Abstract
National Institutes of Health (NIH)-sponsored screening centers provide academic researchers with a special opportunity to pursue small-molecule probes for protein targets that are outside the current interest of, or beyond the standard technologies employed by, the pharmaceutical industry. Here, we describe the outcome of an inhibitor screen for one such target, the enzyme protein phosphatase methylesterase-1 (PME-1), which regulates the methylesterification state of protein phosphatase 2A (PP2A) and is implicated in cancer and neurodegeneration. Inhibitors of PME-1 have not yet been described, which we attribute, at least in part, to a dearth of substrate assays compatible with high-throughput screening. We show that PME-1 is assayable by fluorescence polarization-activity-based protein profiling (fluopol-ABPP) and use this platform to screen the 300,000+ member NIH small-molecule library. This screen identified an unusual class of compounds, the aza-β-lactams (ABLs), as potent (IC50 values of approximately 10 nM), covalent PME-1 inhibitors. Interestingly, ABLs did not derive from a commercial vendor but rather an academic contribution to the public library. We show using competitive-ABPP that ABLs are exquisitely selective for PME-1 in living cells and mice, where enzyme inactivation leads to substantial reductions in demethylated PP2A. In summary, we have combined advanced synthetic and chemoproteomic methods to discover a class of ABL inhibitors that can be used to selectively perturb PME-1 activity in diverse biological systems. More generally, these results illustrate how public screening centers can serve as hubs to create spontaneous collaborative opportunities between synthetic chemistry and chemical biology labs interested in creating first-in-class pharmacological probes for challenging protein targets.
Keywords: proteomics, mass spectrometry, serine hydrolases, SILAC
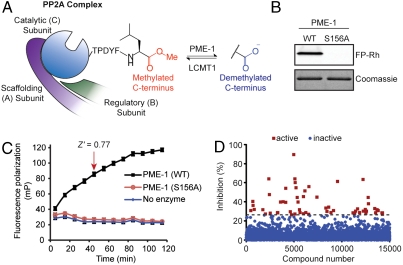
Protein phosphorylation is a pervasive and dynamic posttranslational protein modification in eukaryotic cells. In mammals, more than 500 protein kinases catalyze the phosphorylation of serine, threonine, and tyrosine residues on proteins (1). A much more limited number of phosphatases are responsible for reversing these phosphorylation events (2). For instance, protein phosphatase 2A (PP2A) and PP1 are thought to be responsible together for > 90% of the total serine/threonine phosphatase activity in mammalian cells (3). Specificity is imparted on PP2A activity by multiple mechanisms, including dynamic interactions between the catalytic subunit (C) and different protein-binding partners (B subunits), as well as a variety of posttranslational chemical modifications (2, 4). Within the latter category is an unusual methylesterification event found at the C terminus of the catalytic subunit of PP2A that is introduced and removed by a specific methyltransferase (leucine carbxoylmethyltransferase-1 or LCMT1) (5, 6) and methylesterase (protein phosphatase methylesterase-1 or PME-1) (7), respectively (Fig. 1A). PP2A carboxymethylation (hereafter referred to as “methylation”) has been proposed to regulate PP2A activity, at least in part, by modulating the binding interaction of the C subunit with various regulatory B subunits (8–10). A predicted outcome of these shifts in subunit association is the targeting of PP2A to different protein substrates in cells. PME-1 has also been hypothesized to stabilize inactive forms of nuclear PP2A (11), and recent structural studies have shed light on the physical interactions between PME-1 and the PP2A holoenzyme (12).
Fig. 1.
A fluopol-ABPP assay for PME-1. (A) Schematic representation of the reversible C-terminal methylation of PP2A introduced by LCMT1 and removed by PME-1. (B) FP-Rh (75 nM) labels purified wild-type PME-1 (1 μM) but not the catalytically dead S156A PME-1 mutant (1 μM). Top panel: Fluorescent gel image shown in gray scale. (C) Time course for fluopol signal generated by reactions of wild-type PME-1 (1 μM) with FP-Rh (75 nM). No signal increase is observed in the absence of enzyme or with the S156A PME-1 mutant. The indicated 45-min time point (Z′ = 0.77), prior to reaction completion, was selected for HTS. Data are presented as mean values ± SD. (D) Screening data for a representative 15,000 compounds of the NIH small-molecule library. Compounds that reduced the FP-Rh fluopol signal by > 26.13% were designated as hits for PME-1 (red squares).
Notwithstanding the aforementioned models and findings, the actual functional consequences of perturbing PP2A methylation remain largely unexplored. In yeast, LCMT1 deletion caused severe growth defects under stress conditions, while PME-1 deletion did not result in an observable cellular phenotype (9). Disruption of the PME-1 gene in mice, on other hand, caused early postnatal lethality (13), which has limited the experimental opportunities to explore methylation of PP2A in animals. Recent studies have found that RNA-interference knockdown of PME-1 in cancer cells leads to activation of PP2A and corresponding suppression of protumorigenic phosphorylation cascades (14), indicating that PME-1 could be an attractive drug target in oncology. Changes in PP2A methylation have also been implicated in Alzheimer’s disease, where this modification may stimulate PP2A’s ability to promote neural differentiation (15).
Despite the critical role that PME-1 plays in regulating PP2A structure and function, PME-1 inhibitors have not yet been described. This deficiency may be due to a lack of PME-1 activity assays that are compatible with high-throughput screening (HTS). Assessment of PME-1 activity typically involves either Western blotting with antibodies that recognize specific methylation states of PP2A (7, 13) or monitoring the release of 3H-methanol from radiolabeled-C subunits (16), but neither assay is easily adapted for HTS. PME-1 is, however, a serine hydrolase and therefore susceptible to labeling by active-site-directed fluorophosphonate (FP) probes (17). We have recently shown that FP probes can form the basis for a fluorescence polarization-activity-based protein profiling (fluopol-ABPP) assay suitable for HTS (18). Here, we apply fluopol-ABPP to screen the 300,000+ National Institutes of Health (NIH) compound library for PME-1 inhibitors. From this screen, we identified a set of aza-β-lactam (ABL) compounds that act as remarkably potent and selective PME-1 inhibitors. We show that these ABLs covalently inactivate PME-1 with high specificity in living cells and animals, where disruption of this enzyme leads to substantial decreases in demethylated PP2A.
Results
PME-1 Inhibitor Screening by Fluopol-ABPP.
Because PME-1 is a serine hydrolase that is known to interact with reporter-tagged FP probes (17, 19), we reasoned that this enzyme would be assayable by competitive ABPP methods. However, lower-throughput, gel-based competitive ABPP screens have not succeeded in identifying lead PME-1 inhibitors (20), indicating the need to survey larger compound libraries. We therefore asked whether PME-1 could be assayed using the recently introduced, HTS-compatible fluopol-ABPP platform (18). This technique, where compounds are tested for their ability to block the increase in fluopol signal generated by reaction of a fluorescent activity-based probe with a much larger protein target, has enabled inhibitor screening for a wide range of probe-reactive enzymes (http://pubchem.ncbi.nlm.nih.gov/). We confirmed that purified, recombinant wild-type PME-1, but not a mutant PME-1 in which the serine nucleophile was replaced with alanine (S156A), labels with a fluorophosphonate rhodamine (FP-Rh) (21) probe (Fig. 1B). This reaction generates a strong, time-dependent increase in fluopol signal that is not observed in the absence of enzyme or with the S156A mutant PME-1 enzyme (Fig. 1C). In collaboration with the Molecular Libraries Probe Production Centers Network (MLPCN), we screened 315,002 compounds for PME-1 inhibition using fluopol-ABPP (see Fig. 1D for a representative subset of the primary screening data). Following a confirmation screen on initial hits, we identified 1,068 compounds as potential PME-1 inhibitors. As an initial filter, we selected compounds for follow-up studies that had < 5% hit rates in all other bioassays reported in the PubChem database, < 30% inhibition in three fluopol-ABPP screens performed on other enzymes (http://pubchem.ncbi.nlm.nih.gov/), and > 40% inhibition of PME-1 in the confirmation screen. This filter yielded approximately 300 candidate PME-1 inhibitors.
Discovery of aza-β-lactam (ABL) Inhibitors of PME-1.
The approximately 300 hit compounds were next analyzed by gel-based competitive ABPP (18, 22) in soluble lysates from HEK 293T cells overexpressing PME-1. This convenient selectivity screen assessed in parallel the activity of lead compounds against approximately 25 gel-resolvable, FP-Rh-reactive serine hydrolases expressed in HEK 293T cells and rapidly eliminated false-positive and nonselective compounds. Among the compounds that selectively inhibited PME-1 (Fig. S1) were four ABLs (ABL127, ABL103, ABL105, ABL107) that were similar in structure, all with a branched alkyl group at R1 and with R stereochemistry at this position (Fig. 2A). The MLPCN library contained 22 other ABLs, including the enantiomers of ABL127 (ent-ABL127), ABL103 (ent-ABL103), and ABL105 (ent-ABL105), that were all considerably less active toward PME-1 in the primary screen (Table S1). Intriguingly, the ABLs did not originate from a commercial compound collection but rather were submitted by the academic chemistry laboratory of our coauthor Gregory Fu, who generated these compounds as part of an investigation into the synthetic utility of chiral 4-pyrrolidinopyridine catalysts (23). We further noted that the ABLs are structurally unusual compared to the rest of the MLPCN library, lying a considerable distance in a chemical space plot from the typical structures that populate the compound collection (Fig. 2B).
Fig. 2.
Discovery of selective ABL inhibitors of PME-1. (A) Four ABLs (ABL127, ABL103, ABL105, and ABL107) identified as active in the MLPCN screen. (B) Chemistry space coordinates for compounds (colored red in high occupancy cells; colored blue in low occupancy cells) that were screened against PME-1. The 26 ABLs (shown as enlarged squares; all other compounds are shown as small circles) are located in a sparsely populated region of chemical space. This optimized 6-dimensional BCUTs (31) visualization of chemistry space for the compound library was generated using the standard 3D hydrogen suppressed descriptors with Diverse Solutions (Diverse Solutions, Tripos), with coverage illustrated by compression into an optimized two-dimensional BCUTs space as described in more detail previously (32). (C) Evaluation of compounds by gel-based competitive ABPP with FP-Rh (2 μM) in the soluble proteome (1 mg/mL protein) of MDA-MB-231 cells. (D) Complete PME-1 IC50 curves for ABL127 (4.2 nM; 95% confidence limits 2.3–7.5 nM) and ent-ABL127 (450 nM; 95% confidence limits 240–850 nM) in the soluble proteome of MDA-MB-231 cells (for IC50 curves of ABL103, ABL105, and ABL107, see Fig. S2). Data are presented as mean values ± SEM; n = 3/group. (E) Pretreatment of HEK 293T proteomes with ABL127 (500 nM, 30 min) before addition of purified PME-1 (500 nM, 1 h) blocks PP2A demethylation as determined by Western blotting with antibodies that recognize specific methylation states of PP2A.
The ABL hits were next titrated into the soluble proteome of MDA-MB-231 cells, a cell line that endogenously expresses PME-1, to assess their potency (Fig. 2C). The two compounds bearing cycloalkyl substituents at R1, ABL127 and ABL103, were extraordinarily potent inhibitors of PME-1, with IC50 values of 4.2 and 2.1 nM, respectively (Fig. 2C and Fig. S2). The two compounds with isopropyl substituents, ABL105 and ABL107, exhibited lower IC50 values (92 and 24 nM, respectively) but were still good inhibitors of PME-1. Interestingly, a strong preference for the R enantiomer of ABLs was observed, as the S enantiomer of ABL127 (ent-ABL127) was at least two orders of magnitude less potent at inhibiting PME-1 (Fig. 2 C and D). Indeed, some of the apparent activity of the S enantiomer may be due to the small amount of residual R enantiomer in the > 99% ee sample.
Before proceeding further, we wanted to confirm that ABLs could inhibit the ability of PME-1 to demethylate PP2A. We therefore treated HEK 293T soluble lysates with ABL127 (500 nM, 30 min) or DMSO before adding purified recombinant PME-1 for an additional hour. In DMSO-treated lysates, we observed the expected time-dependent increase in demethylated PP2A and concomitant decrease in methylated PP2A, as determined by immunoblotting with antibodies that specifically recognize either form of the C terminus of PP2A (Fig. 2E). In lysates containing ABL127, however, we observed little or no change in the methylation state of PP2A (Fig. 2E), indicating that ABL127 blocks PME-1’s activity on its physiological substrate. As ABL127 and ABL103 are highly similar structures and emerged as virtually indistinguishable in these assays, we selected one of these compounds, ABL127, for in-depth characterization.
ABLs Covalently Inhibit PME-1.
Based on scientific precedent showing that other serine hydrolases can react with and open β-lactam rings by breaking the amide bond, resulting in covalent acylation/inhibition (24), we hypothesized that the active-site serine (S156) of PME-1 analogously reacted with the ABL ring of ABL127 to produce the enzyme-inhibitor adduct shown in Fig. 3A. Consistent with a covalent mode of inhibition, we observed that blockade of FP-Rh labeling of PME-1 by ABL127 was not reversed by gel filtration (Fig. 3B). The identity of the expected acylation adduct was confirmed by liquid chromatography-mass spectrometry (LC-MS) analysis of purified, recombinant PME-1 treated with ABL127 (Fig. 3C).
Fig. 3.
ABLs are covalent inhibitors of PME-1. (A) Proposed mechanism for covalent PME-1 inhibition by ABL127. (B) PME-1 (500 nM) was incubated with DMSO or ABL127 (5 μM), and each reaction was split into two fractions. One fraction was directly labeled with FP-Rh (left panels), and the other was subjected to gel-filtration to remove free inhibitor and then reacted with FP-Rh (right panels) to determine the reversibility of inhibition. (C) MS1 traces for the PME-1 active site peptide not adducted (top panel) or adducted (bottom panel) to ABL127. See SI Materials and Methods for more information on this experiment. Traces were obtained from tryptic digests of purified, recombinant PME-1 (10 μM) treated with ABL127 (50 μM, red trace) or DMSO (black trace).
ABL127 Selectively Inhibits PME-1 in Cells.
We next investigated whether ABLs could inhibit PME-1 in living cells. We incubated MDA-MB-231 and HEK 293T cells with a concentration range of ABL127 for 1 h, harvested the soluble proteomes, and then reacted these lysates with the FP-Rh probe (Fig. 4A and Fig. S3). In both cell lines, we observed highly potent and selective inhibition of PME-1 with IC50 values of 11.1 nM and 6.4 nM, respectively (Fig. 4B and Fig. S3). Although these gel-based competitive ABPP studies did not reveal any additional serine hydrolase targets of ABL127 at concentrations under 10 μM, we wanted to verify this selectivity profile by higher-resolution LC-MS/MS methods. To accomplish this, we employed an advanced version of our competitive ABPP-MudPIT technology (20, 25) that utilizes stable-isotope labeling of amino acids in cell culture (SILAC) (26). SILAC involves differential labeling of proteins with stable isotopes to generate isotopically “light” and “heavy” samples, which, when pooled and analyzed by MS, yield accurate quantification by comparing intensities of light and heavy peptide peaks. SILAC has previously been used to identify enzymes targets of activity-based probes (27) and small-molecule-binding proteins in cell lysates (28). In our competitive ABPP-SILAC experiments, cells grown in light and heavy media were treated with DMSO or ABL127, respectively, for 1 h. Proteomes were then harvested, combined at a 1∶1 total protein ratio, and treated with the activity-based probe FP-biotin (29). FP-biotin-labeled proteins were then enriched with streptavidin-conjugated beads, digested on-bead with trypsin, and the resulting peptides analyzed by liquid chromatography-high-resolution tandem MS using an LTQ-Orbitrap mass spectrometer. Specifically enriched proteins were identified and quantified based on analysis of MS2 spectra and MS1 profiles, respectively. This analysis revealed complete and selective in situ inhibition of PME-1 by ABL127 with no activity against > 50 other serine hydrolases detected in MDA-MB-231 and HEK 293T cells (Fig. 4C and Fig. S4).
Fig. 4.
ABL127 selectively inactivates PME-1 and decreases the demethylated form of PP2A in cells. (A) Gel-based competitive ABPP of the soluble proteomes from MDA-MB-231 cells treated with ABL127 (0.61–10,000 nM; 1 h) reveals selective blockade of PME-1 with (B) an IC50 value of 11.1 nM. (C) Isotopically “light” and “heavy” MDA-MB-231 cells were treated with DMSO or ABL127 (100 nM), respectively, for 1 h. Proteomes were combined in a 1∶1 total protein ratio (0.5 mg each), analyzed by ABPP-MudPIT, and serine hydrolase activities were quantified by comparing intensities of light and heavy peptide peaks (see Fig. S4 for additional SILAC ABPP-MudPIT analyses). Data are presented as mean values ± SEM for all quantifiable peptides from each serine hydrolase. (D and E) MDA-MB-231 and HEK 293T cells treated with ABL127 (500 nM, 1 h) exhibit significant reductions in demethylated PP2A. (F) Stable overexpression of PME-1 in HEK 293T cells compared to control cells overexpressing GFP. PME-1 is completely inhibited by ABL127 (500 nM, 1 h) in both cell lines. (G and H) Cells overexpressing PME-1 show decreased PP2A methylation, which is reversed by addition of ABL127 (500 nM, 1 h). (I) Time-course for PME-1 inhibition by ABL127 (500 nM) in PME-1-overexpressing HEK 293T cells. For E and I: *p < 0.05, **p < 0.01 for DMSO-treated versus ABL127-treated cells. #p < 0.05, ##p < 0.01 for cells overexpressing GFP versus PME-1. Data are presented as mean values ± SEM; n = 3/group.
We next investigated the impact of ABL127 incubation on the methylation state of PP2A in cells. As expected, in both MDA-MB-231 and HEK 293T cells, we observed significant reductions in the levels of demethylated PP2A (35% and 80%, respectively; Fig. 4 D and E). A trend toward increases in methylated PP2A was also observed in ABL127-treated HEK 293T cells, but this change did not reach statistical significance (p = 0.12) (Fig. 4 D and E). No difference in methylated PP2A was observed in MDA-MB-231 cells treated with ABL127 (Fig. 4 D and E). These outcomes might be expected if the vast majority of PP2A is constitutively methylated under standard cell culture conditions. To investigate this hypothesis, we stably overexpressed PME-1 in HEK 293T cells (Fig. 4F), which resulted in a dramatic increase in demethylated PP2A and a significant decrease in methylated PP2A relative to a control cell line stably expressing GFP (Fig. 4 G and H). Importantly, treatment of PME-1-transfected cells with ABL127 for only 1 h reduced the amount of demethylated PP2A back to the level observed in GFP-overexpressing control cells (Fig. 4 G and H). These ABL127-treated cells also showed a significant increase in methylated PP2A (Fig. 4 G and H). Time course studies revealed that a single treatment of ABL127 resulted in sustained inactivation of PME-1 and reductions in demethylated PP2A for at least 24 h (Fig. 4I). These data, taken together, indicate that ABL127 selectively inactivates PME-1 in living cells, which in turn causes significant changes in the methylation state of PP2A.
A Clickable ABL Confirms Proteome-Wide Selectivity for PME-1.
Our competitive ABPP results showed that ABL127 is highly selective for PME-1 among members of the serine hydrolase family, but they did not address the possibility that ABL127 might react with other proteins in the proteome. To investigate this possibility, we synthesized ABL112, an analog of ABL127 that contains alkyne groups to serve as latent affinity handles amenable to modification by reporter tags using the copper(I)-catalyzed azide–alkyne cycloaddition reaction (“click chemistry”) (30) (Fig. 5A). First, we confirmed that ABL112 retains inhibitory activity for PME-1 by gel-based competitive ABPP in MDA-MB-231 lysates, where it showed only a threefold reduction in potency (IC50 = 13.8 nM; Fig. 5B) compared to the parent inhibitor ABL127 (Fig. S5). Next, we treated MDA-MB-231 cells with ABL127 (100 nM) or DMSO for 30 min before adding ABL112 at a range of concentrations and incubating for another 2 h. Cells were then lysed and the soluble proteomes were subjected to a “click” reaction with an azide-Rh tag (RhN3), separated by SDS/PAGE, and ABL112-labeled proteins were visualized by in-gel fluorescence scanning (Fig. 5C). This analysis identified PME-1 as the only ABL112-labeled protein that could be competed by pretreatment with ABL127. One additional ABL112-reactive protein at approximately 80 kDa was also detected, but the labeling of this protein was not competed by ABL127 or ent-ABL127 (Fig. S5), indicating that it is exclusively a target of the clickable probe ABL112 but not ABL127. Consistent with this premise, competitive ABPP analysis identified an 80 kDa FP-Rh-labeled protein in MDA-MB-231 lysates that was sensitive to inhibition by ABL112 but not ABL127 (Fig. S5). We performed a similar “click” analysis with ABL112 in the soluble proteome of mouse brain, which again revealed selective labeling of PME-1 (Fig. 5D). These results demonstrate that the remarkable selectivity displayed by ABL127 for PME-1 extends not only across the serine hydrolase family but also the greater mammalian proteome.
Fig. 5.
A clickable ABL reveals proteome-wide selectivity for PME-1. (A) Structure of the ABL alkyne probe ABL112. (B) ABL112 inactivates PME-1 (IC50 = 13.8 nM; 95% confidence limits 6.5–29 nM) in the MDA-MB-231 soluble proteome (1 mg/mL protein) as determined by gel-based competitive ABPP (see Fig. S5). (C) MDA-MB-231 cells were incubated with DMSO or ABL127 (100 nM, 30 min) followed by ABL112 (10–200 nM, 2 h). Soluble cell proteomes (0.5 mg/mL) were then subjected to a standard “click” reaction using RhN3 (30) and ABL112-labeled proteins were visualized by in-gel fluorescence scanning. PME-1 was the only protein labeled by ABL112 and competed by ABL127. ABL112 labeled an additional 80 kDa protein that was not competed by ABL127, suggesting it may be a target for ABL112, but not ABL127 (see Fig. S5). (D) Mouse brain soluble lysates (1 mg/mL) were incubated (30 min) with DMSO or ABL127 (100 nM). ABL112 (10 nM) was then added for an additional 30 minutes before analysis by click chemistry-based ABPP.
ABL127 Inactivates PME-1 in Mice.
As mentioned earlier, PME-1(-/-) mice are not viable (13), which has hindered experimental efforts to characterize this enzyme’s function (and the functional significance of PP2A methylation) in animals. Pharmacological inhibition of PME-1 would thus offer a potentially powerful means to study this enzyme in vivo. With this goal in mind, we asked whether ABL127 could inhibit PME-1 in mice. C57Bl/6 mice were treated with ABL127 (50 mg/kg, i.p., 2 h) or vehicle, sacrificed, and their brain proteomes assayed for PME-1 activity by competitive ABPP. Gel-based profiles indicated that brain PME-1 was inactivated by ABL127 (Fig. 6A), but overlapping serine hydrolase activities precluded a confident assessment of the extent of inactivation. For enhanced resolution of the activity state of PME-1 and other brain serine hydrolases, we performed competitive ABPP-MudPIT studies using FP-biotin. These LC-MS profiles confirmed complete inactivation of PME-1 (Fig. 6B) and no substantial reductions in any of the other approximately 40 brain serine hydrolases detected in this experiment. We also observed an approximately 35% reduction in the amount of demethylated PP2A in brain tissue from mice treated with ABL127 (Fig. 6 C and D). These results confirm that ABL127 can selectively inhibit PME-1 in mice and this inhibition alters the methylation state of brain PP2A.
Fig. 6.
ABL127 selectively inhibits PME-1 in vivo. (A) Evaluation of mouse brain soluble proteome from mice treated with vehicle or ABL127 (50 mg kg-1, i.p., 2 h) by gel-based ABPP. In this analysis, proteomes were also treated ex vivo with DMSO or ABL127 (2 μM) before FP-Rh labeling to confirm complete PME-1 inhibition. (B) ABPP-MudPIT analysis of serine hydrolases from brain proteomes of animals treated with vehicle or ABL127. These data confirm selective inactivation of PME-1 in vivo. (C and D) Mice treated with ABL127 exhibited reductions in demethylated PP2A compared with vehicle-treated mice. *p < 0.01, **p < 0.001 for DMSO versus ABL127 groups. Data are presented as mean values ± SEM; n = 3/group.
Discussion
We report herein a class of ABL inhibitors that show remarkable selectivity for PME-1 and equipotent activity in both cell-free and living cell assays. The lead ABL, ABL127 (designated as NIH Probe ML174), is capable of inactivating PME-1 in both human cancer cells and mice, suggesting that it should serve as a versatile pharmacological probe for evaluating PME-1 function in a multitude of living systems. We found that PME-1 inhibition causes a significant reduction in demethylated PP2A and, in cells with high levels of PME-1 activity, also a concomitant increase in methylated PP2A. Future studies with ABL127 should facilitate a more detailed understanding of the role that methylation plays in regulating PP2A function. Will, for instance, alterations in methylation state impact the composition and/or stability of specific PP2A complexes? Structural studies have confirmed that PME-1 is a component of this complex, where its interactions with the catalytic subunit of PP2A appear to be mediated, at least in part, by the PME-1 active site (12). It is thus possible that inhibition of PME-1 will affect PP2A complexes not only through altering methylation but also through disrupting physical interactions between PME-1 and the catalytic subunit of PP2A. Determining how such changes in PP2A complexes affect substrate interactions and the broader phosphoproteome should represent an exciting area of research. On this note, recent studies point to an important role for PME-1 in negatively regulating the tumor-suppressive function of PP2A in cancer cells (14). PME-1 inhibitors may thus have utility as anticancer agents. Finally, we speculate that the net effect of PME-1 inhibition on PP2A methylation state will be dictated by the expression levels of not only PME-1 (see Fig. 3G) but also LCMT1. Imbalances in the relative expression of these two enzymes may thus mark specific cellular states where PME-1 inhibitors will produce their most dramatic pharmacological effects.
There were several keys to the success of our probe development effort. First, screening for inhibitors of PME-1 benefited from the fluopol-ABPP technology, which circumvented the limited throughput of previously described substrate assays for this enzyme. Second, we were fortunate that the NIH compound library contained several members of the ABL class of small molecules. These chiral compounds, which represent an academic contribution to the NIH library, occupy an unusual portion of structural space that is poorly accessed by commercial compound collections. Although at the time of their original synthesis (23) it may not have been possible to predict whether these ABLs would show specific biological activity, their incorporation into the NIH library provided a forum for screening against many proteins and cellular targets, culminating in their identification as PME-1 inhibitors. We then used advanced chemoproteomic assays to confirm the remarkable selectivity displayed by ABLs for PME-1 across (and beyond) the serine hydrolase superfamily. That the mechanism for PME-1 inhibition involves acylation of the enzyme’s conserved serine nucleophile (Fig. 3) suggests that exploration of a more structurally diverse set of ABLs might uncover inhibitors for other serine hydrolases. In this way, the chemical information gained from a single high-throughput screen may be leveraged to initiate probe development programs for additional enzyme targets.
Projecting forward, this research provides an example of how public small-molecule screening centers can serve as a portal for spawning academic collaborations between chemical biology and synthetic chemistry labs. By continuing to develop versatile high-throughput screens and combining them with a small-molecule library of expanding structural diversity conferred by advanced synthetic methodologies, academic biologists and chemists are well-positioned to collaboratively deliver pharmacological probes for a wide range of proteins and pathways in cell biology.
Materials and Methods
PME-1 Protein Expression and Purification.
Human recombinant PME-1 was expressed in BL21(DE3) Escherichia coli and purified at approximately 5 mg/L as detailed in SI Materials and Methods.
PME-1 Fluopol-ABPP Assay.
See SI Materials and Methods for details.
Competitive ABPP Assays in Proteomes.
See SI Materials and Methods for details.
Synthesis of ABLs.
See SI Materials and Methods for details.
Supplementary Material
Acknowledgments.
We thank Tianyang Ji, David Milliken, Kim Masuda, Benjamin Kipper, and Jaclyn M. Murphy for technical assistance. This work was supported by National Institutes of Health grants CA132630 (B.F.C.), MH084512 (H.R.), GM57034 (G.C.F.), and GM086040 (postdoctoral fellowship to J.T.M.); the National Science Foundation (predoctoral fellowship to D.A.B.); the California Breast Cancer Research Program (predoctoral fellowship to D.A.B.); and The Skaggs Institute for Chemical Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015248108/-/DCSupplemental.
References
- 1.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y. Serine/threonine phosphatases: Mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Oliver CJ, Shenolikar S. Physiologic importance of protein phosphatase inhibitors. Front Biosci. 1998;3:D961–972. doi: 10.2741/a336. [DOI] [PubMed] [Google Scholar]
- 4.Janssens V, Goris J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalhor HR, Luk K, Ramos A, Zobel-Thropp P, Clarke S. Protein phosphatase methyltransferase 1 (Ppm1p) is the sole activity responsible for modification of the major forms of protein phosphatase 2A in yeast. Arch Biochem Biophys. 2001;395:239–245. doi: 10.1006/abbi.2001.2558. [DOI] [PubMed] [Google Scholar]
- 6.Leulliot N, et al. Structure of protein phosphatase methyltransferase 1 (PPM1), a leucine carboxyl methyltransferase involved in the regulation of protein phosphatase 2A activity. J Biol Chem. 2004;279:8351–8358. doi: 10.1074/jbc.M311484200. [DOI] [PubMed] [Google Scholar]
- 7.Ogris E, et al. A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J Biol Chem. 1999;274:14382–14391. doi: 10.1074/jbc.274.20.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, et al. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. EMBO J. 2000;19:5672–5681. doi: 10.1093/emboj/19.21.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant JC, Westphal RS, Wadzinski BE. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Balpha subunit. Biochem J. 1999;339((Pt 2)):241–246. [PMC free article] [PubMed] [Google Scholar]
- 11.Longin S, et al. Spatial control of protein phosphatase 2A (de)methylation. Exp Cell Res. 2008;314:68–81. doi: 10.1016/j.yexcr.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Xing Y, et al. Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell. 2008;133:154–163. doi: 10.1016/j.cell.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 13.Ortega-Gutierrez S, Leung D, Ficarro S, Peters EC, Cravatt BF. Targeted disruption of the PME-1 gene causes loss of demethylated PP2A and perinatal lethality in mice. PLoS One. 2008;3:e2486. doi: 10.1371/journal.pone.0002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puustinen P, et al. PME-1 protects extracellular signal-regulated kinase pathway activity from protein phosphatase 2A-mediated inactivation in human malignant glioma. Cancer Res. 2009;69:2870–2877. doi: 10.1158/0008-5472.CAN-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sontag JM, Nunbhakdi-Craig V, Mitterhuber M, Ogris E, Sontag E. Regulation of protein phosphatase 2A methylation by LCMT1 and PME-1 plays a critical role in differentiation of neuroblastoma cells. J Neurochem. 2010;115:1455–1465. doi: 10.1111/j.1471-4159.2010.07049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longin S, et al. An inactive protein phosphatase 2A population is associated with methylesterase and can be re-activated by the phosphotyrosyl phosphataseactivator. Biochem J. 2004;380:111–119. doi: 10.1042/BJ20031643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blankman JL, Simon GS, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachovchin DA, Brown SJ, Rosen H, Cravatt BF. Substrate-free high-throughput screening identifies selective inhibitors for uncharacterized enzymes. Nat Biotechnol. 2009;27:387–394. doi: 10.1038/nbt.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nomura DK, et al. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachovchin DA, et al. A superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proc Natl Acad Sci USA. 2010;107:20941–20946. doi: 10.1073/pnas.1011663107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patricelli MP, Giang DK, Stamp LM, Burbaum JJ. Direct visualization of serine hydrolase activities in complex proteome using fluorescent active site-directed probes. Proteomics. 2001;1:1067–1071. doi: 10.1002/1615-9861(200109)1:9<1067::AID-PROT1067>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Leung D, Hardouin C, Boger DL, Cravatt BF. Discovering potent and selective reversible inhibitors of enzymes in complex proteomes. Nat Biotechnol. 2003;21:687–691. doi: 10.1038/nbt826. [DOI] [PubMed] [Google Scholar]
- 23.Berlin JM, Fu GC. Enantioselective nucleophilic catalysis: The synthesis of aza-β-lactams through [2 + 2] cycloadditions of ketenes with azo compounds. Angew Chem Int Ed Engl. 2008;47:7048–7050. doi: 10.1002/anie.200802439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kluge AF, Petter RC. Acylating drugs: Redesigning natural covalent inhibitors. Curr Opin Chem Biol. 2010;14:421–427. doi: 10.1016/j.cbpa.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Long JZ, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann M. Functional and quantitative proteomics using SILAC. Nat Rev Mol Cell Biol. 2006;7:952–958. doi: 10.1038/nrm2067. [DOI] [PubMed] [Google Scholar]
- 27.Everley PA, et al. Assessing enzyme activities using stable isotope labeling and mass spectrometry. Mol Cell Proteomics. 2007;6:1771–1777. doi: 10.1074/mcp.M700057-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Ong SE, et al. Identifying the proteins to which small-molecule probes and drugs bind in cells. Proc Natl Acad Sci USA. 2009;106:4617–4622. doi: 10.1073/pnas.0900191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Patricelli MP, Cravatt BF. Activity-based protein profiling: The serine hydrolases. Proc Natl Acad Sci USA. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods. Chem Biol. 2004;11:535–546. doi: 10.1016/j.chembiol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Pearlman RS, Smith KM. Novel software tools for chemical diversity. Perspect Drug Discovery Des. 1998;9-11:339–353. [Google Scholar]
- 32.Schurer SC, et al. Ligand-binding pocket shape differences between sphingosine 1-phosphate (S1P) receptors S1P1 and S1P3 determine efficiency of chemical probe identification by ultrahigh-throughput screening. ACS Chem Biol. 2008;3:486–498. doi: 10.1021/cb800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.