Abstract
Schistosomiasis is an infectious disease caused by parasites of the phylum platyhelminthe. Here, we describe the identification and characterization of a Bcl-2–regulated apoptosis pathway in Schistosoma japonicum and S. mansoni. Genomic, biochemical, and cell-based mechanistic studies provide evidence for a tripartite pathway, similar to that in humans including BH3-only proteins that are inhibited by prosurvival Bcl-2–like molecules, and Bax/Bak-like proteins that facilitate mitochondrial outer-membrane permeabilization. Because Bcl-2 proteins have been successfully targeted with “BH3 mimetic” drugs, particularly in the treatment of cancer, we investigated whether schistosome apoptosis pathways could provide targets for future antischistosomal drug discovery efforts. Accordingly, we showed that a schistosome prosurvival protein, sjA, binds ABT-737, a well-characterized BH3 mimetic. A crystal structure of sjA bound to a BH3 peptide provides direct evidence for the feasibility of developing BH3 mimetics to target Bcl-2 prosurvival proteins in schistosomes, suggesting an alternative application for this class of drugs beyond cancer treatment.
Distinct mechanisms have been described for the intrinsic cell death pathways in organisms from different phyla, although all culminate in the activation of caspases, the proteolytic enzymes that destroy vital intracellular substrates. In vertebrates, the Bcl-2 protein family regulates apoptosis through a complex interplay between opposing prosurvival and proapoptotic factions (1). The prosurvival group, including Bcl-2 itself, Bcl-w, Bcl-xL, Mcl-1, and A1, protects cells against various cytotoxic stimuli by binding to proapoptotic family members. The proapoptotic faction comprises two subgroups, the Bax/Bak proteins, which are essential mediators of apoptosis, and the BH3-only proteins (of which there are eight in humans) that trigger the apoptotic cascade. Members of the Bcl-2 protein family contain at least one of four conserved sequence motifs known as Bcl-2 homology domains (BH1–BH4). Interactions between the different factions of the Bcl-2 family are mediated by the BH3 domains of the proapoptotic proteins, which engage a hydrophobic groove on the surface of the prosurvival molecules (2–5).
The nematode Bcl-2 pathway is significantly less complex because there are no Bax/Bak orthologs and only one prosurvival protein (and one caspase with its specific adaptor) (6–8). In insects, a prosurvival protein (Buffy) and a Bax/Bak ortholog (Debcl/dBok) have been described, although the control of the pathway is dominated by proteins of the inhibitor of apoptosis (IAP) class that function by inhibiting caspases (9–11). More recently, Bcl-2 proteins in the fresh water polyp Hydra, from the phylum of cnidaria, have also been described (12, 13). Cnidaria diverged from the metazoan lineage before the appearance of bilaterians, although they appear to have a more complex cell death machinery than either nematodes or insects as putative Bcl-2 prosurvival as well as putative proapoptotic Bax/Bak and BH3-only proteins were identified. However, further characterization of these proteins is required to fully establish their function(s). Multi–BH-domain Bcl-2 proteins have also been described in the most ancient metazoans, sponges (phylum Porifera) (14–16), but their functions are only poorly characterized.
Recently, the genomes of two species of Schistosoma, from the phylum platyhelminthe, were sequenced (17–19). Platyhelminthes, like nematodes, are bilateralian protostomes although of the superphylum lophotrochozoa (sometimes classified platyzoa) rather than ecdysozoa, which also includes insects. Schistosomes are the parasitic flatworms that cause schistosomiasis, one of the most serious and prevalent diseases in tropical and subtropical regions, ranking with malaria and tuberculosis as a major source of human morbidity (20). Widespread use of a single drug, praziquantel, to treat the disease has led to growing concerns about the development of drug-resistant parasites and, hence, there has been considerable urgency for the identification of new drug targets within schistosomes. The Bcl-2–regulated apoptotic pathway is a therapeutic target of interest in the treatment of cancer. Recently, highly potent small organic compounds (BH3 mimetics) capable of disrupting protein:protein interactions within the pathway to trigger apoptosis in tumor cells have entered phase I/II clinical trials (21–23). The availability of these compounds raised the possibility that the Bcl-2 pathway in infectious pathogens, such as schistosomes, could provide unique targets for this class of drugs.
Here, we describe the identification of all of the necessary components of an intrinsic cell death pathway in schistosomes. Characterization of the Bcl-2 family members demonstrated the existence of a tripartite set of regulators similar to that found in humans, providing further insight into the evolution of the cell death machinery. Moreover, we show that one of the schistosome prosurvival Bcl-2 family members can bind a small molecule BH3 mimetic, opening up a potential avenue for the treatment of schistosomiasis.
Results
Identification of Bcl-2 Relatives in Schistosomes.
By mining the annotated schistosome genome databases (17–19) using full-length human Bcl-2 family protein sequences as well as individual Bcl-2-homology (BH) domains as search parameters, we identified proteins containing all BH domains (BH1–BH4) in S. japonicum (e.g., “sjA” and “sjB”) and their homologs (e.g., “smA” and “smB”) in S. mansoni (Fig. 1A). Proteins containing solely the BH3 domain (“BH3-only” proteins), as well as downstream components of the Bcl-2–regulated apoptotic pathway, including homologs of the caspase activator APAF-1 and caspases themselves, were also identified (Fig. 1A and Fig. S1). The presence of these genes in the schistosome genomes suggested the existence of a previously unrecognized Bcl-2–regulated apoptotic pathway.
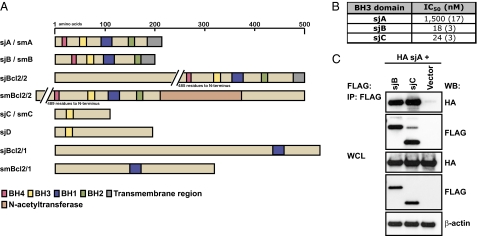
Fig. 1.
Identification of Bcl-2–related proteins in schistosomes. (A) Domain architecture of Bcl-2 family proteins identified in S. mansoni (sm) or S. japonicum (sj). (B) Binding affinity of recombinant sjA for BH3 peptides corresponding to BH3 domains of schistosome proteins. Values are IC50 in nanomolars (SD, n = 2–3). (C) Coimmunoprecipitation of sjA with sjB and sjC. Both full-length sjB and sjC interacted with full-length sjA. Whole-cell lysates (WCL) were also probed to monitor expression levels of each protein.
Biochemical Analysis of Schistosome Bcl-2 Family Proteins.
To understand how cell death might be regulated in schistosomes, we focused on three proteins in S. japonicum: sjA, sjB, and sjC, which are closely related in their domain architecture to human Bcl-2 family members. The presence of multiple BH domains in sjA and sjB suggested that these might be prosurvival Bcl-2–like and/or Bax/Bak–like proapoptotic proteins. Phylogenetic analysis using Bcl-2 proteins from diverse organisms clustered sjA with prosurvival Bcl-2 proteins, whereas sjB was more similar to Bax/Bak-like molecules (Fig. S2). The single BH3 domain in sjC clearly indicated it might be a proapoptotic BH3-only protein. Other family members identified (sjBcl2/2, smBcl2/2) contained multiple BH domains, but also possessed N- and C-terminal elaborations that we thought could complicate an initial analysis of how the pathway is regulated.
Despite attempts with various constructs, we were only able to produce sjA recombinantly for detailed biochemical analysis. Interactions between prosurvival and proapoptotic Bcl-2 proteins are mediated by the BH3 domains within proapoptotic molecules (1). In binding assays, sjA bound peptides corresponding to the BH3 domains from sjB and sjC with high affinity, but weakly to the BH3 sequence of sjA itself (Fig. 1B). In coimmunoprecipitation experiments in which full-length HA-tagged sjA and FLAG-tagged sjB or sjC were coexpressed in HEK293T cells, sjA engaged both sjB and sjC (Fig. 1C). Based on the behavior of mammalian prosurvival proteins in similar assays, and consistent with our phylogenetic analyses, these results suggested that sjA is a prosurvival Bcl-2–like protein because it can engage the BH3 motif of both a putative BH3-only protein (sjC) and a putative multi-BH domain proapoptotic Bax/Bak-like protein (sjB) from schistosomes (1, 24). The binding behavior of sjB together with its multi-BH domain architecture suggested it was a Bax/Bak-like protein, again consistent with the phylogenetic analysis.
Cellular Activity of Proapoptotic Schistosome Bcl-2 Family Members.
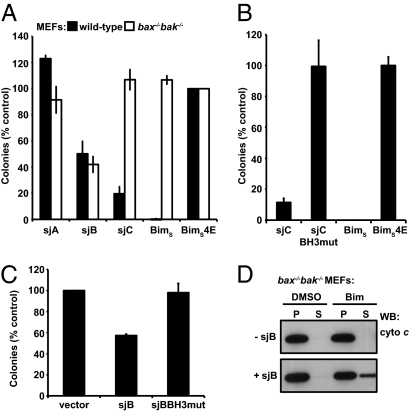
To determine the function of each protein, we examined the effect of their enforced expression in mammalian cells. If sjC is a BH3-only protein, as its sequence and binding activity suggested, it might be expected to induce apoptosis in cells expressing Bax and Bak but not those in which the corresponding genes are deleted (25). Indeed, sjC, like the mammalian BH3-only protein Bim, is a potent killer of wild-type MEFs but not bax−/−bak−/− cells (Fig. 2A). Mutations of conserved BH3 domain residues in sjC abrogated its cell-killing activity (Fig. 2B), indicating that this is the critical functional domain, as in mammalian BH3-only proteins (1, 24).
Fig. 2.
Functional activity of schistosome proapoptotic Bcl-2 proteins in cells. (A) Expression of sjB suppressed colony formation in both wild-type and bax−/−bak−/− MEFs, whereas sjC potently suppressed colony formation only in wild-type but not bax−/−bak−/− MEFs. Expression of sjA had no significant effect. (B) Mutations of conserved residues (L28A and D33A) in the BH3 domain of sjC abrogated its cell killing activity in wild-type MEFs. (C) Mutation of the analogous residues in the sjB BH3 domain (L68A and N73A) also abrogated its killing activity in bax−/−bak−/− MEFs. (D) Permeabilized cells were treated with either DMSO control or BimBH3 peptide and the localization of cytochrome c in the mitochondria and cytosol, respectively, monitored by Western blotting. Only cells expressing sjB released cytochrome c from the pellet (P; containing mitochondria) to the soluble (S; containing cytosol) fraction after treatment with the BimBH3 peptide.
In contrast, significant suppression of colony formation in both wild-type and bax−/−bak−/− MEFs was observed after enforced expression of sjB (Fig. 2A), as occurs when Bax or Bak is overexpressed in such cells, providing evidence that sjB is a Bax/Bax-like protein. This activity of sjB also appeared to be mediated by its BH3 domain as mutations of conserved residues within this region abrogated its cell-killing activity (Fig. 2C). Moreover, reconstitution of bax−/−bak−/− MEFs with sjB enabled the release of cytochrome c from mitochondria upon addition of a Bim BH3 peptide to permeabilized cells (Fig. 2D and Fig. S3). Because cytochrome c release is a hallmark of the activation of the Bcl-2–regulated apoptotic pathway, particularly in mammals, these data further suggest that sjB may function like a Bax/Bak-like protein.
Reconstitution of the Schistosome Bcl-2–Regulated Apoptotic Pathway.
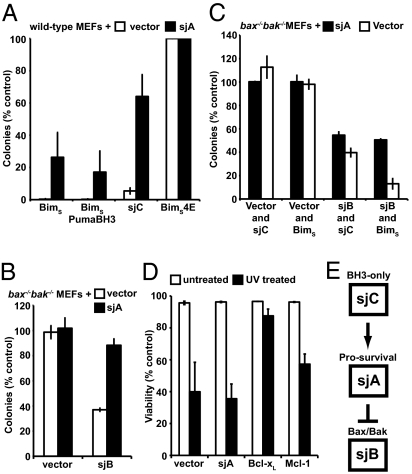
Enforced expression of sjA alone had no discernable effect in any cell type tested (Fig. 2A), therefore we could not conclusively establish its prosurvival function suggested by its binding behavior. However, stable expression of sjA in wild-type MEFs significantly inhibited sjC-mediated cell death (Fig. 3A), demonstrating its prosurvival activity. Some rescue of BimS and BimSPumaBH3-mediated killing was also observed (Fig. 3A). Moreover, suppression of colony formation by sjB expression in bax−/−bak−/− MEFs was inhibited by sjA (Fig. 3B), whereas coexpression of BimS together with sjB enhanced the suppression of colony formation, as did coexpression of sjC with sjB, albeit to a lesser extent (Fig. 3C). Unlike Bcl-xL or Mcl-1, sjA was unable to inhibit apoptosis in response to experimentally applied cytotoxic stressors, such as UV irradiation (Fig. 3D). Most likely this outcome was due to the relatively inefficient binding of sjA to the mammalian BH3-only proteins (e.g., Noxa) (see below) that are the critical initiators for these apoptotic stimuli (26). Collectively, these results reveal the existence of a tripartite Bcl-2–regulated apoptotic pathway in schistosomes similar to that observed for mammals whereby a prosurvival protein, sjA, promotes cell survival by engaging both Bax/Bak-like (sjB) and/or BH3-only proteins (sjC) via their BH3 domains (Fig. 3E).
Fig. 3.
Functional activity of schistosome antiapoptotic Bcl-2 proteins in cells. (A) Wild-type MEFs stably expressing sjA were significantly more resistant to coexpression of sjC and to a lesser extent BimS or a BimSPumaBH3 chimera compared with cells transfected with a control vector. (B) bax−/−bak−/− MEFs stably expressing sjA were more resistant to coexpression of sjB compared with cells transfected with a control vector. (C) Coexpression of sjB and BimS or sjC enhanced suppression of colony formation beyond that seen after expression of sjB alone in bax−/−bak−/− MEFs. (D) Expression of Bcl-xL or Mcl-1 in wild-type MEFs inhibited cell death in response to UV irradiation, whereas enforced expression of sjA had no significant impact. (E) The schistosome Bcl-2 regulated apoptotic pathway appears similar to that in humans where prosurvival proteins bind and inhibit both BH3-only and Bax/Bak-like proteins.
Schistosome Prosurvival Protein, sjA, Binds the BH3-Mimetic ABT-737.
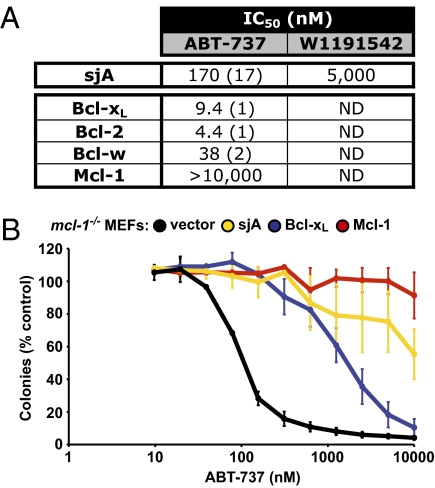
Our discovery of a Bcl-2–regulated apoptotic pathway in schistosomes suggested that drugs that mimic BH3-only proteins (BH3 mimetics) (21–23) might be useful for killing schistosomes by triggering their intrinsic apoptotic pathway. Such BH3 mimetics have recently attracted significant attention as potential anticancer therapeutics (21), but have not been considered as anti-infectives. Because BH3 mimetics function by engaging prosurvival proteins, we examined the ability of ABT-737 (the best characterized BH3 mimetic to date) to bind to sjA. Indeed, ABT-737 binds sjA with moderate affinity (IC50 170 nM), whereas a closely related analog, W1191542 (27), binds significantly weaker (IC50 5 μM), suggesting a highly specific interaction (Fig. 4A).
Fig. 4.
sjA binds to ABT-737. (A) Binding affinity of sjA for ABT-737 or the structurally related compound W1191542 (27). Values represent IC50 in nanomolars (SD, n = 3). ND, not determined. (B) Overexpression of sjA in mcl-1−/− MEFs inhibited cell death mediated by ABT-737, similar to that observed with Bcl-xL and Mcl-1 overexpression.
Cells deficient for Mcl-1 (mcl-1−/− MEFs) are highly sensitive (EC50 ≈ 80 nM) to ABT-737. Significantly, overexpression of sjA in mcl-1−/− MEFs leads to significant resistance to ABT-737, similar to when either Bcl-xL or Mcl-1 are overexpressed (Fig. 4B). This inhibition could be a result of sjA binding to Bax and Bak, which we observe in coimmunoprecipitation experiments (Fig. S4) and to isolated Bax/Bak BH3 peptides (Fig. 5B). Alternatively, sjA might be sequestering the drug within the cells although this possibility appears less likely given the moderate affinity of sjA for ABT-737 (Fig. 4A).
Fig. 5.
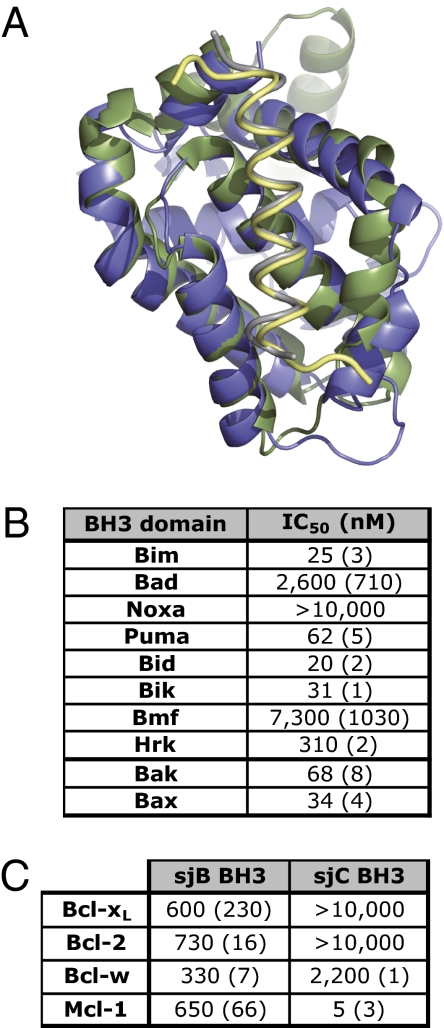
sjA adopts a canonical Bcl-2 protein fold. (A) The BakBH3 peptide (gray) forms a helix that binds into the canonical hydrophobic groove on the surface of sjA (blue) (PDB ID code 3QBR). Data collection and refinement statistics are in Table S1. Overlay of BakBH3:sjA with human BimBH3:Bcl-xL (yellow:green; PDB ID code 3FDL) reveals evolutionary conservation of complex formation. (B) Binding affinities of BH3 domain peptides from mammalian proapoptotic Bcl-2 family proteins were determined by competitive binding assays. Values represent average IC50 (SD, n = 2–4). (C) Binding of sjB and sjC BH3 domain peptides to mammalian prosurvival proteins were determined by solution competition assays. Both sjB BH3 and sjC BH3 bound tightly to sjA (Fig. 1B), whereas binding to the mammalian prosurvival proteins, with the exception of sjC BH3 for Mcl-1, was significantly weaker. Values represent average IC50 (SD, n = 3).
Experiments examining the effect of ABT-737 treatment on adult schistosomes in culture have provided variable results thus far, although in several experiments accelerated parasite death has been observed (at 20 μM) compared with parasites treated with the closely related, weaker binding analog W1191542 (27). It is likely that the moderate affinity of ABT-737 for sjA (IC50 170 nM) compared with the very high affinity (≈1 nM) of ABT-737 for human prosurvival Bcl-2–like proteins (22) accounts for the inconsistent activity. We suspect that ABT-737 binding to sjA is beyond the threshold affinity required to trigger death, hence higher affinity compounds are required if BH3 mimetics are to be pursued as antiparasitic agents.
sjA Adopts the Bcl-2 Protein Fold.
To provide a basis for such future drug development efforts, an X-ray crystal structure (2.6 Å) of sjA complexed with a Bak BH3 domain peptide was determined (Fig. 5A, Fig. S5A, and Table S1). This structure demonstrated that sjA adopts a typical Bcl-2 protein fold, with the BakBH3 peptide binding into the hydrophobic groove of sjA, analogous to BakBH3 binding to Bcl-xL (Fig. 5A) (4). The four signature hydrophobic residues of the Bak BH3 domain are buried within the groove (Fig. S5B), as in all previously determined BH3:prosurvival protein complexes (2–5, 27), and a salt bridge between a critical aspartyl residue on the peptide and an arginyl residue on the BH1 domain of the prosurvival protein, is also evident (Fig. S5C). Hence, this structure unambiguously reveals that sjA is a Bcl-2 family protein and provides a framework for rational drug design efforts, much as the Bcl-xL:BakBH3 complex structure served as a framework for the development of ABT-737 (22).
Schistosome Prosurvival Protein, sjA, Has a Unique BH3 Domain Binding Profile.
For BH3 mimetics to be maximally useful as anti-infectives, they must be able to target parasite prosurvival protein(s) without significant cross-reactivity with the human Bcl-2 counterparts. To address this issue, we examined the ability of sjA to bind a panel of mammalian BH3 domains (Fig. 5B). A complementary experiment measuring mammalian prosurvival protein binding to schistosome BH3 domains was also performed (Fig. 5C). Collectively, these results show that sjA exhibits a unique BH3-binding profile compared with human prosurvival proteins (28). Hence, its BH3 ligand-binding groove is sufficiently different from that of mammalian prosurvival Bcl-2 proteins to potentially allow for its selective targeting.
Discussion
The recently published genomes of S. mansoni and S. japonicum provide an invaluable resource for the identification of new targets for development of antischistosomal drugs (17–19). No previous analysis of a schistosome Bcl-2–regulated apoptotic pathway, beyond characterization of a putative caspase inhibitor (IAP) has been reported (29), although the recent description of Bcl-2 proteins in evolutionarily related nonparasitic Platyhelminthes (planarians) (30) suggested that similar pathways could exist in schistosomes. Here, we showed that schistosomes possess all necessary components of an intrinsic (Bcl-2 regulated) cell death machinery. By focusing on the Bcl-2 proteins, we demonstrated that the pathway is similar to that in humans, consisting of a tripartite cassette architecture: BH3-only proapoptotic proteins, multi-BH domain prosurvival proteins, and multi-BH domain proapoptotic Bax/Bak-like proteins (1). Although our functional characterization involved expression of the various proteins in mammalian cells, we were able to exploit genetically modified cell lines to provide compelling evidence for how the pathway is structured. In particular, the ability of sjB to significantly reduce the clonogenic potential of MEFs deficient in bax and bak indicates its function as a Bax/Bak-like protein. Moreover, bax−/−bak−/− cells stably expressing sjB, unlike the parental cells, release cytochrome c from their mitochondria in response to treatment with a BH3 peptide. Because outer mitochondrial membrane permeabilization, and concomitant release of cytochrome c (and other apoptogenic factors), are the critical functions of Bax/Bak in the apoptotic program (31), these data provide unequivocal evidence for the Bax/Bak-like function of sjB. Importantly, this activity could be inhibited by sjA, and this inhibition could, in turn, be derepressed by BH3-only proteins, (e.g., Bim or sjC). Hence, we were able to reconstitute the entire pathway, as occurs in mammalian Bcl-2 regulated apoptosis. This structure we propose for the schistosome pathway is supported by biochemical data showing prosurvival and proapoptotic members bind one another with nanomolar affinity, similar to that observed for pro- and antiapoptotic Bcl-2 family members in the human pathway (28), and agrees entirely with functional predictions based on phylogenetic analysis of the protein sequences (at least for sjA and sjB). Finally, the sjA:BakBH3 crystal structure provides definitive evidence that sjA exerts its prosurvival function in exactly the same manner as mammalian (and indeed nematode) prosurvival proteins by engaging BH3 sequences within a well-defined ligand binding groove (2–5, 32).
In addition to the proteins characterized here, other Bcl-2–related proteins (based on sequence similarity) we identified in S. japonicum and S. mansoni (Fig. S1) likely participate in this pathway and probably provide some functional redundancy, as occurs in human apoptosis pathways. Phylogenetic analysis suggests that at least one of these proteins (sj/smBcl-2/1) might be a prosurvival molecule while another (sj/smBcl-2/2) is likely a Bax/Bak-like protein, although further study will be required to confirm these functions. Regardless, the pathway in schistosomes is more complex than that observed in nematodes, further supporting the growing evidence that nematodes (and insects) may be evolutionary outliers with a divergent Bcl-2–regulated pathway. A similar complex pathway architecture was recently proposed for Hydra (12, 13), belonging to an even more ancient phylum (cnidaria) than platyhelminthes. That pathway, however, was not fully reconstituted as we have done here with schistosome Bcl-2 family members, and functional experiments with proposed Bax/Bak-like proteins were performed by using cells expressing mammalian Bax/Bak proteins and, hence, could be open to interpretation. Regardless, even cnidaria seem to be more closely related to mammals than nematodes based solely on the number of Bcl-2–related proteins identified.
One of our motivations for identifying a cell death pathway in schistosomes was to uncover potential new drug targets for the treatment of schistosomiasis. By demonstrating that sjA can engage the BH3 mimetic drug ABT-737, we provide some proof-of-principle that molecules of this class could be considered as a basis for the development of novel anti-infectives, although clearly compounds with a higher affinity for the schistosome prosurvival protein(s) will be required to explore this avenue further. Such compounds should be capable of inducing apoptosis in those cells critical for parasite survival. Although we focused on schistosomes in this study, other disease-causing metazoan parasites, including other parasitic flatworms and nematodes, could also be considered for such therapeutic approaches based on BH3 mimetics, provided that they rely on the Bcl-2–regulated apoptotic pathway for their survival and development.
Materials and Methods
Identification of Schistosome Bcl-2 Proteins.
Schistosome Bcl-2–related proteins were identified by using BLAST (33) to search GenBank as well as the SchistoDB (19) using full-length Bcl-2 prosurvival proteins and individual Bcl-2 homology domains as search sequences. Schistosome APAF-1 and caspases were identified by using human homologs as search sequences.
Protein Expression and Purification.
For recombinant sjA production, a synthetic gene, codon optimized for expression in Escherichia coli was obtained from Genscript. A C-terminally deleted construct (ΔC41) of sjA with N-terminal hexahistidine tag was expressed in E. coli. The protein was purified by nickel-affinity chromatography followed by gel-filtration chromatography on a Superdex 75 (16/60) column. Human Bcl-2 proteins for SPR studies were expressed and purified as described (2, 28, 34).
Binding Assays.
Binding affinities were measured by solution competition assays by using the Biacore 3000, as described (35).
Coimmunoprecipitation and Western blotting.
Expression constructs for FLAG-tagged sjB and sjC were cotransfected with constructs encoding HA-tagged sjA into HEK293T cells by using Lipofectamine (Invitrogen). Cell lysates were prepared in lysis buffer [20 mM Tris at pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 10% (vol/vol) glycerol] containing 1% (vol/vol) Triton X-100 and supplemented with protease inhibitors (Roche). FLAG-tagged proteins were immunoprecipitated and associated HA-tagged proteins were detected as described (36). Whole-cell lysates (WCL) were probed with anti-FLAG, anti-HA, and anti-actin (loading control) antibodies. HRP-conjugated anti-rat Ig (SouthernBiotech) was used as the secondary antibody throughout.
Cytochrome c Release Assays.
Cytochrome c release assays were performed on sjB expressing MEFs as described (34).
Other Methods.
Details of the phylogenetic analysis, cell killing assays, and structural determination are provided with SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Prof. David Huang for provision of various cell lines and Prof. Robin Gasser for input into the manuscript. Crystallization trials were performed at the Bio21 Collaborative Crystallisation Centre. Data were collected on the MX2 beamline at the Australian Synchrotron, Victoria, Australia. This work was supported by National Health and Medical Research Council (NHMRC) of Australia Program Grant 461221 (to P.M.C.) and Project Grant 1002227 (to W.D.F.), the Australian Cancer Research Foundation (P.M.C.), the Leukemia and Lymphoma Society (SCOR 7015-02), the Leukaemia Foundation of Australia (Phillip Desbrow Post Doctoral Fellowship; to E.F.L.), ANZ Trustees (William Buckland Foundation) (E.F.L.), and the Contributing to Australian Scholarship and Science (CASS) Foundation (W.D.F.). Infrastructure support from NHMRC Independent Research Institutes Infrastructure Support Scheme Grant 361646 and the Victorian State Government OIS grant is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3QBR).
*This Direct Submission article had a prearranged editor.
See Commentary on page 6695.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100652108/-/DCSupplemental.
References
- 1.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 2.Czabotar PE, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci USA. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day CL, et al. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 4.Sattler M, et al. Structure of Bcl-xL-Bak peptide complex: Recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 5.Smits C, Czabotar PE, Hinds MG, Day CL. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure. 2008;16:818–829. doi: 10.1016/j.str.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 7.Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59(7, Suppl):1701s–1706s. [PubMed] [Google Scholar]
- 8.Yuan JY, Horvitz HR. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol. 1990;138:33–41. doi: 10.1016/0012-1606(90)90174-h. [DOI] [PubMed] [Google Scholar]
- 9.Colussi PA, et al. Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery. J Cell Biol. 2000;148:703–714. doi: 10.1083/jcb.148.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn L, et al. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. EMBO J. 2003;22:3568–3579. doi: 10.1093/emboj/cdg355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo SJ, et al. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat Cell Biol. 2002;4:416–424. doi: 10.1038/ncb793. [DOI] [PubMed] [Google Scholar]
- 12.Lasi M, David CN, Böttger A. Apoptosis in pre-Bilaterians: Hydra as a model. Apoptosis. 2010;15:269–278. doi: 10.1007/s10495-009-0442-7. [DOI] [PubMed] [Google Scholar]
- 13.Lasi M, et al. The molecular cell death machinery in the simple cnidarian Hydra includes an expanded caspase family and pro- and anti-apoptotic Bcl-2 proteins. Cell Res. 2010;20:812–825. doi: 10.1038/cr.2010.66. [DOI] [PubMed] [Google Scholar]
- 14.Wiens M, et al. Axial (apical-basal) expression of pro-apoptotic and pro-survival genes in the lake baikal demosponge Lubomirskia baicalensis. DNA Cell Biol. 2006;25:152–164. doi: 10.1089/dna.2006.25.152. [DOI] [PubMed] [Google Scholar]
- 15.Wiens M, Diehl-Seifert B, Müller WE. Sponge Bcl-2 homologous protein (BHP2-GC) confers distinct stress resistance to human HEK-293 cells. Cell Death Differ. 2001;8:887–898. doi: 10.1038/sj.cdd.4400906. [DOI] [PubMed] [Google Scholar]
- 16.Wiens M, Krasko A, Müller CI, Müller WE. Molecular evolution of apoptotic pathways: Cloning of key domains from sponges (Bcl-2 homology domains and death domains) and their phylogenetic relationships. J Mol Evol. 2000;50:520–531. doi: 10.1007/s002390010055. [DOI] [PubMed] [Google Scholar]
- 17.Berriman M, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, et al. Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zerlotini A, et al. SchistoDB: A Schistosoma mansoni genome resource. Nucleic Acids Res. 2009;37(Database issue):D579–D582. doi: 10.1093/nar/gkn681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 21.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 22.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 23.Tse C, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 24.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 25.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naik E, Michalak EM, Villunger A, Adams JM, Strasser A. Ultraviolet radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J Cell Biol. 2007;176:415–424. doi: 10.1083/jcb.200608070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee EF, et al. Conformational changes in Bcl-2 pro-survival proteins determine their capacity to bind ligands. J Biol Chem. 2009;284:30508–30517. doi: 10.1074/jbc.M109.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Peng J, Yang Y, Feng X, Cheng G, Lin J. Molecular characterizations of an inhibitor of apoptosis from Schistosoma japonicum. Parasitol Res. 2010;106:967–976. doi: 10.1007/s00436-010-1752-y. [DOI] [PubMed] [Google Scholar]
- 30.Pellettieri J, et al. Cell death and tissue remodeling in planarian regeneration. Dev Biol. 2010;338:76–85. doi: 10.1016/j.ydbio.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 32.Yan N, et al. Structural, biochemical, and functional analyses of CED-9 recognition by the proapoptotic proteins EGL-1 and CED-4. Mol Cell. 2004;15:999–1006. doi: 10.1016/j.molcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 34.Lee EF, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180:341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee EF, et al. Novel Bcl-2 homology-3 domain-like sequences identified from screening randomized peptide libraries for inhibitors of the pro-survival Bcl-2 proteins. J Biol Chem. 2009;284:31315–31326. doi: 10.1074/jbc.M109.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czabotar P.E, et al. Mutations to Bax beyond the BH3 domain disrupts interactions with pro-survival proteins and promotes apoptosis. J Biol Chem. 2011;286:7123–7131. doi: 10.1074/jbc.M110.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.