Abstract
Oxidative stress is known to cause tumorigenesis through induction of DNA and lipid damage. It also promotes cancer progression through a largely unknown mechanism. Sulfiredoxin (Srx) is a novel oxidative stress-induced antioxidant protein whose function in tumorigenesis and cancer progression has not been well studied. We report that Srx is highly expressed in human lung cancer. Knockdown of Srx reduces anchorage-independent colony formation, cell migration, and invasion of human lung cancer cells. Srx preferentially interacts with Peroxiredoxin (Prx) IV relative to other Prxs due to its intrinsic higher binding affinity. Knockdown of Prx IV recapitulates the phenotypic changes of depleting Srx. Disruption or enhancement of the Srx–Prx IV axis leads respectively to reduction or acceleration of tumor growth and metastasis formation in vivo. Through identification and validation of the downstream mediators we unraveled the Srx-mediated signaling network that traverses AP-1–activating and other phosphokinase signaling cascades. Our work reveals that the Srx–Prx IV axis is critical for lung cancer maintenance and metastasis, suggesting that targeting the Srx–Prx IV axis may provide unique effective strategies for cancer prevention and treatment.
Keywords: oncogene, signal transduction
Lung cancer is the most common form of human cancer and is the leading cause of cancer mortality worldwide. Despite recent progress in early detection and combined therapeutic strategies, the overall survival rate of patients has not been significantly improved (1). Many risk factors, including the exposure to tobacco smoke, have been identified as causally related to lung carcinogenesis in patients. Tobacco smoke contains a number of carcinogens, and metabolism of these carcinogens generates active metabolites directly leading to formation of DNA adducts and the accumulation of oxygen-free radicals that induce oxidative stress that contributes significantly to tumorigenesis and cancer progression (2).
Oxygen-free radicals, including reactive oxygen species (ROS) and reactive nitrogen species, have been shown to be causally related to cancer progression and metastasis. However, the molecular mechanisms of oxidative stress in cancer maintenance and metastases formation are still not well understood, due in part to the dual roles of ROS as both deleterious and beneficial species in cancer cells. Under physiological conditions ROS-induced oxidative stress response causes cellular senescence and apoptosis. Cancer cells have an enhanced defense system characterized by intrinsically higher expression of antioxidant proteins, and ROS are more likely to function as mediators of intracellular signaling cascades that are critical for maintenance of tumor phenotypes (3). Sulfiredoxin (Srx), or neoplastic progression 3, was previously identified as a differentially expressed gene with unknown function that distinguished transformed and transformation-sensitive from transformation-resistant mouse epidermal cells (4). More recent studies substantiate that Srx is an oxidation-induced antioxidant protein that catalyzes the reduction of hyperoxidized Peroxiredoxins (Prxs) to the reduced form to restore their peroxidase activity (5, 6). In addition, Srx may also contribute to protein deglutathionylation (7, 8).
Our previous study demonstrates that Srx is a unique target of activator protein-1 (AP-1) activation and TAM67 (a dominant-negative form of c-Jun) inhibition that is functionally significant in skin carcinogenesis (9). Transcriptional activation of Srx by AP-1 and nuclear factor erythroid 2-related factor (Nrf2) signaling has also been demonstrated in other cell types including neurons and pancreatic cells, and Srx expression can be induced by tobacco smoke in lung epithelial cells (10). Our current work elucidates the essential function of Srx and the Srx–Prx IV axis in human lung cancer cell migration, invasion, and metastasis and reveals an important oncogenic role of Srx in cancer maintenance and progression through modulation of specific phosphokinase signaling cascades. Our findings also suggest that targeting the Srx–Prx IV axis may provide unique strategies for cancer prevention and treatment.
Results
Srx Is Highly Expressed in Human Lung Cancer and Is Required for Anchorage-Independent Colony Formation, Cell Migration, and Invasion of Lung Cancer Cells.
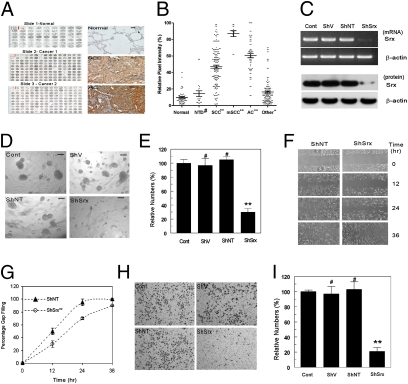
To study the function of Srx in lung cancer, tissue microarray analysis using samples from human normal lung as well as lung diseases was performed. A total of 463 tissue cores representing 271 individuals were stained for Srx expression (Table S1). As shown in Fig. 1, Srx-positive staining is rarely found in human normal or inflammatory lung tissues; in contrast, strong staining of Srx is found in tumor samples from patients with squamous cell carcinoma or adenocarcinoma. In particular, Srx staining intensity is strong in tumor tissues of advanced or metastatic squamous cell carcinoma, possibly indicating a positive correlation of Srx expression with human lung cancer progression (Fig. 1B).
Fig. 1.
Srx is highly expressed in patients with lung cancer and is required for anchorage-independent colony formation, migration, and invasion of cancer cells. (A) Screenshots of representative tissue microarray images (Left) and microscopic images of Srx expression in normal, squamous cell carcinoma (SCC) and adenocarcinoma (AC) (Right). (Scale bar, 10 μm.) (B) Quantitative results of A. NTD, nontumor diseases; mSCC, metastatic SCC. Data are presented as means ± SD. Compared with normal, #P > 0.05, *P < 0.05, **P < 0.01 (t test). See also Table S1 for detailed tissue information. (C) Knockdown of endogenous Srx indicated at the transcript level by RT-PCR and at the protein level by Western blot. (D–I) Srx knockdown cells form fewer colonies in anchorage-independent growth in soft agar (D and E), migrate slower in the wound-healing assay (F and G), and are less invasive in the matrigel invasion assay (H and I). Representative images from sextuplicates are shown. (D, F, and H) Colonies with diameter >100 μm (scale bar in D) were counted. All data are presented as means ± SD (n = 6). (E, G, and I) Compared with the control parental cells, #P > 0.05 and **P < 0.01 (t test in E and I, paired t test in G).
The A549 cell line was originally derived from a patient with lung nonsmall cell adenocarcinoma and Srx is moderately expressed in these cells. A lentiviral-mediated ShRNA knockdown strategy was used to deplete the endogenous expression of Srx in these cells. Infection of cells with lentiviral particles expressing the ShRNA targeting the protein-encoding region of the human Srx gene (ShSrx) effectively depleted the endogenous expression of Srx at both transcript and protein levels. In contrast, Srx expression was not affected by infection of viral particles expressing the vector (ShV) or the nontarget control ShRNA (ShNT) (Fig. 1C). Knockdown of Srx slightly delays cell-cycle progression at the early phase of serum induction but is unable to produce a cumulative effect on cell growth and proliferation under adherent conditions (Fig. S1 A and B). However, compared with parental cells or control cells, Srx knockdown cells formed fewer anchorage-independent colonies when cultured in soft agar (Fig. 1 D and E), migrated more slowly in the chamber wound-healing assay (Fig. 1 F and G), and were significantly less invasive in the transwell matrigel invasion assay (Fig. 1 H and I). These observations suggest that Srx is required for the maintenance of the tumor phenotype of human lung cancer cells.
Identification of Prx IV as the Major Protein That Interacts with Srx.
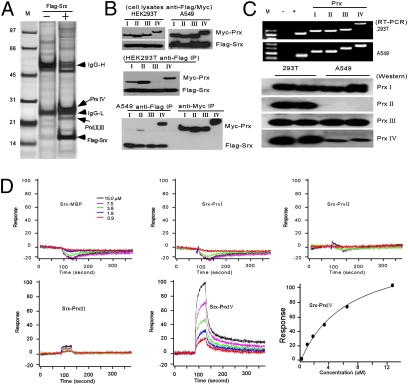
Immunoprecipitation (IP) and reverse-phase liquid chromatography–mass spectrometry (RPLC-MS) methods were applied to identify proteins that may interact with Srx, using a combination of different cell lines and strategies (SI Materials and Methods). Numerous proteins were identified to interact with Srx (see Dataset S1 for a sample list). A summary of all MS identifications reveals that Prx I, II, III, or IV may interact with Srx or be the component of the complex (Fig. 2A). To our surprise, Prx IV was the most abundant protein that was identified in all assays, indicated by the highest numbers of total and unique peptides in MS (Fig. 2A and Table S2). For example, in HEK293T cells, a total of 70 peptides representing nine unique fragments of Prx IV were identified by RPLC-MS (Table S2). Other members, such as Prxs I, II, and III, were also identified, but at lower abundance and with cell type specificity. In particular, Prxs I and II were found by IP using mouse epidermal JB6 cells and HEK293T cells, and Prx III was found by IP using A549 cells. Therefore, Srx may differentially interact with members of the Prx family and the interactions may be cell-context dependent. This prediction is further confirmed by a traditional IP/Western blotting procedure using HEK293T and A549 cells expressing Srx and Prxs (Fig. 2B). In both cell types, Srx–Prx IV interaction is confirmed and there may be cell type-specific differences in regard to Srx interaction with other Prxs. The inability of anti-Myc antibody to pull down Srx may result from the spatial inaccessibility of the antibody to the Myc epitope due to the structural or stoichiometric feature of the complex.
Fig. 2.
Prx IV is the major protein that interacts with Srx. (A) Silver staining of proteins that pull down with Flag-Srx in HEK293T cells. Arrows indicate major proteins of interest identified. (B) Differential interaction of Srx to Prxs by IP in HEK293T and A549 cells. (C) (Upper) Endogenous expression of Prxs in HEK293T and A549 cells at the mRNA level; (Lower) Prx protein expression obtained from the same membrane. (D) Binding kinetics of Srx to different Prxs in vitro as measured by SPR. Under the conditions used, the binding Kd of Srx to Prx IV is ∼4.0–7.0 μM.
The formation of the Srx protein complex may also be affected by the availability/expression level of the partner protein. To clarify whether the abundance of Prx IV identified in MS resulted from its high expression level, we examined the endogenous expression of different Prxs in HEK293T and A549 cells. As shown in Fig. 2C, there were no obvious differences at the transcript levels of Prxs I–IV in these two cell lines. At the protein level, Prxs (I–IV) were relatively abundant and correlated well with their transcript levels in HEK293T cells. In A549 cells, Prxs I and III were expressed similarly to those in HEK293T cells. However, Prx II was not detected in A549 cells and the Prx IV level was significantly less than that in HEK293T cells, which may indicate a posttranscriptional or posttranslational regulation of Prx II and Prx IV expression in A549 cells. The finding of Prx IV as the most abundant Srx-interacting protein in both cell lines, despite the lower expression of Prx IV in A549 cells, suggests that Srx may bind preferentially to Prx IV.
Srx Has a Higher Binding Affinity to Prx IV.
To test the hypothesis that Srx binds preferentially to Prx IV relative to other Prxs, we investigated the Srx–Prx interactions using the surface plasmon resonance (SPR) technique with purified recombinant proteins. The expression and solubility of recombinant Srx and Prxs in Escherichia coli were evaluated (Fig. S2A) and a tandem affinity purification method was used to generate large amounts of purified recombinant Srx and Prxs (Fig. S2B). The final purified proteins were >95% pure, indicated by a single band in Coomassie staining (Fig. S2C). The interaction of Srx with individual Prxs was analyzed on a Biacore Chip by SPR and the binding kinetics were modeled and calculated (11). As a control, Srx did not interact with purified maltose binding protein (MBP) even at the high concentration (Fig. 2D). Under the same conditions, complex formation was not detected between Srx and Prx I or II. A temporary weak formation of complexes was noted between Srx and Prx III. In contrast, a robust and strong association of Srx to Prx IV was detected by SPR and the formation of the complex was concentration dependent (Fig. 2D). The association constant that measures the binding affinity (Ka) of Srx to Prx IV from two independent experiments (on independent chips) was 4 μm and 7 μm, respectively. Therefore, Srx has an intrinsically higher binding affinity to Prx IV than to the other members of the Prx family. These data suggest that there is a hierarchy of Srx–Prx interactions and Srx may exist mainly in the form of Srx–Prx IV complex in human lung cancer cells.
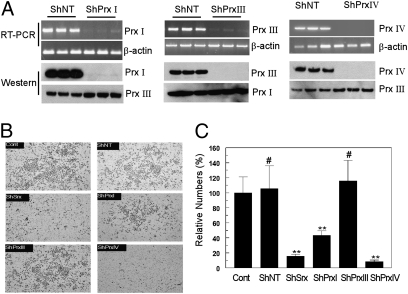
Knockdown of Prx IV Recapitulates the Phenotypic Changes of Knockdown of Srx in A549 Cells.
In A549 cells, endogenous Prx II protein was undetectable by Western blot (Fig. 2C), and Srx was not found to interact with Prx II by mass spectrometry (Table S2). Therefore, we asked whether Prx I, III, or IV is required for the Srx-mediated function in A549 cells. Using the lentiviral-based ShRNA knockdown strategy, the endogenous expression of individual Prxs in A549 cells was efficiently depleted, and knockdown of one Prx did not interfere with the expression of other Prxs (Fig. 3A). In the transwell matrigel invasion assay knockdown of Prx IV significantly reduced the number of cells invading through matrigel to a similar extent to that observed in Srx knockdown cells. Knockdown of Prx III did not cause changes in cell invasion and knockdown of Prx I led to a lesser reduction than that of Srx or Prx IV knockdown (Fig. 3 B and C). When cultured in soft agar, Prx IV knockdown cells formed significantly fewer anchorage-independent colonies, similar to Srx knockdown cells (Fig. S3). Knockdown of Prx III did not cause changes in colony formation and knockdown of Prx I led to a lesser reduction than that seen with Srx or Prx IV knockdown (Fig. S3). Taken together, these findings suggest that knockdown of Prx IV recapitulates the phenotypic changes of Srx knockdown in A549 cells.
Fig. 3.
Knockdown of Prx IV recapitulates the phenotypic changes of Srx knockdown in A549 cells. (A) Efficient and specific knockdown of individual Prx in A549 cells occurred at the transcript and protein levels. (B and C) Prx IV knockdown cells were less invasive. Compared with the parental control, #P > 0.05; **P < 0.01; ShPrx IV compared with ShPrx I, P < 0.01 (n = 6, t test).
Disruption or Enhancement of the Srx–Prx IV Axis Leads to Corresponding Reduction or Acceleration of Tumor Growth in Mouse Xenograft and Metastasis.
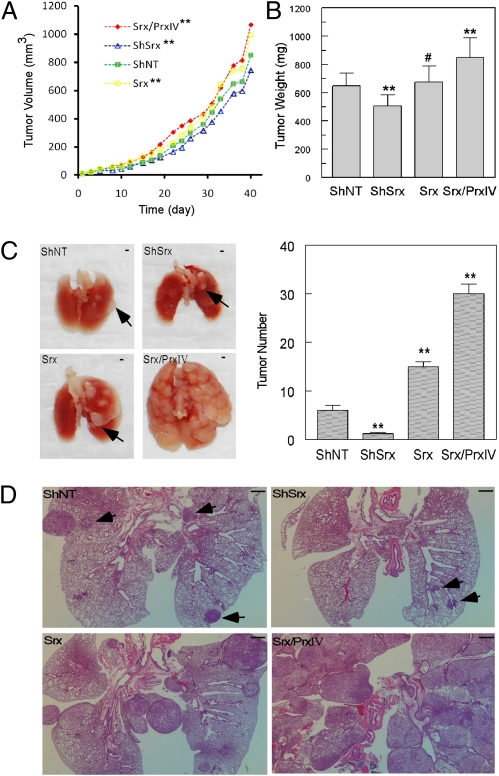
As knockdown of Srx or Prx IV significantly reduces colony formation, we predicted that knockdown of Srx may also affect tumor growth in vivo. To test this hypothesis, cells expressing ShNT (control), ShSrx (knockdown), Srx (overexpression), or Srx/Prx IV (overexpression of Srx plus Prx IV) were injected s.c. into SCID mice. As shown in Fig. 4A, tumor xenografts originating from cells expressing Srx (or Srx plus Prx IV) grew faster than those from control cells. In contrast, tumors originating from the Srx knockdown cells grew slower than those from control cells. To exclude the variations in the measurement of tumor volume, all tumors were extracted and the average tumor weight of the Srx/Prx IV group was found to be significantly heavier than that of the control group, whereas the average tumor weight of the knockdown group was less than that of the control group (Fig. 4B). Therefore, loss of Srx or enhancement of the Srx–Prx axis in A549 cells causes a moderate reduction or acceleration of tumor growth in mouse xenograft in vivo.
Fig. 4.
Disruption (or enhancement) of the Srx–Prx IV axis reduces (or accelerates) tumor growth in mouse xenografts and lung metastasis formation. (A) SCID mice were s.c. injected with A549 cells stably expressing ShNT, ShSrx (knockdown), Srx (overexpression), or Srx/Prx IV (coexpression of Srx and Prx IV). Compared with ShNT, **P < 0.05 (n = 10, paired t test). (B) The final average tumor weights from different groups were compared. Compared with ShNT, #P > 0.05, **P < 0.01 (n = 10, t test). (C) Lung metastasis formation at 13 wk after the injection of A549 cells into the tail veins of SCID mice. Arrows indicate the position of macroscopic tumor nodules. (Scale bar, 1 mm.) Tumor nodules with a diameter >1 mm were counted and tumor numbers from different groups were compared. Compared with ShNT, **P < 0.01 (n = 10, t test). (D) H&E staining of lung tissues from C; arrows indicate the position of microscopic tumor nodules with diameter <1 mm. (Scale bar in D, 1 mm.)
The significant reduction of transwell invasion in Srx (or Prx IV) knockdown cells suggests that the Srx–Prx IV axis may also play a positive role in the metastasis of A549 cells in vivo. To test this hypothesis, cells described above were injected into SCID mice through the tail vein. Ten weeks later, the group injected with cells expressing Srx/Prx IV showed symptoms of developing tumors, such as loss of body weight, difficulty in breathing, and lack of activity. In fact, the unexpected death of one mouse in the Srx/Prx IV group led to the euthanizing of all mice at 13 wk (3 mo) after tail vein injection, despite the fact that mice in other groups were still symptom-free. Examination of mouse lungs revealed that overexpression of Srx alone or in combination with Prx IV in A549 cells dramatically increased the numbers of visible tumor nodules. In contrast, knockdown of Srx significantly reduced the formation of metastatic tumor nodules (Fig. 4C). Microscopically, the reduction of tumor size/numbers in mice injected with Srx knockdown cells, or the exacerbation of tumors (with loss of normal lung structure) in mice injected with Srx or Srx/Prx IV cells, was even more evident (Fig. 4D). Therefore, these observations suggest that the disruption/enhancement of the Srx–Prx IV axis reduces/accelerates the formation of tumor metastases in a mouse model of lung tumor metastasis.
Srx–Prx IV Axis Is Required for the Activation of Specific Intracellular Phosphokinase Signaling Including the AP-1/MMP9 Axis and MAPK Signaling.
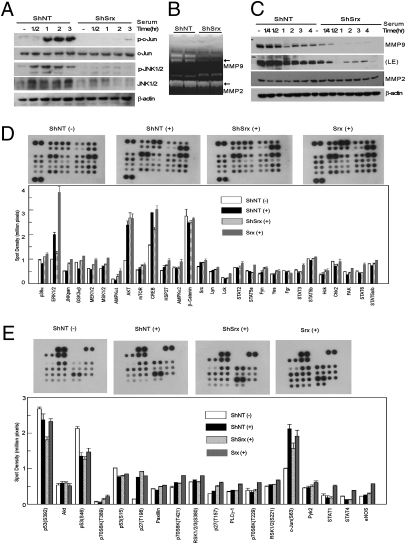
In mouse epidermal JB6 cells, Srx is required for the positive feedback regulation of AP-1 activity (9). This is also true in A549 cells because knockdown of Srx significantly reduced serum-induced c-Jun phosphorylation (Fig. 5A). Members of the matrix metalloproteinase (MMP) protein family are known to be transcriptional targets of oncogenic AP-1 activation and are required for cancer invasion and metastasis (12). Therefore, we asked whether Srx is required for MMP activity in A549 cells. Gelatin zymography was used to examine the enzyme activities of MMPs in the cell culture medium. Compared with control, a significant loss of MMP9 but not MMP2 activity was found in the culture medium of the Srx knockdown cells (Fig. 5B). The loss of activity was likely due to the absence of MMP9 protein, indicated by a significant down-regulation of MMP9 protein expression in Srx knockdown cells (Fig. 5C). Taken together, these observations demonstrate that the oncogenic or tumor-promoting function of Srx is mediated at least in part through the regulation of the AP-1/MMP9 axis.
Fig. 5.
The Srx–Prx IV axis is required for sufficient activation and/or amplification of specific phosphokinase signaling including the AP-1/MMP9 and MAPK cascades. (A) Reduction of the serum-induced JNK and c-Jun activation in Srx knockdown cells. (B) Reduction of MMP9 activity in the culture medium of the Srx knockdown cells measured by gelatin zymography. (C) Down-regulation of MMP9 protein expression in Srx knockdown cells, indicated by Western blot. LE, longer exposure. (D and E) Proteome profiler human phosphokinase array. −, no stimulation; +, serum stimulation.
To further explore the molecular mechanisms of Srx in human lung cancer cell invasion and metastasis, we examined the global phosphokinase signaling changes mediated by Srx in A549 cells. Proteome profiler human phosphokinase arrays were performed using cells expressing ShNT, ShSrx, or Srx. Each phosphokinase assay detects the levels of multiple phosphorylated proteins in duplicate, along with several spots of predesigned negative and positive controls (Fig. S4 A and B). Of all of the phosphorylated proteins examined, ERK1/2, CREB, and c-Jun showed significantly attenuated serum-induced phosphorylation levels upon knockdown of Srx (Fig. 5 D and E). The antibody to p-ERK used in this assay recognizes both p-ERK1 and p-ERK2. Therefore, to clarify which of the p-ERKs was affected by the knockdown of Srx, further proteome profiler human phospho-MAPK arrays were performed. In this assay, specific antibodies recognizing either p-ERK1 or p-ERK2 were used (Fig. S4 C and D). Compared with the control, the induction of both p-ERK1 and p-ERK2 levels was significantly reduced in Srx knockdown cells (Fig. S5 A and B).
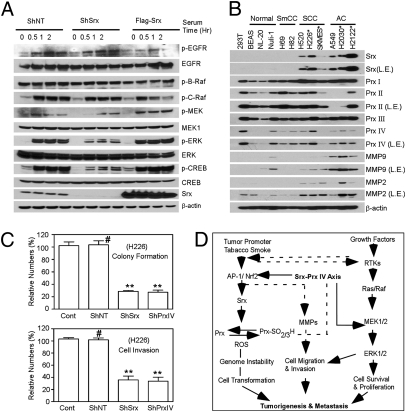
The signaling changes identified from the phosphokinase array were also validated by Western blotting. As shown in Fig. 6A, Srx knockdown cells showed significant reduction in the phosphorylation levels of ERK1/2 and CREB at multiple time points in response to stimulation, and Srx-overexpressing cells showed slightly enhanced phosphorylation of these proteins. The regulation of these phosphorylation events did not occur at the EGF receptor or downstream Ras/Raf activation, because only the phosphorylation of MEK1/2 was significantly reduced in Srx knockdown cells and enhanced in Srx-overexpressing cells (Fig. 6A). Therefore, the reduced activation of MEK1/2 may contribute to the attenuation of ERK1/2 activation by Srx knockdown.
Fig. 6.
Examination of the Srx–Prx axis in human lung normal and tumor cell lines. (A) Validation of Srx-related cell signaling changes in A549 cells. (B) Expression of Srx, Prxs, and MMPs in lung normal and tumor cells. *, cell line derived from cancer metastasis. (C) Knockdown of Srx or Prx IV reduces colony formation and cell invasion in H226 cells. Compared with control, #P > 0.05, **P < 0.01 (n = 6, t test). (D) A schematic model of the Srx–Prx axis in tumorigenesis and cancer progression.
Because knockdown of Prx IV recapitulates the phenotypic changes of Srx knockdown cells (Fig. 3), we asked whether knockdown of Prx IV would have a similar effect in intracellular cell signaling. Proteome profiler human phosphokinase arrays were analyzed using ShNT-, ShPrxIV-, and Prx IV-overexpressing cells. As shown in Fig. S5 C and D, knockdown of Prx IV led to a kinase profiling change similar to those observed in Srx knockdown cells, such as the insufficient phosphorylation of ERK1/2, AKT, CREB, and c-Jun (Fig. S5 C and D). Additionally, knockdown of Prx IV has a broader effect. For example, the phosphorylation levels of p38α, JNK1/2, GSK3α/β, MEK1/2, MSK1/2, AMPKα, HSP27, Src, and Fyn were somewhat reduced compared with those in ShNT cells. Taken together, these observations further demonstrate that the integrity of the Srx–Prx IV axis is required for the sufficient activation and/or amplification of specific kinase signaling pathways. The compromised signaling pathways in Srx knockdown cells may collectively contribute to the reduced rate of tumor growth in mouse xenografts and their inability to form lung metastases in vivo.
Systematic Evaluation of Srx and Prx Expression in Multiple Cell Lines and a Model of the Srx–Prx IV Axis in Human Cancer.
To generalize our findings in human lung cancer, we examined the expression of Srx, Prxs, and MMPs in multiple cell lines derived from human normal lung epithelium (BEAS-2B, NL20, and Nuli-1), small cell carcinoma (H69 and H82), squamous cell carcinoma (H520, H226, and SK-Mes-1), and adenocarcinoma (A549, H2030, and H2122). As shown in Fig. 6B, Srx is not expressed in cells from human normal lung epithelium and small cell carcinoma, but is highly expressed in cells from squamous cell carcinoma or adenocarcinoma. In particular, strong expression is found in cells derived from metastasis of squamous cell carcinoma or adenocarcinoma (H226, H2030, and H2122 cells). Moreover, cell lines from lung squamous cell carcinoma or adenocarcinoma express a much higher level of Prx IV, which is not detected in two of three lung normal epithelial cell lines. The expression of Prx I is also higher in lung cancer cells but the levels of Prx II and Prx III are variable compared with lung normal epithelial cells. These data further demonstrate the importance of the Srx–Prx IV axis in human lung squamous cell carcinoma and adenocarcinoma. Furthermore, knockdown of Srx or Prx IV in the H226 cell line, which is derived from the pleural effusion of lung squamous cell carcinoma, significantly reduces colony formation in soft agar and cell invasion in matrigel transwell assay (Fig. 6C). The importance of the Srx–Prx IV axis is also demonstrated from the Oncomine database (Fig. S6). On the basis of all of these findings, a model is proposed for the function of the Srx–Prx IV axis in human cancer (Fig. 6D).
Discussion
Initially identified in transformation-sensitive mouse JB6 cells (4) and subsequently found to be targeted by AP-1 blockade when it inhibits carcinogenesis (9), Srx now emerges as required not only for anchorage-independent colony formation, cell migration, and invasion of lung cancer cells in vitro, but also as pivotal for these cells to form metastases in vivo. Examination of the downstream mediators of Srx function revealed that Srx preferentially binds to Prx IV and that the Srx–Prx IV axis contributes significantly to the maintenance of tumor cell phenotype in vitro and the formation of metastases in vivo. Disruption or enhancement of this axis leads to corresponding reduction or acceleration of tumor growth in mouse models. From a systematic screening of Srx expression in human primary cancer tissues, we discovered that Srx is expressed in human tumors including cancers of the skin, lung, and rectum, but not in normal tissue (9). In particular, increased Srx expression was found in lung squamous cell carcinoma and adenocarcinoma. Mechanistic studies further demonstrated that the integrity of the Srx–Prx IV axis is required for sufficient activation and/or amplification of certain phosphokinase signaling cascades including the AP-1/MMP9 axis, CREB, and MAPK pathways. This study thereby reveals a unique oncogenic function of Srx in human cancer that appears to be mediated through the Srx–Prx IV axis and its positive regulation of specific phosphokinase signaling cascades.
Srx is an oxidative stress-induced protein and a master enzyme to catalyze the reduction of hyperoxidized Prxs (5, 6). In addition, Srx may also contribute to protein deglutathionylation (7, 8). Although Prxs bear the common function of H2O2 scavenging, previous studies indicate that they are not simply redundant proteins and they may not even share the same potential in cancer development. In fact, different Prxs may have distinctive signaling specificity that can also be cell context dependent. For example, Prx I interacts with the oncoprotein c-Abl to inhibit its tyrosine kinase activity (13) and regulates AKT activation through preserving PTEN activity (14). In prostate cancer cells, Prx I interacts with androgen receptor, enhances its transactivation activity, and contributes to the aggressive cancer phenotype (15). Prx II is a negative regulator of platelet-derived growth factor (PDGF) receptor-mediated cell signaling (16) and a negative regulator of nuclear factor-kappa B (NF-κB) activation in mouse fibroblasts (17). Little is known about the function of Prx IV in cell signaling except that it may act as a negative regulator of NF-κB activation in HeLa cells (18). These studies clearly show that members of the Prx family are actively involved in the regulation of multiple signal cascades in addition to maintaining cellular redox balance. By incorporating into specific intracellular signaling pathways, individual Prxs may have distinctive functions that are important for physiological as well as pathological signaling activities.
The complexity of Prxs in cell signaling reflected in cancer development is that they may function as double-edged swords, i.e., being either tumor activators or suppressors. Several lines of evidence indicate that members of the Prx family may function as activators in cancer development. First, Prxs are often found highly expressed in human tumors, in particular in cancers from breast (Prx I, II, and III), lung (Prx I, III, and IV), bladder (Prx I and VI), thyroid (Prx I), and tongue (Prx I) (19). Second, expression of Prxs promotes cell survival under oxidative stress conditions, and depletion of Prxs sensitizes cancer cells to apoptosis. For example, overexpression of Prx I facilitates cell growth and proliferation by protecting them from oxidant-induced cell death, and Prx III is required for Myc-mediated rat fibroblast transformation and proliferation of breast cancer MCF7 cells (20). On the other hand, Prxs, in particular Prx I, can also function as tumor suppressors. In Myc-transformed mouse fibroblasts, overexpression of Prx I reduces colony formation in soft agar and tumorigenesis in mouse xenografts (21). Depletion of Prx I also significantly enhances cell susceptibility to Ras-induced neoplastic transformation (22). In mouse studies, knockout of Prx I causes spontaneous tumor formation in aging mice in one study (23), and the tumor suppressor function of Prx I may result from the inhibition of AKT activity through maintaining PTEN activity (14). The unique function of Prx I may be partially due to the presence of the cysteine residue 83, which is not present in other Prxs (24). It is noteworthy, though, that Prx I knockout mice are normal in another study (25). Mice with a single knockout of other Prxs, such as Prx II, III, IV, or VI, are all viable and have a normal phenotype (23, 25–29), which may in part reflect their redundant function in redox signaling. However, whether these mice are tumor prone or tumor resistant has not been investigated.
In this study, we identified the Srx–Prx IV axis and its critical role in human cancer maintenance and metastasis. The preferential interaction of Srx with Prx IV may be attributable to the unique structural features of Prx IV, as even the minor differences in Prx I and II are able to cause dramatic differences in protein–protein interaction as well as cell signaling (30, 31). By the proteome profiler kinase array, we found that the Srx–Prx IV axis is critical for specific phosphokinase-mediated signaling in human lung cancer cells. The activation of CREB and c-Jun can be induced by growth factor-mediated MAPK signaling activation (32, 33). Therefore, the insufficient activation of MAPK signaling cascade in Srx-deficient cells, together with the reduction of JNK activity, may lead to the insufficient activation of CREB and AP-1 activity. The MAPK cascade has been well established as a major signaling pathway that drives tumor cell invasion and metastasis. Our data suggest that one mechanism by which Srx promotes tumor progression is through the regulation of the MAPK/AP-1/MMP9 axis. Due to the broader inhibitory effect of Prx IV depletion on phosphokinase signaling, we may expect a larger scale of importance for the Srx–Prx IV axis in tumor invasion and metastasis as shown from the results in vivo. However, whether blockage of Prx IV alone is sufficient to inhibit lung tumorigenesis or cancer invasion and metastasis remains to be answered. Further mechanistic understanding of Srx and Prxs in cell signaling may provide unique strategies for the design of improved therapeutic approaches for human lung cancer.
Our research also sheds light on upstream aspects of Srx-mediated cell signaling. The effects of Srx knockdown on multiple phosphokinases may result from inhibiting MAPK signaling. The MAPK cascade is mainly composed of the receptor tyrosine kinases (RTKs), Ras, Raf, MEK, ERK, and downstream transcriptional targets. Other modulators, such as scaffolding protein KSR, protein kinase C (PKC), and protein phosphatases, have also been identified as playing critical roles in determining the strength and duration of growth factor-mediated MAPK signaling. A close examination of the MAPK signaling cascade and various modulators in Srx knockdown cells revealed that the regulation by Srx does not occur at the level of RTKs (such as the phosphorylation of EGF receptor) or the Ras/Raf activation, but at the level of MEK activation. Moreover, recent findings suggest that the sustained activation of MAPK signaling requires localized accumulation of H2O2 in the plasma microdomain (30) and the formation of an active complex of KSR/Raf/MEK (34). In fact, under physiological conditions in which Srx is not induced, the local inactivation of Prx I at the plasma membrane by growth factor-stimulated tyrosine phosphorylation is required for sufficient activation of MAPK signaling cascades (30). In cancer cells where Srx and most Prxs are highly expressed, how the cells reconcile the need for sustained activation of RTK signaling and simultaneous removing of the excessive H2O2 still remains to be understood. In the future, it will be of interest to investigate whether the Srx–Prx IV axis plays a significant role in normal physiological signaling processes such as wound healing and tissue regeneration.
Materials and Methods
Tissue Microarray, RT-PCR, Plasmids, and Lentivirus Production.
Tissue microarray slides were commercially obtained (Biomax). Immunohistochemistry staining was performed as previously reported (9). The relative stain intensities were manually quantitated using the image-J software, according to a combination of previously described methods (35). Detailed methods for viral production are provided in SI Materials and Methods.
Cell Culture, Anchorage-Independent Growth, Wound Healing, Transwell Matrigel Invasion Assay, Western Blotting, IP, Gelatin Zymography, and Phosphokinase Profiling.
All cells were cultured in standard conditions and detailed methods are provided in SI Materials and Methods.
RPLC-MS, Recombinant Protein Purification, and SPR.
Detailed RPLC-MS and SPR methods are provided in SI Materials and Methods.
Mouse Xenografts and Lung Metastasis.
All mouse experiments were agreed to and regulated by the Animal Care and Use Committee of the National Cancer Institute (Frederick, MD) and detailed methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. D. Esposito, T. Troy, W. Gillette, and A. Stephen for protein purification and SPR analysis; Ms. L. Dodge and Mr. D. Logsdon for mouse experiments (Science Applications International Corporation); Ms. K. Noer for flow cytometry analysis (National Cancer Institute); and Dr. K. D. Tew for discussion and communication (Medical University of South Carolina). This work was supported by funding from the National Cancer Institute Intramural Research Program (to N.H.C.) and the National Cancer Institute Howard Temin Pathway to Independence Award K99/R00 (to Q.W). Q.W. is a Cancer Research Training Award fellow and a Cancer Genetics and Signaling fellow. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health under Contract HHSN261200800001E.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013012108/-/DCSupplemental.
References
- 1.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59:27–41. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Hegamyer G, Colburn NH. Molecular cloning of five messenger RNAs differentially expressed in preneoplastic or neoplastic JB6 mouse epidermal cells: One is homologous to human tissue inhibitor of metalloproteinases-3. Cancer Res. 1994;54:1139–1144. [PubMed] [Google Scholar]
- 5.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 6.Chang TS, et al. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 7.Findlay VJ, et al. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66:6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei K, Townsend DM, Tew KD. Protein cysteine sulfinic acid reductase (sulfiredoxin) as a regulator of cell proliferation and drug response. Oncogene. 2008;27:4877–4887. doi: 10.1038/onc.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Q, Jiang H, Matthews CP, Colburn NH. Sulfiredoxin is an AP-1 target gene that is required for transformation and shows elevated expression in human skin malignancies. Proc Natl Acad Sci USA. 2008;105:19738–19743. doi: 10.1073/pnas.0810676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh A, et al. Nrf2-dependent sulfiredoxin-1 expression protects against cigarette smoke-induced oxidative stress in lungs. Free Radic Biol Med. 2009;46:376–386. doi: 10.1016/j.freeradbiomed.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuck P. Use of surface plasmon resonance to probe the equilibrium and dynamic aspects of interactions between biological macromolecules. Annu Rev Biophys Biomol Struct. 1997;26:541–566. doi: 10.1146/annurev.biophys.26.1.541. [DOI] [PubMed] [Google Scholar]
- 12.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 13.Prospéri MT, Ferbus D, Rouillard D, Goubin G. The pag gene product, a physiological inhibitor of c-abl tyrosine kinase, is overexpressed in cells entering S phase and by contact with agents inducing oxidative stress. FEBS Lett. 1998;423:39–44. doi: 10.1016/s0014-5793(98)00057-x. [DOI] [PubMed] [Google Scholar]
- 14.Cao J, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SY, et al. Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer Res. 2007;67:9294–9303. doi: 10.1158/0008-5472.CAN-07-0651. [DOI] [PubMed] [Google Scholar]
- 16.Choi MH, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 17.Han YH, Kwon JH, Yu DY, Moon EY. Inhibitory effect of peroxiredoxin II (Prx II) on Ras-ERK-NFkappaB pathway in mouse embryonic fibroblast (MEF) senescence. Free Radic Res. 2006;40:1182–1189. doi: 10.1080/10715760600868552. [DOI] [PubMed] [Google Scholar]
- 18.Jin DY, Chae HZ, Rhee SG, Jeang KT. Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation. J Biol Chem. 1997;272:30952–30961. doi: 10.1074/jbc.272.49.30952. [DOI] [PubMed] [Google Scholar]
- 19.Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins—structures, mechanisms and functions. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wonsey DR, Zeller KI, Dang CV. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc Natl Acad Sci USA. 2002;99:6649–6654. doi: 10.1073/pnas.102523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mu ZM, Yin XY, Prochownik EV. Pag, a putative tumor suppressor, interacts with the Myc Box II domain of c-Myc and selectively alters its biological function and target gene expression. J Biol Chem. 2002;277:43175–43184. doi: 10.1074/jbc.M206066200. [DOI] [PubMed] [Google Scholar]
- 22.Egler RA, et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 23.Neumann CA, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 24.Lee W, et al. Human peroxiredoxin 1 and 2 are not duplicate proteins: The unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J Biol Chem. 2007;282:22011–22022. doi: 10.1074/jbc.M610330200. [DOI] [PubMed] [Google Scholar]
- 25.Uwayama J, et al. Tissue Prx I in the protection against Fe-NTA and the reduction of nitroxyl radicals. Biochem Biophys Res Commun. 2006;339:226–231. doi: 10.1016/j.bbrc.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 26.Iuchi Y, et al. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J. 2009;419:149–158. doi: 10.1042/BJ20081526. [DOI] [PubMed] [Google Scholar]
- 27.Li L, et al. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem Biophys Res Commun. 2007;355:715–721. doi: 10.1016/j.bbrc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Lee TH, et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, et al. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–25190. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- 30.Woo HA, et al. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Jönsson TJ, Johnson LC, Lowther WT. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature. 2008;451:98–101. doi: 10.1038/nature06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 33.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 34.McKay MM, Ritt DA, Morrison DK. Signaling dynamics of the KSR1 scaffold complex. Proc Natl Acad Sci USA. 2009;106:11022–11027. doi: 10.1073/pnas.0901590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halushka MK, Selvin E, Lu J, Macgregor AM, Cornish TC. Use of human vascular tissue microarrays for measurement of advanced glycation endproducts. J Histochem Cytochem. 2009;57:559–566. doi: 10.1369/jhc.2009.953273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.