 4-methyl and 4,8-dimethylcoumarins. Eur J Pharm Sci. 2010;39:134–140. doi: 10.1016/j.ejps.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Vergeer M, Stroes ES. The pharmacology and off-target effects of some cholesterol ester transfer protein inhibitors. Am J Cardiol. 2009;104:32E–38E. doi: 10.1016/j.amjcard.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Barter PJ, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 40.Metcalfe C, Wheeler BW, Gunnell D, Martin RM. International regulatory activity restricting COX-2 inhibitor use and deaths due to gastrointestinal haemorrhage and myocardial infarction. Pharmacoepidem Dr S. 2010;19:778–785. doi: 10.1002/pds.1957. [DOI] [PubMed] [Google Scholar]
- 41.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 42.Lukacs NW, et al. Intercellular adhesion molecule-1 mediates the expression of monocyte-derived MIP-1 alpha during monocyte-endothelial cell interactions. Blood. 1994;83:1174–1178. [PubMed] [Google Scholar]
- 43.Ball CP, et al. Chameleon catches in combinatorial chemistry: Tebbe olefination of polymer supported esters and the synthesis of amines, cyclohexanones, enones, methyl ketones and thiazoles. Chem Commun. 1998:2019–2020. [Google Scholar]
- 44.Barrett AMG, Procopiou PA, Voigtmann U. Solid-phase synthesis of isoxazoles using vinyl ethers as chameleon catches. Org Lett. 2001;3:3165–3168. doi: 10.1021/ol016479x. [DOI] [PubMed] [Google Scholar]
- 45.Fukuyama T, Cheung M, Kan T. N-carboalkoxy-2-nitrobenzenesulfonamides: A practical preparation of n-Boc, N-Alloc, and N-Cbz-protected primary amines. Synlett. 1999:1301–1303. [Google Scholar]
- 46.Zhou Y, et al. Library synthesis using 5,6,7,8-tetrahydro-1,6-naphthyridinesas scaffolds. J Comb Chem. 2008;10:534–540. doi: 10.1021/cc800038r. [DOI] [PubMed] [Google Scholar]
- 47.Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Direct viewing of atherosclerosis in vivo: Plaque invasion by leukocytes is initiated by the endothelial selectins. FASEB J. 2001;15:1149–1157. doi: 10.1096/fj.00-0537com. [DOI] [PubMed] [Google Scholar]
- 48.Rossi B, Constantin G. Anti-selectin therapy for the treatment of inflammatory diseases. Inflamm Allergy Drug Targets. 2008;7:85–93. doi: 10.2174/187152808785107633. [DOI] [PubMed] [Google Scholar]
- 49.Laffer CL, Elijovich F. Inflammation and therapy for hypertension. Curr Hypertens Rep. 2010;12:233–242. doi: 10.1007/s11906-010-0125-3. [DOI] [PubMed] [Google Scholar]
- 50.Short KR, Blackett PR, Gardner AW, Copeland KC. Vascular health in children and adolescents: Effects of obesity and diabetes. Vasc Health Risk Manag. 2009;5:973–990. doi: 10.2147/vhrm.s7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cocchi F, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 52.Nakashima E, et al. A candidate for cancer gene therapy: MIP-1 alpha gene transfer to an adenocarcinoma cell line reduced tumorigenicity and induced protective immunity in immunocompetent mice. Pharm Res. 1996;13:1896–1901. doi: 10.1023/a:1016057830271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.