Abstract
Trastuzumab, a monoclonal antibody targeting human epidermal growth factor receptor-2 (HER2/ErbB-2), has become the mainstay of treatment for HER2-positive breast cancer. Nevertheless, its exact mechanism of action has not been fully elucidated. Although several studies suggest that Fc receptor-expressing immune cells are involved in trastuzumab therapy, the relative contribution of lymphocyte-mediated cellular cytotoxicity and antitumor cytokines remains unknown. We report here that anti–ErbB-2 mAb therapy is dependent on the release of type I and type II IFNs but is independent of perforin or FasL. Our study thus challenges the notion that classical antibody-dependent, lymphocyte-mediated cellular cytotoxicity is important for trastuzumab. We demonstrate that anti–ErbB-2 mAb therapy of experimental tumors derived from MMTV-ErbB-2 transgenic mice triggers MyD88-dependent signaling and primes IFN-γ–producing CD8+ T cells. Adoptive cell transfer of purified T cell subsets confirmed the essential role of IFN-γ–producing CD8+ T cells. Notably, anti–ErbB-2 mAb therapy was independent of IL-1R or IL-17Ra signaling. Finally, we investigated whether immunostimulatory approaches with antibodies against programmed death-1 (PD-1) or 41BB (CD137) could be used to capitalize on the immune-mediated effects of trastuzumab. We demonstrate that anti–PD-1 or anti-CD137 mAb can significantly improve the therapeutic activity of anti–ErbB-2 mAb in immunocompetent mice.
Keywords: adaptive, innate, natural killer cell, tumor immunology
The use of trastuzumab (Herceptin), a humanized IgG1 monoclonal antibody (mAb) against human epidermal growth factor receptor-2 (HER2/ErbB-2), has substantially improved the standard of care of HER2-positive breast cancer (1–3). Accordingly, trastuzumab has become the mainstay of treatment for HER2-overexpressing breast cancer. Nevertheless, its exact mechanism of action has not been fully elucidated.
HER2 is overexpressed in 20–30% of patients with breast cancer; HER2 promotes tumor growth by activating several prosurvival pathways, including the phosphatidylinositol 3-kinase (PI-3K)-AKT pathway, the MAPK pathway, the STAT pathway, and the NF-κB pathway (1, 2). Several studies have shown that the activity of trastuzumab depends on both signaling blockade of HER2 and immune-mediated tumor destruction through activation of antibody-binding Fc receptors (FcR) (1–9). It is believed that the function of FcR+ immune cells, largely natural killer (NK) cells and monocytes, is to perform antibody-dependent cellular cytotoxicity (4–9). However, the importance of antibody-dependent cellular cytotoxicity per se and the role of other immune effector pathways in the therapeutic activity of trastuzumab have not been formally investigated.
One aspect that has been investigated is the contribution of FcR+ immune cells. The importance of FcR+ immune cells for trastuzumab therapy is supported by the fact that anti-HER2 antibodies incapable of binding FcR are ineffective against tumor xenografts (4), whereas antibodies with increased FcR binding show greater activity (9). It is further supported by the fact that FcR-deficient mice fail to respond to anti–ErbB-2 mAb therapy (10). In addition, human breast cancer biopsy samples have revealed an increase in tumor infiltrating FcR+ cells, mainly NK cells, after trastuzumab treatment (6, 7). Finally, for some patients, specific Fcr genotypes are associated with improved progression-free survival in response to trastuzumab (5). Taken together, these studies strongly suggest that FcR+ innate immune cells are instrumental to trastuzumab's activity.
Although the role of innate immune cells has been studied, the potential role of adaptive immunity has not been thoroughly investigated. In collaboration with others, we have previously shown that some chemotherapeutic drugs, such as anthracyclines, can kill tumor cells in a manner that activates the NLRP3 inflammasome in dendritic cells (DCs), thereby triggering tumor-specific adaptive immunity via IL-1β (11). Most notably, adaptive immune responses generated in response to these drugs were shown to be essential to their therapeutic activity. In the context of antibody therapy, however, it remains unclear whether tumor cell death can trigger adaptive antitumor immune responses and whether these significantly contribute to treatment activity. Using an immunocompetent murine model of ErbB-2 breast cancer, Park et al. recently demonstrated that to achieve optimal therapeutic effects, anti–ErbB-2 mAb requires CD8α+ cells, MyD88 signaling, and RAG-dependent adaptive immunity (10). Although Park et al. demonstrated a role for RAG-dependent adaptive immunity, the subset of the immune cells and the nature of the effector mechanisms that actually reduce tumor growth remains unknown. It our contention that the identification of these immune effector pathways will potentially allow the development of more effective therapies against HER2-positive breast cancer.
We here describe the function of innate and adaptive immune responses, cellular cytotoxic molecules, and antitumor cytokines in the therapeutic activity of anti–ErbB-2 mAb in mice. Our study questions whether, in fact, classical lymphocyte-mediated cellular cytotoxicity is important for trastuzumab's activity, and suggests key roles for type I and type II IFN responses. We further provide experimental evidence that immunostimulating anti–PD-1 or anti-CD137 mAbs can be used to capitalize on the immune effects of trastuzumab and to enhance its therapeutic activity.
Results
Anti–ErbB-2 mAb Therapy Requires NK, CD8αα+, and CD8αβ+ Cells.
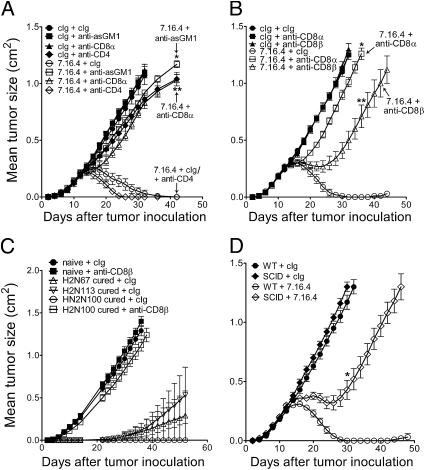
To investigate the immune effector mechanisms required for anti–ErbB-2 mAb therapy, we used the anti–ErbB-2 mAb clone 7.16.4 (12) and tumor cell lines derived from BALB/c transgenic mice expressing oncogenic rat ErbB-2 (13, 14) implanted in BALB/c mice, BALB/c-ErbB-2 transgenic mice, gene-targeted mice or mice treated with previously described depleting or neutralizing antibodies (11, 15–18). BALB/c-ErbB-2 transgenic mice develop spontaneous mammary carcinomas with a latency of ∼100 d that can be harvested, cultured and transplanted into immunocompetent syngeneic mice for analysis. In the BALB/c background, mice are tolerant to oncogenic rat ErbB-2 and adaptive tumor-specific immunity can be evaluated. We first assessed in our model the role of NK cells, CD8+ cells, and CD4+ cells (experimental design in Fig. S1A). As shown in Fig. 1A, anti–ErbB-2 mAb therapy of ErbB-2 tumors induced sustained tumor regression in syngeneic immunocompetent mice. In contrast, mice depleted of NK cells, or mice depleted of CD8α+ cells, failed to respond to treatment, whereas mice depleted of CD4+ cells showed a minor improvement in responsiveness (Fig. 1A). The role of CD8α+ cells was further confirmed in two additional tumor models derived from ErbB-2 transgenic mice (Fig. S1B). We next compared the relative importance of CD8αα+ cells—largely dendritic cells (DCs) specialized in antigen cross-presentation—and CD8αβ+ T cells. As shown in Fig. 1B, we observed that depletion of CD8β+ T cells also significantly abrogated treatment efficacy, albeit to a lesser extent than depletion of all CD8α+ cells. Consistent with a role for adaptive antitumor immunity, anti–ErbB-2 mAb therapy was initially effective at controlling tumor growth in mice depleted of CD8β+ T cells, but eventually failed to do so over time (Fig. 1B).
Fig. 1.
Anti–ErbB-2 mAb therapy requires NK, CD8α+, and CD8β+ cells. (A) H2N100 tumor cells (5 × 105 cells) were injected s.c. into WT and immunodeficient BALB/c mice, and treated with 100 μg of anti–ErbB-2 mAb (clone 7.16.4) or control Ig (clone MAC4) injected i.p. on days 12, 16, 19, and 23. Immunodeficient mice consisted of mice depleted of NK cells (anti-asialo GM1; **P = 0.0097 vs. 7.16.4 + cIg), CD8α+ cells (mAb 53.6.7; *P = 0.0095 vs. 7.16.4 + cIg), or CD4+ cells (mAb GK1.5). (B) Same as A, except that mice were depleted of CD8α+ (*P = 0.0097 vs. 7.16.4 + cIg) or CD8β+ cells (mAb 53.5.8; **P = 0.0097 vs. 7.16.4 + cIg; * vs. **P = 0.0079). (C) Same as A; mice cured of H2N100 tumors were challenged on the opposite flank with 5 × 105 H2N100, H2N67, or H2N113 cells injected s.c. between 42 and 56 d after primary injection (new day 0 on graph). Mice received either 100 μg control Ig (MAC4) or anti-CD8β+ mAb (53.5.8) i.p. on days −1, 0, 7, 14, and 21. (D) Same as A, except that SCID mice were used (*P = 0.0097 SCID + 7.16.4 vs. WT + 7.16.4). Data are mean ± SE of five mice per group. Statistical analyses were performed at time points indicated in figure using the Mann–Whitney test. Depleting or neutralizing mAbs were injected i.p. on days 11, 12, 19, and 26.
We next tested whether mice cured of their primary tumor were protected against a secondary tumor challenge. Fig. 1C shows the growth of H2N67, H2N113, and H2N100 tumors in naive mice and mice cured of H2N100 tumors following anti–ErbB-2 mAb treatment. As shown, mice that were cured of their primary tumors were protected against a distant secondary tumor in the absence of treatment. Notably, mice cured of H2N100 tumors were protected against a secondary tumor of a different origin (i.e., protected against H2N67 and H2N113 tumors). This suggested induction of cross-reactive antigenic responses against different ErbB-2 tumors. Importantly, CD8β+ T cells were fully responsible for the protection against secondary tumors (Fig. 1C), consistent with the generation of adaptive memory responses. Furthermore, anti–ErbB-2 mAb therapy was impaired in SCID mice, with tumor growth kinetics comparable to those observed in mice depleted of CD8β+ T cells (Fig. 1D and Fig. S2). Taken together, our experiments strongly suggest that innate NK cells, CD8αα+ DCs and adaptive CD8αβ+ T cells are critical to the therapeutic activity of anti–ErbB-2 mAb therapy.
Anti–ErbB-2 mAb Therapy Requires Type I and II Interferons but Not Lymphocyte-Mediated Cytotoxicity.
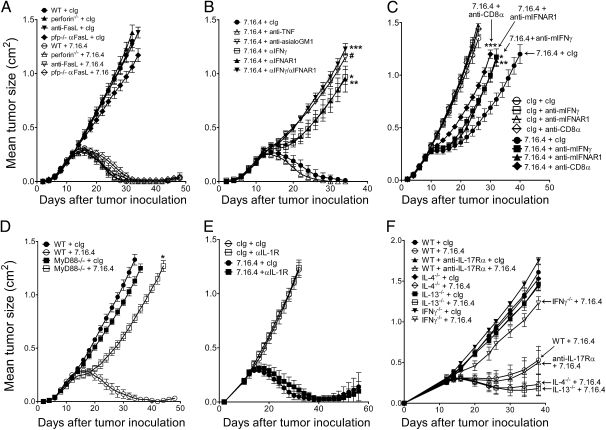
We next assessed the role of specific immune effector pathways on the activity of anti–ErbB-2 mAb therapy. Using gene-targeted mice, we first observed that the pore-forming protein perforin was totally dispensable for anti–ErbB-2 mAb therapy (Fig. 2A). This result was quite unexpected, as perforin is required for granule-mediated cellular cytotoxicity (17). We further assessed the role of FasL and TNF-α. Neutralization of FasL, including in perforin-deficient mice, did not impair anti–ErbB-2 mAb therapy (Fig. 2A). Similarly, neutralization of TNF had no effect on the activity of anti–ErbB-2 mAb (Fig. 2B). We next assessed the role of IFNs. Using neutralizing antibodies, our data demonstrated that IFNAR1, which is the receptor of type I IFNs in mice, and IFN-γ are both required for anti–ErbB-2 mAb treatment (Fig. 2B). The role of type I IFNs and IFN-γ was confirmed in another tumor model derived from ErbB-2 transgenic mice (Fig. S3). We next assessed whether type I and type II IFNs had redundant antitumor effects. As shown in Fig. 2B, combined neutralization of both IFNAR1 and IFN-γ suggested nonredundant antitumor effects.
Fig. 2.
Immune effector mechanism of anti–ErbB-2 mAb therapy. (A) H2N100 tumor cells (5 × 105 cells) were injected s.c. into WT and gene-targeted perforin-deficient BALB/c mice, and treated with 100 μg anti–ErbB-2 mAb (clone 7.16.4) or control Ig (clone MAC4) injected i.p. on days 12, 16, 19, and 23 (perforin−/− + 7.16.4 vs. WT + 7.16.4, P = 1.0). In some groups, WT and perforin-deficient mice were also injected with neutralizing anti-FasL mAb (clone MLF1; P = 1.0 vs. cIg). (B) Same as A; mice were depleted of NK cells (#P = 0.0097 vs. 7.16.4 + cIg) or treated with neutralizing mAbs to TNF (clone TN3-19.12; P = 1.0), IFNAR1 (clone MAR1-5A3; *P = 0.0095 vs. 7.16.4 + cIg), or IFN-γ (clone H22; **P = 0.0095 vs. 7.16.4 + cIg; ***P = 0.031 vs. 7.16.4 + αIFN-γ). (C) Same as A, except that H2N100 tumors were established in BALB/c-MMTV-neu transgenic mice, and mice were injected with 7.16.4 or control Ig on days 8, 10, 12, 14, 16, 18, 20, and 22 (*P = 0.0317; **P = 0.0159; ***P = 0.0079 vs. 7.16.4 + cIg). (D) Same as A except that gene-targeted, MyD88-deficient mice were used (*P = 0.0112 vs. WT + 7.16.4). (E) Same as A; mice were treated with neutralizing mAbs against IL-1R (clone JAMA147; results of one of two representative experiments are shown). (F) Same as A; WT or gene-targeted IL-4–deficient, IL-13–deficient, or IFN-γ–deficient mice were used. WT mice were treated with neutralizing mAbs against IL-17Rα (clone M751; results of one of two representative experiments are shown). Data are mean ± SE of five mice per group. Statistical analyses were performed at the time points indicated in figure using the Mann–Whitney test. Depleting or neutralizing mAbs were injected i.p. on days 11, 12, 19, and 26.
We further investigated the role of IFNAR1, IFN-γ, and CD8+ cells in transgenic BALB/c-ErbB-2 recipient mice. As shown in Fig. 2C, treatment of transplanted tumors in ErbB-2 transgenic mice also required the presence of CD8α+ cells and depended on both type I and type II IFNs. Our data thus strongly suggest that trastuzumab relies, at least in part, on the antitumor effects of IFNs.
Anti–ErbB-2 mAb Therapy Requires MyD88 Signaling but Is Independent of IL-1R or IL-17Ra.
We then assessed the role of Toll-like receptors (TLR), which regulate secretion of type I IFNs (19). Using gene-targeted mice deficient in MyD88, our data demonstrated anti–ErbB-2 mAb therapy was dependent on TLR signaling via MyD88 (Fig. 2D). We next assessed the mechanism by which anti–ErbB-2 mAb generates adaptive immunity. Ghiringhelli et al. (11) demonstrated that tumor cell death following anthracycline therapy activated the NLRP3 inflammasome, which primed IFN-γ–mediated adaptive immunity via IL-1β. We thus investigated the role of IL-1R signaling in anti–ErbB-2 mAb therapy. In contrast to chemotherapy, anti–ErbB-2 mAb therapy was independent of IL-1R (Fig. 2E). Anti–ErbB-2 mAb therapy was also not affected by IL-17Rα neutralization, whereas IL-4 or IL-13 neutralization showed a minor improvement in responsiveness (Fig. 2F). We finally assessed the role of plasmacytoid DCs (pDCs) and monocytes/macrophages. Our data suggest that CD11b+ cells and macrophages, but not pDC, are important for anti-ErbB2 mAb therapy (Fig. S4). Taken together, our studies are consistent with a model whereby trastuzumab activates NK cells; stimulates MyD88-dependent TLR, thereby triggering the release of type I IFNs and primes adaptive CD8+ T cells in an IL-1R–independent manner, which then mediate tumor cell destruction via IFN-γ, independently of perforin, FasL, or TNF.
Anti–ErbB-2 mAb Therapy Requires IFN-γ–Producing CD8+ T cells.
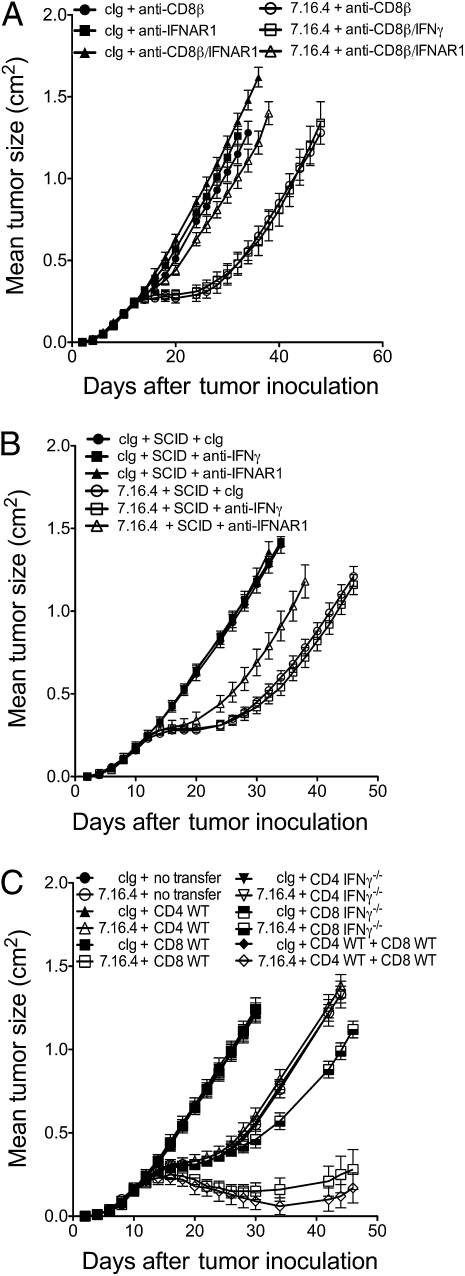
To test whether CD8+ T cells were the source of IFN-γ, we initially compared the activity of anti–ErbB-2 mAb in mice depleted of CD8β+ cells with or without additional neutralization of IFN-γ or IFNAR1. Our hypothesis was that if CD8+ T cells were the source of IFN-γ, then neutralization of IFN-γ would have no further effect in CD8β+ cell-depleted mice. As shown in Fig. 3A, neutralization of IFN-γ did not further reduce treatment efficacy in mice depleted of CD8β+ cells, whereas neutralization of IFNAR1 did further reduce treatment efficacy. Consistently, neutralization of IFN-γ did not further reduce treatment efficacy in SCID mice (Fig. 3B). These experiments suggested that IFN-γ indeed came from CD8+ T cells. Consistently, flow cytometry analyses revealed that anti–ErbB-2 mAb increased the frequency of tumor-infiltrating IFN-γ–producing CD8+ T cells (Fig. S5). To clearly establish the role of IFN-γ production by CD8+ T cells, we performed adoptive cell transfer (ACT) of purified CD8+ T cells and/or CD4+ T cells from WT or IFN-γ–deficient mice into SCID mice. As shown in Fig. 3C, ACT experiments demonstrated that CD8+ T cells producing IFN-γ are required for the therapeutic activity of anti–ErbB-2 mAb. To investigate the effect of IFN-γ on ErbB-2+ tumor cells, we treated H2N tumor cell lines with increasing doses of recombinant (rec) IFN-γ in vitro and measured proliferation after 4 d. H2N100 tumor cells were significantly inhibited by 1 U/mL rec IFN-γ, whereas H2N67 and H2N113 were inhibited by 10 U/mL and 1,000 U/mL rec IFN-γ, respectively (Fig. S6 A–C). Notably, rec IFN-γ significantly enhanced the antiproliferative effect of anti–ErbB-2 mAb in vitro (Fig. S6 A–C). Rec IFN-γ (5 and 200 U/mL) also enhanced MHC class I and PD-L1 expression on the tumor cells (Fig. S6D).
Fig. 3.
IFN-γ–producing CD8+ T cells are required for anti–ErbB-2 mAb therapy. (A) H2N113 tumor cells (5 × 105 cells) were injected s.c. into WT BALB/c mice and treated with 100 μg anti–ErbB-2 mAb (clone 7.16.4) or control Ig (clone MAC4) injected i.p. on days 12, 16, 19, and 23. Mice were injected i.p. with control Ig or anti-CD8β mAb (53.5.8) and/or anti–IFN-γ mAb (H22) or IFNAR1 mAb (MAR1-5A3). (B) Same as A, except that tumor-bearing SCID mice were treated. (C) SCID mice were injected with 5 × 105 H2N113 tumor cells on day 0, then received on day 7 one of the following: (i) no transfer, (ii) 106 CD4+ WT cells, (iii) 106 CD8+ WT cells, (iv) 106 CD4+ IFN-γ-deficient cells, (v) 106 CD8+ IFN-γ-deficient cells, or (vi) 106 CD4+ WT cells + 106 CD8+ WT cells. Data are mean ± SE of five mice per group.
Our murine model thus suggested that IFN responses are essential to the therapeutic activity of trastuzumab. Hence, we hypothesized that IFN-γ levels, but not perforin, IL-1β, or IL-17 levels, would predict clinical responses to trastuzumab in HER2 overexpressing human tumors. To test our hypothesis, mRNA expression levels of IFN-γ, perforin (PRF1), and IL-1β of 45 HER2+ tumor biopsy samples taken before treatment with trastuzumab and chemotherapy (20) were correlated with pathological complete response (pCR) after 4 mo of treatment. The area under the curve (AUC), where the null hypothesis is that the true AUC = 0.5, was used to assess correlation with pCR. This method avoids having to determine an arbitrary cutoff of gene expression levels. Our analysis revealed that mRNA expression levels of IFN-γ positively and significantly correlated with pCR for HER2+ breast cancer patients (Fig. S7). In contrast, no relationship with pCR was observed for perforin, IL-1β, and IL-17. This supports our murine studies and strongly suggests that IFN-γ is important for the therapeutic activity of trastuzumab in some women with HER2-overexpressing breast cancer.
Anti–ErbB-2 mAb Therapy Synergizes with Anti–PD-1 mAb or Anti-CD137 mAb.
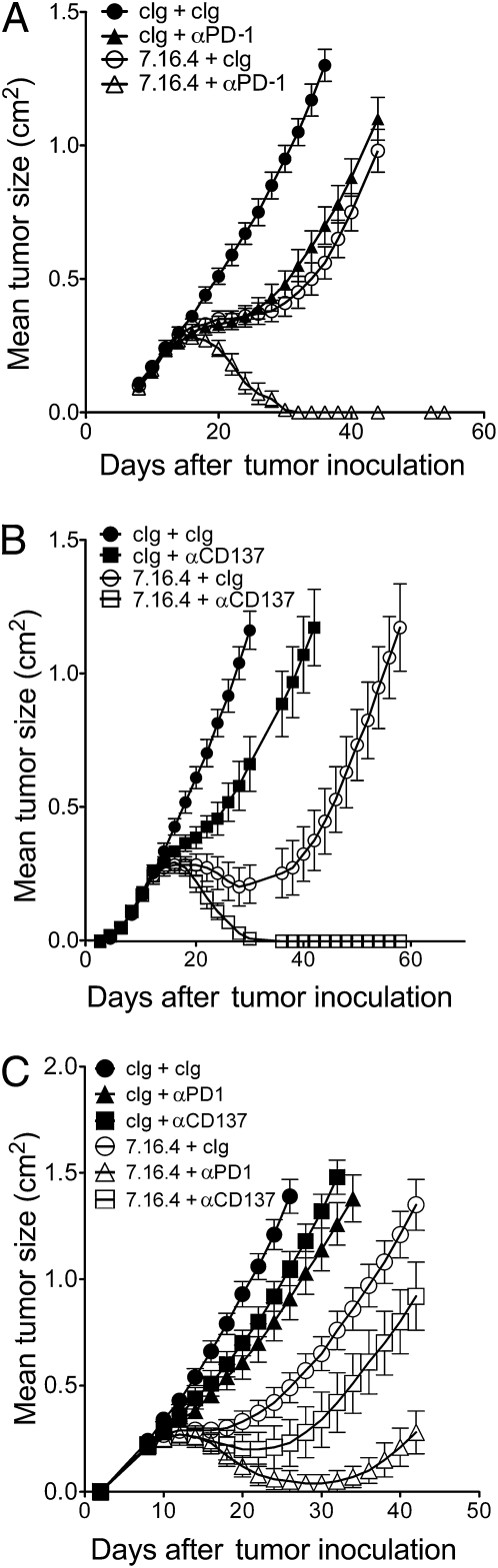
Our study raises the possibility that therapeutic strategies could be used to capitalize on the immune-mediated responses of trastuzumab. To investigate this hypothesis, we used our murine model to test whether administration of immunostimulating mAbs could improve anti–ErbB-2 mAb treatment. We tested the therapeutic activity of our anti–ErbB-2 mAb in combination with agonistic anti-CD137 mAb (clone 3H3) or blocking anti–PD-1 mAb (clone RMP1-14). Anti-CD137 and anti–PD-1 mAbs are potent activators of antitumor immune response that are currently under clinical development for cancer treatment (21, 22). We titered down the dose of anti–ErbB-2 mAb to assess the therapeutic activity of combination regimens. As shown in Fig. 4, the addition of anti-CD137 mAb (Fig. 4A) or anti–PD-1 mAb (Fig. 4B) significantly improved the therapeutic activity of anti–ErbB-2 mAb. Our data further demonstrated the enhanced efficacy of anti–ErbB-2 with anti-CD137 mAb or anti–PD-1 mAb in BALB/c-ErbB-2 transgenic mice (Fig. 4C).
Fig. 4.
Anti–PD-1 and anti-CD137 mAbs improve therapeutic activity of anti–ErbB-2 mAb. (A) H2N113 tumor cells (5 × 105 cells) were injected s.c. into BALB/c mice and treated with 50 μg anti–ErbB-2 mAb (clone 7.16.4) or control Ig and/or 100 μg anti-PD1 mAb (clone RMP1-14) injected i.p. on days 12, 16, 19, and 23 (data are mean ± SE of of one of two representative experiments with five mice per group). (B) Same as A, except that mice were treated with 50 μg anti–ErbB-2 mAb (clone 7.16.4) or control Ig and/or 100 μg of anti-CD137 mAb (clone 3H3). Data are mean ± SE of five mice per group. At day 42: 7.16.4 + cIg vs. 7.16.4 + anti–PD-1, P = 0.0097; 7.16.4 + cIg vs. 7.16.4 + anti-CD137, P = 0.0097; anti–PD-1 vs. anti–PD-1 + 7.16.4, P = 0.0097; anti-CD137 vs. anti-CD137 + 7.16.4, P = 0.0097 (using Mann–Whitney test). P = 0.0003 with Kruskal–Wallis test in all groups. (C) H2N113 tumor cells (5 × 105 cells) were established in BALB/c-MMTV-neu transgenic mice and mice were injected on days 8, 10, 12, 14, 16, 18, 20, and 22 with 100 μg anti–ErbB-2 mAb (clone 7.16.4) or control Ig and/or 100 μg anti-CD137 mAb or anti–PD-1 mAb on days 8, 12, 16, and 20. Data are mean ± SE of five mice per group.
Discussion
Although correlative studies have suggested that FcR+ immune cells are involved in the therapeutic activity of trastuzumab, the contribution of downstream immune effectors pathways, such as lymphocyte-mediated cellular cytotoxicity and the production of regulatory cytokines, has not been addressed. The data presented here question whether, in fact, classical lymphocyte-mediated cellular cytotoxicity is important for trastuzumab's activity, rather suggesting a key role for type I IFNs and IFN-γ–producing CD8+ T cells. We do not rule out, however, that antibody-dependent cellular cytotoxicity might occur independently of the classical perforin and FasL pathways. Indeed, there is substantial evidence that human FcR+ cells can perform antibody-dependent cellular cytotoxicity of trastuzumab-bound tumor cells in vitro (7, 8). FcR+ cells might further promote an inflammatory cascade culminating in the generation of type I IFNs and IFN-γ–producing CD8+ T cells. Our study supports recent work describing that anti–ErbB-2 mAb treatment can induce adaptive antitumor immune responses in murine models and in human patients (7, 10, 23, 24). Our study significantly advances the work by Park et al. (10) by revealing a previously unrecognized role for type I and II IFNs. Furthermore, we clearly define a distinct role for both CD8α and CD8β+ cells and, specifically, the critical function of CD8+ T cells producing IFN-γ. We also have shown that cytotoxic pathways do not appear to be important in the mechanism of action of anti--ErbB-2. The combinatorial antitumor effects of anti--ErbB-2 and anti-PD1 or anti-CD137 are important proof-of-principle experiments that the antitumor T-cell immunity provoked by anti--ErbB-2 can be capitalized upon, providing a new paradigm of potential combination treatment for women receiving trastuzumab therapy.
We showed that anti–ErbB-2 mAb therapy was not affected by deficiencies in perforin, FasL, TNF-α, IL-17Rα, and IL-1R, but was to a great extent dependent on IFNAR1 and IFN-γ. Our study findings also suggest that IL-4 and IL-13 can limit the activity of anti–ErbB-2 mAb, perhaps by restricting the generation of Th1 IFN-γ responses. We demonstrated that CD8+ T cells were the main source of IFN-γ. Although we have not identified the cellular source of type I IFNs, we demonstrated a requirement for MyD88-dependent TLR signaling, consistent with the production of type I IFNs. Various cell types, including pDCs and monocytes/macrophages, may be activated by TLRs to produce type I IFNs. Our data suggest a role for the latter, not the former, in the activity of anti–ErbB-2 mAb (although pDC cannot be definitely ruled out due to the absence of a specific pDC-depleting mAb). Our data thus support a model whereby trastuzumab activates MyD88-dependent TLR signaling, stimulates the release of type I IFNs, and then primes adaptive IFN-γ–producing CD8+ T cells. Based on CD8α+ and CD8β+ cell depletion studies, we hypothesize that CD8αα+ cross-presenting DCs might also play a critical role in this immune cascade. Further studies should clarify the mechanism of antigen presentation and the role of DC subsets. Of particular interest, zum Büschenfelde et al. (23) have previously reported that trastuzumab can enhance HER2 antigen processing and presentation onto MHC class I molecules. The ability of trastuzumab to enhance HER2 peptide presentation was suggested to take place after saturation of HER2 molecules by trastuzumab and following internalization and proteosomal degradation of HER2 (25). Consistent with this notion, a recent clinical trial revealed that women who had been treated with trastuzumab had superior anti-HER2 CD8+ T-cell response after vaccination against HER2 (26). This suggests that trastuzumab is able to generate long-lasting immunological responses.
It is becoming more evident that certain forms of cancer cell death, such as triggered by specific classes of chemotherapeutic drugs and, as described here, targeted mAbs, can be potently immunogenic and can induce adaptive antitumor immunity. Recently, Ghiringhelli et al. (11) demonstrated that the antitumor immune responses induced by certain chemotherapeutic drugs such as oxaliplatin were essential to the therapeutic activity of these drugs in mice. Importantly, Ghiringhelli et al. demonstrated that IL-1β production, via activation of NLRP3 inflammasome, is a critical event in the induction of IFN-γ–producing CD8+ antitumor T cells following chemotherapy. Because we observed that anti–ErbB-2 mAb therapy also triggered IFN-γ–producing CD8+ antitumor T cells, we hypothesized that the two forms of immunogenic tumor cell death (i.e., chemotherapy-induced and mAb-induced) would similarly depend on IL-1 signaling. In contrast, we observed that anti–ErbB-2 mAb therapy was independent of IL-1R. This suggests that the mechanism of immunogenic tumor cell death leading to the generation of adaptive antitumor immune responses is different for mAbs and chemotherapy.
Our analysis of human breast cancer biopsy samples revealed that tumor IFN-γ levels (as measured by microarray) before treatment significantly associated with pCR. Several clinical trials have established that pCR (i.e., the absence of residual tumor cells in the breast and lymph node as evaluated by pathologists) is an excellent surrogate for disease-free survival and overall survival in breast cancer (27). Our analysis suggests that tumor IFN-γ levels may act as a biomarker to identify those patients who will most clearly respond to trastuzumab (28). Nevertheless, prospective studies assessing tumor IFN-γ levels and its correlation with clinical outcome in larger cohorts of patients are needed to clarify whether IFN-γ is an effective predictive biomarker of trastuzumab activity.
We observed that recombinant IFN-γ directly inhibited the proliferation of our ErbB-2+ tumor cell lines in vitro. Moreover, recombinant IFN-γ significantly enhanced the antiproliferative effect of anti–ErbB-2 mAb. Together with the fact that IFN-γ significantly enhanced MHC class I expression on tumor cells, this may explain the importance of IFN-γ for trastuzumab therapy. Interestingly, tumor cells exposed to IFN-γ also acquired expression of the immune-inhibitory ligand PD-L1. Our data raise the possibility that blocking PD-L1/PD-1 interactions could be used to capitalize on the immune-mediated responses of trastuzumab. PD-1 is an inhibitory coreceptor expressed on activated and exhausted T cells. Administration of a blocking anti–PD-1 mAb can significantly enhance adaptive antitumor immune responses (29). We here demonstrated that anti–PD-1 mAb can synergize with anti–ErbB-2 mAb therapy. Human anti–PD-1 mAbs have been developed and are currently being tested in clinical trials (30). Our study also suggests that an agonistic anti-CD137 mAb could be combined with trastuzumab. CD137 is an important mediator of lymphocyte survival, particularly in CD8+ T cells, which express CD137 upon activation. The robust effects of anti-CD137 mAb therapy are dependent on enhanced proliferation and function of tumor-specific CD8+ T cells (31). However, anti-CD137 mAb can also enhance the activation and function of NK cells and DCs (32), and may thus potentiate anti–ErbB-2 mAb therapy by acting on both FcR+ cells and adaptive immune cells. Anti-CD137 mAbs are currently being used in clinical trials (33). An alternative strategy to the use of immunostimulatory mAbs might be to combine trastuzumab with HER2-targeted vaccination (34). Recently it has been reported that trastuzumab can potentiate HER2 E75 peptide vaccination in human cancer patients (26). The potential of combining HER2 peptide vaccination with trastuzumab is currently being evaluated in independent clinical trials (ClinicalTrials.gov identifiers NCT00791037, NCT00343109, and NCT00524277).
In conclusion, our study demonstrated that anti–ErbB-2 mAb therapy is dependent on type I and II IFN responses but not on lymphocyte-mediated cytotoxicity. Our findings suggest that immunostimulatory approaches such as anti-CD137 mAb and anti–PD-1 mAb therapy could be used to capitalize on the immune-mediated effects of trastuzumab.
Materials and Methods
Animals and Cell Lines.
All mice were bred and maintained at the Peter MacCallum Cancer Centre. H2N100, H2N113, and H2N67 tumor cell lines were generated as previously described (13). Briefly, lobular breast carcinomas were excised from 20- to 23-wk-old female BALB/c MMTV-ErbB-2/neu transgenic mice and dissociated with DNaseI and collagenase. ErbB2/neu expression was confirmed by flow cytometry using purified anti-rat ErbB-2 mAb (10 μg/mL clone 7.16.4, mouse IgG2a) and phycoerythrin-conjugated antimouse IgF(ab)2 fragment (Chemicon).
Antibodies.
The anti-rat ErbB-2mAb (clone 7.16.4) hybridoma was generously provided by Mark I. Greene, University of Pennsylvania, and anti-IFNAR1 (MAR1-5A3) and anti–IFN-γ (H22) were generously provided by Robert D. Schreiber, Washington University School of Medicine. Rabbit anti-asialoGM1 antibody was purchased from Wako Chemicals, resuspended in PBS, and injected according to the manufacturer's instructions at the indicated time points. All monoclonal antibodies were produced in-house as previously described (15–18)
In Vivo Treatments.
Tumor cells (5 × 105 cells) were injected s.c. into WT BALB/c, gene-targeted BALB/c, SCID BALB/c, mAb-treated BALB/c, or transgenic MMTV-neu BALB/c mice. The following mAbs were injected at 100 μg per dose at the indicated time points: anti-asialoGM1, anti-CD8α, anti-CD8β. Previously described neutralizing mAbs against IL-1R (11), IFN-γ (15), IFNAR (16), FasL (17), and TNF (18) were injected at 250 μg per dose at the indicated time points. All antibodies were injected i.p.. For adoptive cell transfers, CD4+ and CD8+ cells were isolated from the spleens of WT or IFN-γ KO mice by negative selection using AutoMACS isolation (Miltenyi) according to the manufacturer's instructions. Cells were at least 95% pure as determined by flow cytometry.
Supplementary Material
Acknowledgments
We thank Bianca von Scheidt, Nicole McLaughlin, and Michelle Stirling for technical support. We thank L. Putzai and W. F. Symmans for assistance with the gene expression data. We also acknowledge funding support from the National Health and Medical Research Council of Australia, the Susan Komen Breast Cancer Foundation, and the Victorian Breast Cancer Research Consortium.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016569108/-/DCSupplemental.
References
- 1.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 2.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 3.Untch M, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: Results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 4.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 5.Musolino A, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 6.Varchetta S, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991–11999. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- 7.Arnould L, et al. Trastuzumab-based treatment of HER2-positive breast cancer: An antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gennari R, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 9.Junttila TT, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70:4481–4489. doi: 10.1158/0008-5472.CAN-09-3704. [DOI] [PubMed] [Google Scholar]
- 10.Park S, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, et al. Shared antigenic epitopes and pathobiological functions of anti-p185(her2/neu) monoclonal antibodies. Exp Mol Pathol. 1999;67:15–25. doi: 10.1006/exmp.1999.2266. [DOI] [PubMed] [Google Scholar]
- 13.Stagg J, et al. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci USA. 2008;105:16254–16259. doi: 10.1073/pnas.0806849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Carlo E, et al. Analysis of mammary carcinoma onset and progression in HER-2/neu oncogene transgenic mice reveals a lobular origin. Lab Invest. 1999;79:1261–1269. [PubMed] [Google Scholar]
- 15.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 16.Swann JB, et al. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 2007;178:7540–7549. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 18.van Dommelen SL, et al. Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity. 2006;25:835–848. doi: 10.1016/j.immuni.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Swann JB, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteva FJ, et al. CD40 signaling predicts response to preoperative trastuzumab and concomitant paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide in HER-2-overexpressing breast cancer. Breast Cancer Res. 2007;9:R87. doi: 10.1186/bcr1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westwood JA, et al. Toll-like receptor triggering and T-cell costimulation induce potent antitumor immunity in mice. Clin Cancer Res. 2009;15:7624–7633. doi: 10.1158/1078-0432.CCR-09-2201. [DOI] [PubMed] [Google Scholar]
- 23.zum Büschenfelde CM, Hermann C, Schmidt B, Peschel C, Bernhard H. Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Res. 2002;62:2244–2247. [PubMed] [Google Scholar]
- 24.Taylor C, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol. 2006;13:1085–1098. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 26.Benavides LC, et al. The impact of HER2/neu expression level on response to the E75 vaccine: From U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2009;15:2895–2904. doi: 10.1158/1078-0432.CCR-08-1126. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann M, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: Review and recommendations. J Clin Oncol. 2003;21:2600–2608. doi: 10.1200/JCO.2003.01.136. [DOI] [PubMed] [Google Scholar]
- 28.Burstein HJ, Winer EP. Refining therapy for human epidermal growth factor receptor 2-positive breast cancer: T stands for trastuzumab, tumor size, and treatment strategy. J Clin Oncol. 2009;27:5671–5673. doi: 10.1200/JCO.2009.24.2222. [DOI] [PubMed] [Google Scholar]
- 29.Li B, et al. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor–secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 30.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 31.Uno T, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Okumura K, Smyth MJ. Combination antibody-based cancer immunotherapy. Cancer Sci. 2007;98:1297–1302. doi: 10.1111/j.1349-7006.2007.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sznol M. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients with advanced cancer. ASCO Annual Meeting Proceedings. J Clin Oncol. 2008;26(15S Suppl):3007. [Google Scholar]
- 34.Kim PS, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118:1700–1711. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.