Abstract
We compared species mean data on the size of functionally distinct brain regions to test the relative rates at which investment in higher-order cognitive processing (mushroom body calyces) versus peripheral sensory processing (optic and antennal lobes) increased with increasing brain size. Subjects were eusocial paper wasps from queen and worker castes of 10 species from different genera. Relative investment in central processing tissue increased with brain size at a higher rate than peripheral structure investment, demonstrating that tissue devoted to higher-order cognitive processing is more constrained by brain size. This pattern held for raw data and for phylogenetically independent contrasts. These findings suggest that there is a minimum necessary investment in peripheral sensory processing brain tissue, with little to gain from additional investment. In contrast, increased brain size provides opportunities to invest in additional higher-order cognitive processing tissue. Reproductive castes differed within species in brain tissue investment, with higher central-to-peripheral brain tissue ratios in queens than in workers. Coupled with previous findings that paper wasp queen, but not worker, brain architecture corresponds to ecological and social variation, queen brain evolution appears to be most strongly shaped by cognitive demands, such as social interactions. These evolutionary patterns of neural investment echo findings in other animal lineages and have important implications, given that a greater investment in higher-order processing has been shown to increase the prevalence of complex and flexible behaviors across the animal kingdom.
Keywords: Hymenoptera, mushroom bodies, social brain, Vespidae
As brains evolve, both relative expansion of specific brain regions and general increases in absolute brain size can enable changes in animal cognitive capacity (1). Of particular note is the frequent convergent evolution of central brain regions that perform higher-order cognitive processing. Larger central processing centers are associated with enhanced capacity for learning, memory, and behavioral innovation among species (1–3). In primates, increased investment in higher-order cognitive processing structures, such as the neocortex and hippocampus, has been linked to increased cognitive performance (4), and the neocortex is five times larger in primates than in insectivores after accounting for differences in whole brain size (5). Increased hippocampal size in passerine birds has been associated with improved memory and the advent of food-storing behavior (6). Increased size and complexity of the mushroom bodies (MB), the neural centers of higher-order cognitive processing in insects, are paired with an increase in the prevalence of generalist feeding ecologies across species (7) and with social dominance status within species (8–10).
Molecular evidence supports a single origin of the CNS in bilaterally symmetrical animals, including insects and vertebrates, occurring before the protostome–deuterostome split (11). Although a great deal of structural homology is seen in all brains that arose from this common ancestor, the higher-order cognitive processing centers in many modern animal brains are not homologous (11). Across millennia of evolutionary divergence, novel structural subcompartments for higher-order processing have evolved multiple times in both vertebrates and invertebrates (7, 11, 12). Given that brain tissue is energetically costly and that neural investment is strongly coupled with functional necessity (13), this is likely a case of convergence: brains yield similar forms under evolutionary pressure for similar cognitive functions.
The evolution of greater degrees of sociality appears to place especially strong cognitive demands on animals, and increased levels of sociality are associated with greater brain investment in diverse animal taxa (14–16). Larger relative brain sizes in three orders of mammals (ungulates, carnivores, and primates) are correlated with an increase in sociality and the cognitively demanding behaviors that come with it (17).
The diversity of paper wasps (Vespidae: Polistinae) makes these wasps important insect models for the evolution of cooperative behavior and division of labor (18, 19). Paper wasp colonies include reproductive queens and sterile workers. Although all paper wasps are eusocial, they have great interspecific variation in social complexity. For example, colony size, nest architecture, and division of labor differ between primitively eusocial independent founding species and advanced eusocial swarm founding species (20–22). (Fig. 1) Like other eusocial Hymenoptera, paper wasps show behavior-related individual variation in adult brain structure (23–25). Paper wasp species also differ in the relative amounts of tissue invested in brain regions that process input from different sensory modalities (26). The strongest species differences in brain investment are seen in the reproductive, or queen, caste (26).
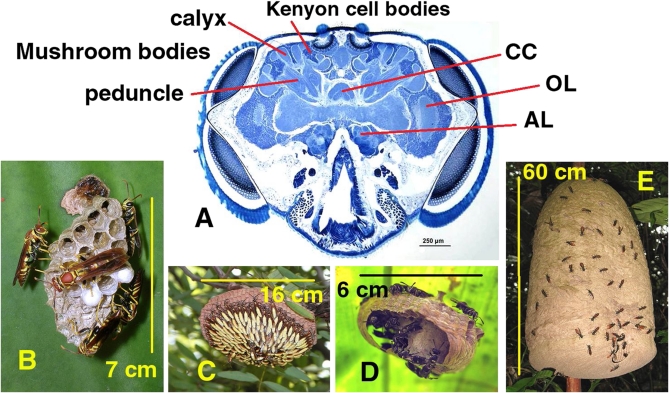
Fig. 1.
Representative wasp brain section and subject wasp colonies. (A) Frontal section of a Mischocyttarus mastigophorus head capsule showing the brain regions that we quantified. Labeled are part of the MB calyx, peduncle, and Kenyon cell body region. CC, central complex; OL, optic lobe, AL, antennal lobe. (B–E) Subject paper wasp species nests. Note the range of founding modes, mature colony sizes, and nest architectures. (B) Polistes instabilis: Independent founding, ~10 adults, open comb. (C) Apoica pallens: Swarm founding, several hundred adults, open comb. (D) Protopolybia exigua: Swarm founding, several dozen adults, enveloped nest. (E) Polybia dimidiata: Swarm founding, tens of thousands of adults, enveloped nest. Approximate head capsule size (A) and nest sizes (B–E) are indicated by the scale bars and their lengths.
We asked whether the relative investment in peripheral versus central processing capacities, as indicated by brain region volumes, was uniform among paper wasp species. In paper wasps, the MB calyces are central processing neuropils involved in learning, memory, and sensory integration, and the calyces show stronger behavior-related changes in volume compared with other MB structures (8, 23). Brain regions associated with the compound eyes (optic lobes) and antennae (antennal lobes) are dedicated to peripheral sensory processing of visual and olfactory information, respectively (9, 26). We tested whether species differences in the ratio of investment in these central and peripheral processing brain regions was related to total brain volume. We then tested whether overall brain size covaried allometrically with investment in the central and peripheral brain regions separately. Finally, we asked whether the ratio of central to peripheral region volumes differed between reproductive queen and sterile worker castes.
We used previously published generic phylogenies of paper wasps (27, 28) to analyze whether the patterns of brain tissue investment were significantly affected by species relationships. Our results may help reconcile the bodies of evidence that advocate for whole brain measurements (1) and those that advocate for higher-order brain center measurements (4, 5) as the best metrics for higher cognition and behavioral complexity.
Results
In the species that we studied, the mean brain size (total volume of brain structures that we measured) correlated positively with mean body size (as indicated by wing length, R2 = 0.61, F1,8 = 12.5, P = 0.008). Within most species, brain size correlated weakly and nonsignificantly with body size, suggesting weak body size constraints on total brain volume within species (Pearson correlations, r = 0.02–0.91, df = 2–9, P = 0.09–0.96); the only exception was Mischocyttarus mastigophorus (r = 0.74, df = 7, P = 0.02).
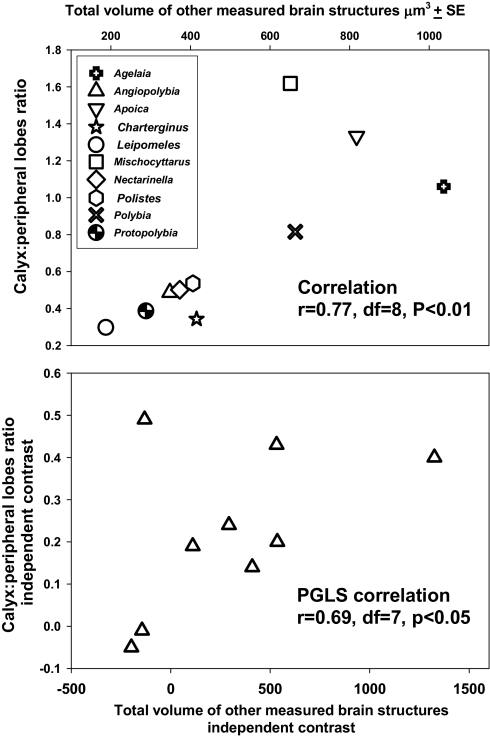
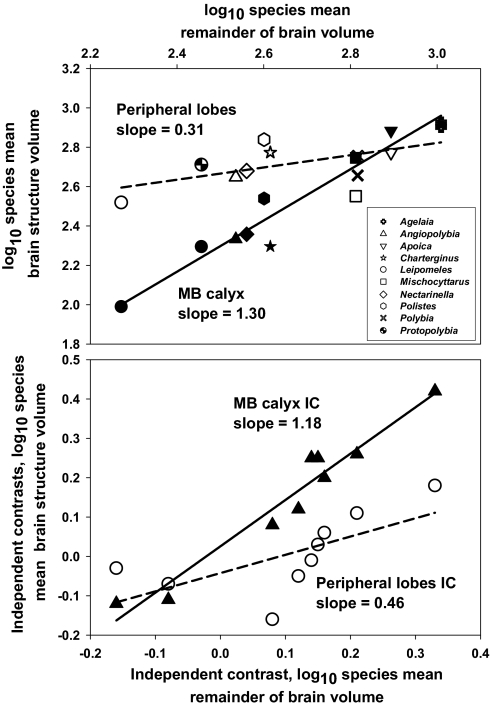
The ratio of MB calyx:peripheral lobe volumes increased with overall brain size. Species mean values of the MB calyx:peripheral lobe ratio were significantly positively correlated with brain size for both raw data (r = 0.77, df = 8, P < 0.01) and phylogenetically independent contrasts (phylogenetic generalized least squares correlations, r = 0.69 for all phylogenies and branch lengths, df = 7, P < 0.05) (Fig. 2). This pattern was due to a relatively rapid increase in MB calyx volume with brain size. The volumes of both the peripheral lobes and the MB calyx increased with brain size (ANCOVA, main effect term, F1,16 = 68.0, P < 0.0001) (Fig. 3). However, the rates of increase with brain size differed dramatically between the two regions (ANCOVA interaction term, F1,16 = 26.1, P = 0.0001); the slope of the MB calyx increase was >1 (1.3 a on log-log plot), whereas the peripheral lobe slope was positive but relatively shallow (0.3 on a log-log plot). These patterns were not driven by differential relatedness among species; we obtained similar results when analyzing the independent contrasts data [Wenzel and Carpenter (28) phylogeny ANCOVA: region effect, F1,16 = 22.0, P = 0.0003; remainder of brain effect, F1,16 = 55.6, P < 0.0001; interaction term, F1,16 = 10.5, P = 0.006; Pickett and Carpenter (27) phylogeny ANCOVA: region effect not significant, F1,16 = 1.8, P = 0.20; remainder of brain effect, F1,16 = 46.2, P < 0.0001; interaction term, F1,16 = 22.7, P = 0.0003] (Fig. 3).
Fig. 2.
Scatterplots showing the relationships between the ratio of the volumes of the MB calyx and peripheral lobes and the size of the remaining brain structures measured. Each data point represents the mean value for a species of paper wasp. (Upper) Species mean brain volume data. (Lower) Phylogenetically independent contrasts values of species mean volumes, calculated using a paper wasp phylogeny from Wenzel and Carpenter (28), with all branch lengths set to 1.
Fig. 3.
Scatterplots (log-log) showing the relationships between the volumes of central processing brain regions (filled symbols, MB calyx) and peripheral processing regions (open symbols, pooled optic and antennal lobes) with the size of the remaining brain structures that were measured. Each data point represents the mean value for a species of paper wasp. (Upper) Species mean volume data. (Lower) Phylogenetically independent contrasts values of species mean volumes, calculated using a paper wasp phylogeny from Wenzel and Carpenter (28), with all branch lengths set to 1.
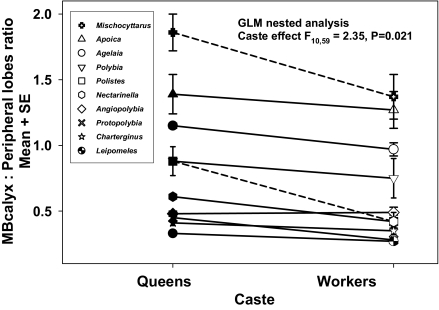
Within species, reproductive castes had different MB calyx:peripheral lobe ratios. The ratio was significantly higher for queens [generalized linear model (GLM) nested analysis, caste effect, F10,59 = 2.35, P = 0.021] (Fig. 4). The pattern of greater queen MB calyx:peripheral lobe investment held for all genera except Angiopolybia. Queen MB calyces tended to be larger than worker MB calyces, and queen peripheral lobes tended to be smaller, but neither of these differences alone was significant (ANCOVA, MB calyx caste difference, F1,75 = 0.10, P = 0.75; peripheral lobe caste difference, F1,75 = 2.17, P = 0.15). The greater central:peripheral ratio in queens was not due to caste differences in brain size; total brain volume ratio (pooling all structures measured) did not differ significantly between castes (nested ANOVA, caste nested within genus effect, F10,59 = 1.45, P = 0.18). Furthermore, the magnitude of the difference in ratio between queens and workers did not correlate with overall brain size (GLM; raw queen–worker difference, F1,8 = 2.5, P = 0.15; weighted queen–worker difference, F1,8 = 0.0, P = 0.95).
Fig. 4.
Interaction plot showing species mean MB calyx: peripheral lobe ratios, by caste, for 10 species of Neotropical paper wasps. Lines connect the values for the two castes within species. Dashed lines indicate independent founding species; solid line, swarm founding species. Filled symbols represent queens; open symbols, workers. Error bars are ± 1 SEM.
Independent founding species had significantly greater differences in central:peripheral investment ratio between queens and workers than swarm founding species (GLM; raw queen–worker difference, F1,8 = 47.6, P = 0.0001; weighted queen–worker difference, F1,8 = 5.4, P = 0.049), but species with and without morphologically distinct queens did not differ significantly (GLM; raw queen–worker difference, F1,8 = 0.24, P = 0.63; weighted queen–worker difference, F1,8 = 1.4, P = 0.26).
Discussion
We found that the ratio of investment in central processing to peripheral processing brain tissue increased with brain size for Neotropical paper wasp species. The investment in central cognitive processing tissue was more strongly related to total brain size. The volume of the MB calyx increased rapidly with total brain size (log-log slope >1), whereas the volume of peripheral lobes increased with brain size at a much slower rate (log-log slope <1). This suggests that for paper wasps, a certain minimum investment in peripheral lobes is necessary to meet sensory processing demands, with relatively little advantage to increased investment. In contrast, investment in MB calyces is strongly constrained by total brain volume, and increases in total brain size permit allometric increases in MB calyx size. Changes in overall brain volume are a major determinant of opportunities for investment in central processing tissue, such that only species with large brains are capable of relatively heavy investment in MB calyces. Our results echo findings in other animal taxa, notably mammals, indicating that this pattern of constrained higher-order neural investment may hold true across the vertebrate–invertebrate divide (29).
Queens invested relatively more heavily in central processing due to a combination of larger MB calyces and smaller peripheral lobes. This pattern suggests that queens are far from being cognitively impaired by their largely nest-bound existence (26). Greater relative investment in central processing tissue suggests that queens face cognitive challenges that require MB solutions. These likely include social interactions, such as social aggression and reproductive competition. The pattern of greater central:peripheral investment ratios for queens was consistent across most (9/10) species, and overall brain size had little effect on the magnitude of queen–worker differences. However, the mode of colony founding was associated with the magnitude of queen–worker differences. Independent founders showed greater queen–worker differences than swarm founders. Queens and other dominant females in independent founding paper wasp colonies species often engage in high rates of aggressive interactions and confront strong reproductive competition (8, 9). Less is known about queen–worker interactions in swarm founding Epiponini. West-Eberhard showed that swarm founding wasp queens are challenged by workers in ritualized behavioral contests (30), and low-quality queens can be eliminated from swarm founding paper wasp colonies (31). Our comparative data, and those of Molina et al. (25), suggest that queens of all paper wasps may face especially strong social cognition challenges (26). Whether social aggression is the driving force behind species-typical caste differences in brain architecture remains to be tested.
Other findings correlating complex behaviors in mammals, birds, and other animal groups to larger overall brain size may be attributed to increased investment in brain areas involved in higher-order processing (32–34). Furthermore, if the pattern that we found holds true in vertebrates, it may provide an alternative explanation for why both whole brain size and the size of a higher-order brain center (i.e., neocortex) predict cognitive ability to such a similar extent among primates and in humans (1). Contrary to the view that different regions of the brain are so functionally well integrated that the whole brain acts as the unit of cognition (1), higher-order brain structures like the neocortex might represent a larger relative portion of larger brains. Our results also provide evidence for absolute brain size as an important factor in cognitive evolution (1, 35).
Methods
We used histology and light microscopy to measure volumes of selected brain regions. Subjects were from 10 genera of Neotropical eusocial paper wasps (Polistinae). We asked how species differences, brain size, and reproductive caste are related to relative investment in central processing regions (MB calyx neuropils) versus peripheral processing regions (optic lobes and antennal lobes) (25) (Fig. 1).
Specimen Collection.
We collected entire colonies of paper wasp specimens from several Neotropical field sites. Active nests were sealed in plastic bags with a cotton ball soaked in ethyl ether or halothane. Anesthetized wasps were placed immediately into 15-mL or 50-mL vials of aldehyde-based fixative (Prefer fixative, active ingredient glyoxal; Anatech); wasp volume never exceeded 10% of the fixative volume. Specimens were stored in fixative under refrigeration at 4 °C until dissection and embedding of head capsules. Collection sites and dates were as follows: Polistes instabilis, July 2005 near Cañas, Costa Rica, 10°27.2'N, 85°7.5'W; Mischocyttarus mastigophorus and Agelaia xanthopus, August 2006, Monteverde, Costa Rica, 10°18.1'N, 84°47.9'W; Nectarinella championi, August 2006 near Monteverde, Costa Rica, 10°14.4'N, 84°54.3'W; Apoica pallens, Angiopolybia zischkai, Charterginus fulvus, Leipomeles dorsata, Polybia dimidiata, and Protopolybia exigua, June 2007, Yasuni Research Station, Ecuador, 0°40.3'S, 76°24.0'W. Representative nests of some subject paper wasp species are shown in Fig. 1.
Determining Subjects' Age and Caste.
To determine the subjects' caste, we dissected their gasters (the terminal body region in aculeate Hymenoptera) in fixative with tenotomy scissors. We exposed the ovaries and examined them at 10× under a binocular dissecting scope. Workers had filamentous ovarioles with no visible opaque oocyte swellings. Queens had robust, well-developed ovaries with at least one fully opaque, oblong oocyte per ovariole. Although individuals with intermediate ovaries were observed, we used only the two extreme phenotypes as subjects for this study. Only mature wasps with fully hardened, deeply colored cuticles served as subjects. In species in which eye color changes with age, only adults with mature eye coloration were used (the compound eyes often grow paler with age) (26). We collected neuroanatomical data on 4–12 wasps per species, with 1–7 individuals from each caste.
Determining Body Size.
We used the length of the rigid vein along the leading edge of the front pair of wings (costa vein) as an index of body size. We dissected wings from the thorax and mounted them flat on microscope slides with transparent tape. We photographed the wings through a dissecting scope with a digital camera and used the ruler tool of Adobe Photoshop 5.0, calibrated to a photograph of a 2-mm stage micrometer, to measure costa vein length. Whenever possible, we measured both forewings and averaged their lengths as a body size index for each wasp.
Histology.
We dissected wasps' head capsules off of the body and removed the antennae. We dehydrated each head in individual Eppendorf tubes using an ethanol series, acetone, and then a 1:1 mixture of acetone and plastic resin [resin composition: 5.5 g of EMbed 812 (a mixture of bisphenolA/epichchlorohydrin epoxy resin; (CAS #25068–38-6), epoxy modifier (CAS #2425–79-8), 5.7 g of dodecenyl succinic anhydride, 0.65 g pf dibutyl phthalate, and 0.31g of 2,4,6-tri(dimethylaminoethyl)phenol]. We allowed this to penetrate each head for 24–48 h and then repeated the process with a 3:1 resin-to-acetone mixture and then with pure resin. We transferred individual heads in resin to 0.5-mL silicon molds and incubated them in an electric convection oven at 60 °C for 72 h.
After the 72 h elapsed, we allowed the hardened resin to cool for 24 h. We sectioned each head in to 15-μm-thick sections using a rotary microtome with disposable steel histology blades. We floated single sections on drops of 15% acetone on gelatin-coated microscope slides, and evaporated the liquid by placing the slides on a heating block.
To stain the sections, we placed the slides on a heating block and placed a drop of toluidine blue stain on each head section, incubating for 7 min and replacing stain that evaporated. We then took the slides through a dehydrating series of ethanol in water, xylenes, and histoclear. We dried the cleared slides in the oven at 60 °C for 10 min, then coverslipped the sections under Permount mounting medium (Fisher Scientific).
Neuroanatomy and Brain Region Categorization.
We used a microscope-mounted digital camera and Q Capture version 2.68 (QImaging) to photograph the slides at a 2,048 × 1,536 pixel resolution. We used either the 2× or the 4× scope objective, depending on the size of the head. For each head, we began photographing in the section where brain tissue first became visible and continued to the last section with brain tissue. We used GIMP version 2.6 imaging software (GNU Image Manipulation Program Development Team) to quantify the volume of each brain structure, measuring every other section. We did so by outlining the target brain regions and quantifying the number of image pixels in the structure, converting the pixel counts to area (square microns) using a photograph of a stage micrometer as a size reference, and then multiplying the areas by distance between sections (30 μm) to yield total volume. We measured the volumes of brain subregions as follows (25). For peripheral sensory processing structure volume, we pooled part of the optic lobes (summing the medulla and lobula) and the antennal lobes (only the glomeruli). For central processing structure volume, we pooled MB calyx neuropils (lip, collar, and basal ring). For the other brain regions used for brain size comparisons, we pooled MB Kenyon cell body regions, MB peduncle and lobes, and the central complex (Fig. 1).
Comparative Analyses.
We performed comparative analyses accounting for the effect of phylogeny on relationships among variables using Compare 4.6b software (E.P. Martins, Department of Biology, Indiana University; http://compare.bio.indiana.edu/). For most analyses, we used phylogenetic generalized least squares regression to estimate phylogenetically independent relationships among species mean brain variables. We used two fully resolved generic-level phylogenies for social paper wasps (Vespidae) for our comparative analyses. The first phylogeny was based on behavioral and morphological data and included all taxa from our analysis (27). The second, more recent phylogeny incorporated molecular data, but did not include all of the genera that we sampled (28). When a genus that we sampled was missing from the phylogeny, we assigned it to the position of closest related genus in Wenzel and Carpenter's phylogeny (27). For the taxa that we sampled, these phylogenies were similar except for the relative position of the two basal independent founding genera (Polistes and Mischocyttarus), and the relationships within the clade including the swarm founders Protopolybia, Polybia, and Charterginus. For some analyses and for plotting the data in figures, we calculated phylogenetically independent contrasts using Compare 4.6b.
We used DNA base sequence data to estimate branch lengths on the phylogenies. Nucleotide sequence data were 378-bp fragments of the mitochondrial COI gene (36). We used the sequence data for the species in our study whenever possible (5/10 species). For two species, we used COI sequence data from congeners (Agelaia multipicta and Polybia emaciata). If congeneric sequence data were not available, we used COI sequence data from the most closely related genus according to Wenzel and Carpenter (27): Brachygastra augusti for Charterginus and Parachartergus colobopterus for Nectarinella and Leipomeles. We estimated branch lengths using DNAml version 3.69 in the Phylip software package (Joe Felsenstein, University of Washington; http://evolution.genetics.washington.edu/phylip.html). We entered the two phylogenies as user-defined trees and estimated branch lengths from the COI data using the default program settings in Phylip. Branch distances within the Nectarinella–Leipomeles clade could not be resolved, because we used a sequence from the same related genus for both genera. We modeled two extreme conditions of branch lengths for this clade by assigning all of the genetic distance to the tips in one case and assigning all of the genetic distance to the branch from the common ancestor to the node in the other case. Thus, we ran four comparative analyses, one for each Nectarinella–Leipomeles clade branch length condition for both the Wenzel and Carpenter (28) and the Pickett and Carpenter (27) phylogenies.
We performed ANCOVA using SAS version 9.2 (SAS Institute, Cary, NC) to analyze the relationships of species means of MB calyx volume and peripheral lobe volume with the volume of remaining brain structures. We performed these analyses on log10-transformed species mean data. The main-effects terms of the ANCOVA tested whether the central and peripheral region volumes correlated with the size of the remainder of the brain. The region × remainder interaction term tested whether the slopes of the two region–remainder correlations differed. We repeated this analysis on phylogenetically independent contrast values for the species mean brain region volumes (calculated with Compare 4.6b). We used the Pickett and Carpenter (27) and Wenzel and Carpenter (28) phylogenies for these analyses, with all branch lengths set to 1 for both trees.
Analysis of Caste Differences.
We used two response variables to identify factors that could influence the magnitude of caste differences in the ratio of central:peripheral tissue investment: the raw difference between queen and worker mean ratios for each species, and a weighted difference (queen mean ratio – worker mean ratio/worker mean ratio). We analyzed relationships of the magnitude of the queen–worker differences with two behavioral and developmental covariates related to species differences in social complexity (22): mode of colony founding [independent vs. swarm founding (20)] and whether or not queens are morphologically distinct from workers [data from Noll et al. (17)]. We also analyzed whether the magnitude of caste differences covaried with overall brain size, as indicated by the volume of all brain structures we measured. We used generalized linear models to analyze these relationships.
Acknowledgments
Wasp specimens were collected under research and collecting permits from the governments of Costa Rica and Ecuador, in accordance with the laws of those countries. S.O. was funded by National Science Foundation Grants IBN 0347315 and IOS 0923680. Y.M. was funded by a grant from the Society for Integrative and Comparative Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Deaner RO, Isler K, Burkart J, van Schaik C. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav Evol. 2007;70:115–124. doi: 10.1159/000102973. [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre L, Reader SM, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav Evol. 2004;63:233–246. doi: 10.1159/000076784. [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre L, Sol D. Brains, lifestyles and cognition: Are there general trends? Brain Behav Evol. 2008;72:135–144. doi: 10.1159/000151473. [DOI] [PubMed] [Google Scholar]
- 4.Shultz S, Dunbar RIM. Species differences in executive function correlate with hippocampus volume and neocortex ratio across nonhuman primates. J Comp Psychol. 2010;124:252–260. doi: 10.1037/a0018894. [DOI] [PubMed] [Google Scholar]
- 5.Barton RA, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- 6.Krebs JR. Food-storing birds: Adaptive specialization in brain and behavior? Philos Trans R Soc Lond B Biol Sci. 1990;329:153–160. doi: 10.1098/rstb.1990.0160. [DOI] [PubMed] [Google Scholar]
- 7.Farris SM, Roberts NS. Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proc Natl Acad Sci USA. 2005;102:17394–17399. doi: 10.1073/pnas.0508430102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina Y, O'Donnell S. Mushroom body volume is related to social aggression and ovary development in the paperwasp Polistes instabilis. Brain Behav Evol. 2007;70:137–144. doi: 10.1159/000102975. [DOI] [PubMed] [Google Scholar]
- 9.Smith AR, Seid MA, Jiménez LC, Wcislo WT. Socially induced brain development in a facultatively eusocial sweat bee Megalopta genalis (Halictidae) Proc Biol Sci. 2010;277:2157–2163. doi: 10.1098/rspb.2010.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farris SM. Evolutionary convergence of higher brain centers spanning the protostome–deuterostome boundary. Brain Behav Evol. 2008;72:106–122. doi: 10.1159/000151471. [DOI] [PubMed] [Google Scholar]
- 11.Farris SM. Structural, functional and developmental convergence of the insect mushroom bodies with higher brain centers of vertebrates. Brain Behav Evol. 2008;72:1–15. doi: 10.1159/000139457. [DOI] [PubMed] [Google Scholar]
- 12.Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- 13.Dunbar RIM. The social brain hypothesis and its implications for social evolution. Ann Hum Biol. 2009;36:562–572. doi: 10.1080/03014460902960289. [DOI] [PubMed] [Google Scholar]
- 14.Dunbar RIM, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- 15.Shumway CA. Habitat complexity, brain, and behavior. Brain Behav Evol. 2008;72:123–134. doi: 10.1159/000151472. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Barbería FJ, Shultz S, Dunbar RIM. Evidence for coevolution of sociality and relative brain size in three orders of mammals. Evolution. 2007;61:2811–2821. doi: 10.1111/j.1558-5646.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 17.Noll FB, Wenzel JW, Zucchi R. Evolution of caste in Neotropical swarm-founding wasps (Hymenoptera: Vespidae; Epiponini) Am Mus Novit. 2004;3467:1–24. [Google Scholar]
- 18.O'Donnell S. Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae) Annu Rev Entomol. 1998;43:323–346. doi: 10.1146/annurev.ento.43.1.323. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter JM. Phylogenetic relationships and the origin of social behavior in the Vespidae. In: Ross KG, Matthews RW, editors. The Social Biology of Wasps. Ithaca, NY: Cornell Univ Press; 1991. pp. 7–32. [Google Scholar]
- 20.Jeanne RL. The swarm-founding Polistinae. In: Ross KG, Matthews RW, editors. The Social Biology of Wasps. Ithaca, NY: Cornell Univ Press; 1991. pp. 191–231. [Google Scholar]
- 21.Jeanne RL. Polyethism. In: Ross KG, Matthews RW, editors. The Social Biology of Wasps. Ithaca, NY: Cornell Univ Press; 1991. pp. 389–425. [Google Scholar]
- 22.Jeanne RL. Social complexity in the Hymenoptera, with special attention to wasps. In: Kitkuchi T, Azuma N, Higashi S, editors. Genes, Behaviors and Evolution of Social Insects. Sapporo, Japan: Hokkaido Univ Press; 2003. pp. 81–131. [Google Scholar]
- 23.O'Donnell S, Donlan NA, Jones TA. Mushroom body structural plasticity is associated with temporal polyethism in eusocial wasp workers. Neurosci Lett. 2004;356:159–162. doi: 10.1016/j.neulet.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 24.O'Donnell S, Donlan NA, Jones TA. Developmental and dominance-associated differences in mushroom body structure in the paper wasp Mischocyttarus mastigophorus. Dev Neurobiol. 2007;67:39–46. doi: 10.1002/dneu.20324. [DOI] [PubMed] [Google Scholar]
- 25.Molina Y, Harris R, O'Donnell S. Brain organization mirrors the evolution of caste determination, colony founding and nest architecture in paper wasps (Hymenoptera: Vespidae) Proc Biol Sci. 2009;276:3345–3351. doi: 10.1098/rspb.2009.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina Y, O'Donnell S. Age, sex, and dominance-related mushroom body plasticity in the paperwasp Mischocyttarus mastigophorus. Dev Neurobiol. 2008;68:950–959. doi: 10.1002/dneu.20633. [DOI] [PubMed] [Google Scholar]
- 27.Pickett KM, Carpenter JM. Simultaneous analysis and the origin of eusociality in the Vespidae (Insecta: Hymenoptera) Arth Syst Phyl. 2010;68:3–33. [Google Scholar]
- 28.Wenzel JW, Carpenter JM. Comparing methods: Adaptive traits and tests of adaptation. In: Eggleton P, Vane-Wright RI, editors. Phylogenetics and Ecology. London: Academic; 1994. pp. 79–101. [Google Scholar]
- 29.de Winter W, Oxnard CE. Evolutionary radiations and convergences in the structural organization of mammalian brains. Nature. 2001;409:710–714. doi: 10.1038/35055547. [DOI] [PubMed] [Google Scholar]
- 30.West-Eberhard MJ. Temporary queens in metapolybia wasps: Nonreproductive helpers without altruism? Science. 1978;200:441–443. doi: 10.1126/science.200.4340.441. [DOI] [PubMed] [Google Scholar]
- 31.Noll FB, Zucchi R. Increasing caste differences related to life cycle progression in some Neotropical swarm-founding polygynic polistine wasps (Hymenoptera: Vespidae; Epiponini) Ethol Ecol Evol. 2000;12:43–65. [Google Scholar]
- 32.Sol D, Timmermans S, Lefebvre L. Behavioural flexibility and invasion success in birds. Anim Behav. 2001;63:495–502. [Google Scholar]
- 33.Lefebvre L, Whittle P, Lascaris E, Finkelstein A. Feeding innovations and forebrain size in birds. Anim Behav. 1997;53:549–560. [Google Scholar]
- 34.McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- 35.Marino L. Absolute brain size: Did we throw the baby out with the bathwater? Proc Natl Acad Sci USA. 2006;103:13563–13564. doi: 10.1073/pnas.0606337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arevalo E, Zhu Y, Carpenter JM, Strassmann JE. The phylogeny of the social wasp subfamily Polistinae: Evidence from microsatellite flanking sequences, mitochondrial COI sequence, and morphological characters. BMC Evol Biol. 2004;4:8. doi: 10.1186/1471-2148-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]