Abstract
An essential stage in endocytic coated vesicle recycling is the dissociation of clathrin from the vesicle coat by the molecular chaperone, 70-kDa heat-shock cognate protein (Hsc70), and the J-domain-containing protein, auxilin, in an ATP-dependent process. We present a detailed mechanistic analysis of clathrin disassembly catalyzed by Hsc70 and auxilin, using loss of perpendicular light scattering to monitor the process. We report that a single auxilin per clathrin triskelion is required for maximal rate of disassembly, that ATP is hydrolyzed at the same rate that disassembly occurs, and that three ATP molecules are hydrolyzed per clathrin triskelion released. Stopped-flow measurements revealed a lag phase in which the scattering intensity increased owing to association of Hsc70 with clathrin cages followed by serial rounds of ATP hydrolysis prior to triskelion removal. Global fit of stopped-flow data to several physically plausible mechanisms showed the best fit to a model in which sequential hydrolysis of three separate ATP molecules is required for the eventual release of a triskelion from the clathrin–auxilin cage.
Keywords: clathrin-mediated endocytosis, kinetic mechanism, macromolecular assemblies, vesicle uncoating, mathematical model
Endocytosis is at the center of a hub of cellular processes that include nutrient uptake, receptor down-regulation, synaptic vesicle recycling, signaling, and developmental processes (1). During clathrin-mediated endocytosis, the cell membrane invaginates to form a bud in which receptors with specific cargo accumulate. The bud encloses the cargo and forms a vesicle that becomes detached from the membrane, moving on to fuse with its target compartment. This process is directed by a network of proteins that dictate how and when the bud forms, which receptors are included in the vesicle, and which ensure that the vesicle is completed and detached from the membrane. Some of these proteins, including clathrin and AP2, form a coat around the vesicle while it is forming and help to select the cargo that is enclosed (2–5). After detachment, the protein coat is quickly removed, and the vesicle goes on to fuse with its target membrane. This process of uncoating is essential and primarily involves the molecular chaperone, 70-kDa heat-shock cognate protein (Hsc70), and its DnaJ cofactor, auxilin/cyclin-G-associated kinase. As well as their role in uncoating, Hsc70 and auxilin interact with other proteins, indicating their possible involvement in related processes such as vesicle movement and vesicle formation (6).
Clathrin can be purified and assembled, in vitro, into polyhedral cages that resemble the clathrin coats observed in cells. Monitoring of cage disassembly in vitro has allowed the disassembly of clathrin cages into individual clathrin triskelions by Hsc70 and auxilin to be well-characterized in biochemical terms. Through the work of a number of different groups, the essential domains of auxilin and Hsc70 required for clathrin disassembly have been established (7, 8), affinities of Hsc70 for auxilin (9, 10) and nucleotides (11, 12) have been determined, and the stoichiometric relationships between clathrin, Hsc70 and auxilin during disassembly have been investigated. It has been proposed that three molecules of Hsc70 are involved in removing one triskelion from a coated vesicle (13). This is supported by electron microscopy and gel filtration data that showed three Hsc70 molecules bound to the released triskelia (14, 15) and by demonstration of maximal binding to cages of three Hsc70s per triskelion (16). However, interestingly, Xing et al. (17) report a stoichiometry of about one Hsc70 molecule per threefold clathrin vertex in their recent cryoelectron microscopy study of Hsc70 bound to clathrin cages. Maximal binding of auxilin to clathrin cages has been shown to occur at a ratio of three molecules per triskelion (7, 18), yet substoichiometric amounts of auxilin can support complete cage disassembly (19, 20). Intriguingly, only a single auxilin per triskelion is required for maximal stimulation of ATP hydrolysis by Hsc70 (9) or maximal binding of Hsc70 to clathrin (16).
In light of these data, a model for disassembly was proposed by Ungewickell et al. (16) that depicts a single auxilin molecule binding per clathrin triskelion, each of which recruits three molecules of Hsc70, and upon hydrolysis of ATP, conformational changes distort the triskelia and cage disassembly occurs. Given that auxilin and Hsc70 are known to interact at a 1∶1 stoichiometry in solution (7, 10), this model raises an important question: By what mechanism can a single auxilin recruit three Hsc70 molecules?
In this paper, we address this problem via kinetic analysis of cage disassembly based on light-scattering measurements. It was recently demonstrated that dynamic light scattering can be used effectively to monitor clathrin cage disassembly (21, 22), thus providing better time resolution than previous studies that were predominantly based on centrifugation and densitometry of SDS-PAGE (19, 20). We have further increased the time resolution by which disassembly kinetics can be measured, by monitoring simple perpendicular light scattering using stopped-flow methods to capture events on the milliseconds-to-seconds timescale. This has allowed us to observe a previously unseen stage in the recruitment of Hsc70 to clathrin cages. We have also determined the amount of phosphate released per triskelion while cage disassembly is taking place, and, in addition, we show that only a single auxilin per triskelion is required for the maximum rate of clathrin cage disassembly by Hsc70, thus demonstrating the functional significance of the stoichiometry of the interaction of auxilin with clathrin and Hsc70 shown previously. Statistical analysis of our data according to five physically plausible mechanistic models revealed that a three-step sequential mechanism fitted the data most accurately. Based on these results, we propose a sequential recruitment model for the action of Hsc70 on clathrin cages, which explains both our observations and previously published data.
Results
The Light-Scattering Assay.
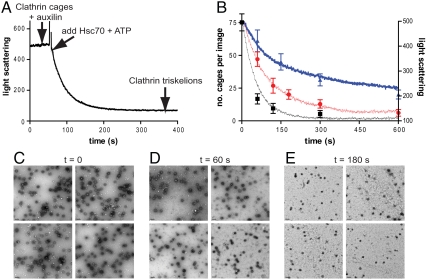
In these measurements, perpendicular light scattering is used to monitor the real-time disassembly of clathrin cages by Hsc70, ATP, and auxilin (GSTaux401–910). Upon addition of Hsc70 and ATP to clathrin cages with auxilin bound, the scattering signal decreases rapidly as the cages are disassembled into triskelia (Fig. 1A). We clarified the meaning of this signal by imaging the disassembly process using transmission electron microscopy. Samples were taken at specific time points during disassembly reactions and negatively stained EM grids prepared. For each time point, multiple images were obtained and the number of cages per image counted. These assays were conducted under conditions where the Hsc70 concentration was low so that sufficient intermediate time points could be captured using negative staining. Fig. 1 C–E shows representative images from three grids prepared at different time points during a single disassembly reaction. The average results of the EM cage-counting assay for three different concentrations of Hsc70 in Fig. 1B show an excellent correlation between the decay in cage numbers counted and the decay in light scattering.
Fig. 1.
A real-time in vitro assay for clathrin cage disassembly and correlation with electron microscopy images of clathrin cages. (A) Representative trace of the right-angle light-scattering assay for clathrin cage disassembly. Clathrin cages (0.09 μM triskelia) were premixed with 0.1 μM auxilin, and after 60 s, cage disassembly was initiated by addition of 1 μM Hsc70 and 500 μM ATP. (B) Average results for three different disassembly assays monitored both by light scattering as in A and compared with electron microscopy images as in C–E. The single points represent the average number of cages counted per image, initiated with 0.1 μM (triangles), 0.2 μM (circles), or 0.5 μM (squares) Hsc70. Data are mean ± SD. The single lines represent the light-scattering results obtained under the same conditions. (C–E) Representative transmission electron micrographs of negatively stained grids prepared at 0, 60, and 180 s during a disassembly assay containing clathrin cages (0.09 μM triskelia), 0.1 μM auxilin, 500 μM ATP, and initiated with 0.2 μM Hsc70. The scale bar in the bottom left-hand corner of each image represents 0.2 μm.
The Dependence of Cage Disassembly on Hsc70 and Auxilin Concentrations.
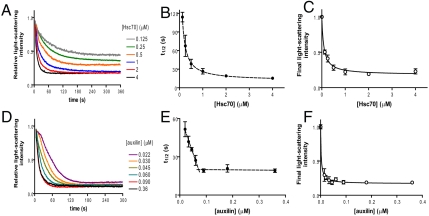
We then used the light-scattering assay to monitor the effect of varying the concentrations of auxilin and Hsc70 on the time-course of cage disassembly. Representative data for these measurements are shown in Fig. 2 A and D. At fixed concentrations of clathrin (0.09 μM triskelia) and auxilin (0.3 μM), increasing the concentration of Hsc70 leads to an increase in the rate at which disassembly occurs (Fig. 2A). Using the time taken to disassemble half of the clathrin cages, t1/2, as a measurement for the rate of disassembly (Fig. 2B) it can be seen that an excess of Hsc70 is required to reach the maximal rate of disassembly.
Fig. 2.
The effect of [Hsc70] or [auxilin] on clathrin cage disassembly. (A) Representative light-scattering curves containing clathrin cages (0.09 μM triskelia), auxilin (0.3 μM), and ATP (500 μM) with disassembly initiated by various concentrations of Hsc70 (0.15–4 μM). (D) Representative disassembly curves with clathrin cages (0.09 μM triskelia) premixed with varying [auxilin] (0.022–0.36 μM) and 500 μM ATP, and initiated by addition of 2 μM Hsc70. (B and E) Symbol t1/2 on the vertical axis denotes the average time taken for disassembly of 50% of clathrin cages obtained from raw data traces as in A and D, respectively. Data points and error bars, respectively, are mean ± standard error from replicated measurements (n≥4). (C and F) Average amplitude (total amount of disassembly) at the end of each assay from curves obtained as in A and D, respectively. Data points and error bars, respectively, are mean ± standard error from replicated measurements (n≥4). The smooth curves in panels B, C, and F represent a fit to the hyperbolic saturation function (Eq. S1), which serves as an empirical description of the data.
The amplitude plot shown in Fig. 2C demonstrates that at low concentrations of Hsc70, the disassembly curves do not decay to zero; i.e., disassembly of clathrin cages under conditions of limiting Hsc70 is incomplete. This result suggests that the Hsc70 is not recycled and that, after cage disassembly driven by ATP hydrolysis, the resultant Hsc70∶ADP(Pi) species remains tightly bound to the dissociated triskelia. To support this conclusion, subsequent addition of Hsc70 allows disassembly to proceed to completion (Fig. S1).
When the dependence of disassembly rate on auxilin concentration is analyzed (Fig. 2E), we observe a linear relationship between rate and auxilin concentration until a distinct break point is reached. This result shows there is a very tight interaction between auxilin and the assembled clathrin cages. This functional assay is relevant to the stoichiometry that governs the rate of the uncoating process. The maximum rate is achieved at a stoichiometry of 1 mol of auxilin to 1 mol of clathrin triskelion. It is also evident that complete disassembly occurs even at very low, substoichiometric concentrations of auxilin (Fig. 2F). This suggests that auxilin is recycled during the disassembly process.
The Role of Nucleotide Hydrolysis in Clathrin Cage Disassembly.
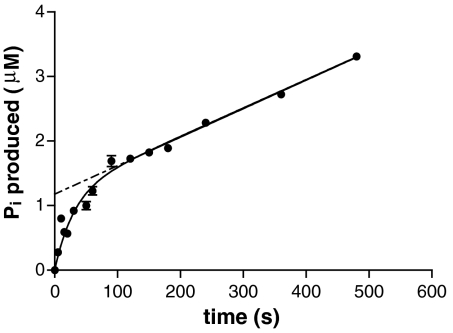
The hydrolysis of ATP is an obligatory step in clathrin cage disassembly and disassembly does not occur in the presence of nonhydrolyzable ATP analogues (Fig. S2). The rate of ATP hydrolysis by Hsc70 alone is very slow (k = 0.0011 ± 0.0002 s-1, Table S1), and it has been previously reported that interaction with both auxilin and clathrin can stimulate hydrolysis (7, 9, 10), as we also find (Table S1). However, these previous measurements have been carried out at pH 6, under conditions where disassembly is not observed. In order to understand how ATP hydrolysis might be coupled to clathrin cage disassembly, we measured the ATP hydrolysis that occurred during a clathrin cage disassembly reaction when the concentration of Hsc70 is in excess. Fig. 3 shows the amount of Pi produced under disassembly conditions (i.e., when the system is functionally coupled). The curve shows a rapid initial phase followed by a slow steady-state rate. The initial nonlinear region corresponds to the activity during cage disassembly, and the rate at which ATP is hydrolyzed during this time is comparable to the rate of clathrin disassembly (t1/2 ≈ 15 s). The later, linear steady-state rate is simply the basal activity of Hsc70 plus any stimulation from the low concentration of auxilin present, and this rate agrees well with our steady-state data (Table S1). Interestingly, these data show that approximately 1 mol of ATP is hydrolyzed during the disassembly of 1 mol of clathrin heavy chain, or 3 mol of ATP per clathrin triskelion released. This stoichiometry suggests that either three Hsc70s bind to a triskelion and each hydrolyzes a single ATP, or a single Hsc70 binds per triskelion and hydrolyzes three ATPs. If three Hsc70s bind, they might bind clathrin and each hydrolyze one ATP at the same time or in series. If the process happened in series, ATP ligands would be hydrolyzed one after the other with disassembly only occurring after the final hydrolysis. If a single Hsc70 is required to turn over three ATPs consecutively, or if three Hsc70s work in series, this would result in a lag phase early in the disassembly process while the first two rounds of hydrolysis occurred.
Fig. 3.
The hydrolysis of ATP during clathrin cage disassembly. The amount of Pi produced from ATP hydrolysis by Hsc70 during clathrin cage disassembly was monitored. Clathrin (0.33 μM triskelia), 0.35 μM auxilin, and 8 μM Hsc70 were mixed together. The reaction was initiated by addition of ATP (50 μM), and at specific time points samples were removed, quenched, and assayed for Pi content with malachite green solution. The data are fitted to a single exponential superimposed on a linear function (Eq. S2).
Early Events in Clathrin Cage Disassembly.
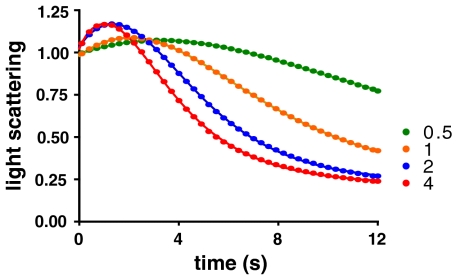
To study the early events in disassembly and ascertain if there is a lag phase, we performed the scattering assay in a stopped-flow fluorimeter, which allowed us to decrease the dead time from 4–6 s in the standard fluorimeter to less than 10 ms. Results from the stopped-flow experiments are shown in Fig. 4, and demonstrate that there is indeed a lag phase before disassembly begins. Strikingly, in addition to the lag phase, there is a significant increase in the scattering signal before the disassembly process begins. Both the rate and amplitude of this initial increase in scattering are dependent on the concentration of Hsc70. Factors that give rise to changes in light-scattering signal include changes in molecular weight or in radius of gyration. Because we observe a process that depends upon Hsc70 concentration and yet is not indicative of a simple binding interaction, we propose that this increase in scatter represents Hsc70 recruitment to the cage complex, accompanied by a conformational change in the complex that alters its radius of gyration. We interpret the lag phase as being due to the first two rounds of ATP hydrolysis per triskelion by Hsc70, after which, upon the third round of ATP hydrolysis, the triskelion dissociates and the scattering signal starts to decrease.
Fig. 4.
Stopped-flow measurements of light scattering to examine the early stages of clathrin cage disassembly. Clathrin cages (0.09 μM triskelia) premixed with 0.1 μM auxilin were mixed with Hsc70 (concentrations in μM shown on graph) and 500 μM ATP, and perpendicular light scattering was measured using stopped-flow techniques. The closely spaced raw data was reduced to a frequency of 3.3 s-1 (closed circles) and fitted (dashed lines) to a system of first-order ordinary differential equations corresponding to the reaction mechanism shown in Scheme 1, using the software DYNAFIT (29, 30). Data corresponding to 0 and 0.25 μM Hsc70 were omitted from the fit because of their low information content, as was data collected after 12 s.
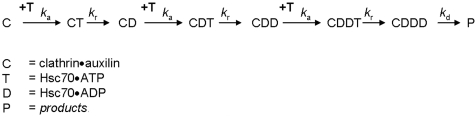
In order to test our conclusions, we fitted five alternative kinetic mechanisms (Schemes S3–S7) to the stopped-flow data. The fit of the simplest plausible mechanism consistent with the data (Scheme 1) is shown in Fig. 4. The light-scattering data agree well with a mechanism in which an initial binding event is accompanied by an increase in molar scattering followed by two more binding events and a loss of scattering on disassembly (Scheme 1 and Table 1). Three unique rate constants, ka, kr and kd, are required to describe the data taking the values of 0.69 μM-1 s-1, 6.5 s-1, and 0.38 s-1, respectively. In Scheme 1, the three unique rate constants appear altogether in seven separate steps. The first six steps are pairs of consecutive association-hydrolysis reactions, each characterized by rate constants ka and kr, respectively. The final step is the cage disassembly itself, characterized by the rate constant kd. Although we initially allowed every binding and hydrolysis step to take different values, it became apparent that this offered no statistical advantage over a simpler scheme where two rate constants described the binding of Hsc70 and turnover of ATP (see SI Text). Reducing the number of binding/hydrolysis steps from three to two (Scheme S5) resulted in a 20% increase in the residual sum of the squares and was not favored by the Akaike information criterion (23), which properly accounts for differing number of adjustable parameters used in the models (Table S2). Variations on the three-step model depicted in Scheme 1 were tested, but we were unable to confidently distinguish between “semiconcerted” models that allowed binding of two Hsc70 molecules before the first hydrolysis step and the fully sequential Scheme 1. A “fully concerted” scheme, Scheme S6, where binding of three Hsc70s takes place before hydrolysis was not favored, further supporting a mechanism involving a stepwise process of Hsc70 binding and ATP hydrolysis. The fact that three microscopic rate constants, ka, kr, and kd, are required to describe the data would suggest that the clathrin disassembly progress curves should be fitted well by a triple exponential model, and this is indeed the case (Fig. S3).
Scheme 1.
The three-step sequential mechanism for clathrin cage disassembly that fitted the kinetic data most accurately when five alternate kinetic mechanisms were tested (Schemes S3–S7). Each step consists of an association event between clathrin.auxilin (C) and Hsc70.ATP (T) with rate constant ka, followed by hydrolysis of ATP to ADP with rate constant kr. Once the three steps have taken place, the clathrin.auxilin cage disassembles with rate constant kd.
Table 1.
Kinetic parameters for the reaction mechanism depicted in Scheme 1
| Parameter | Best-fit value | 99% confidence interval |
| ka, μM-1 s-1 | 0.69 | 0.67–0.72 |
| kr, s-1 | 6.5 | 5.3–8.4 |
| kd, s-1 | 0.38 | 0.37–0.39 |
Discussion
Using a simple perpendicular light-scattering assay we have measured the in vitro disassembly of clathrin cages by Hsc70 and auxilin, which occurs rapidly with a t1/2 of approximately 10 s. This is comparable to recent results from experiments that used dynamic light scattering to monitor clathrin cage disassembly (21, 22) but faster than earlier centrifugation-based studies that had t1/2 values ranging from 2–10 min (19, 20, 24) but that also contained adaptor proteins such as AP180 or AP2, which are known to stabilize the cages and may consequently have slowed down disassembly. In this study, we have increased the time resolution beyond that of dynamic light scattering by monitoring perpendicular light scattering, and analysis of these data has allowed us to isolate and describe individual steps in the chaperone-mediated disassembly of cages that hitherto have remained invisible.
The veracity of the scattering signal in representing the true disassembly reaction was established by correlating the cage count with the scattering intensity. This required using time-resolved sampling with electron microscopy and comparing this with the scattering intensities. Our data show an excellent correlation and demonstrate that the decrease in scattering signal is proportional to the number of cages throughout the progress of the reaction.
The data we have collected on the concentration dependence of disassembly kinetics (Fig. 2) reveal that, whereas an excess of Hsc70 is required to achieve the maximal rate of uncoating, a ratio of only one auxilin molecule per triskelion is required to achieve this. Our phosphate release experiments (Fig. 3) show that three ATP molecules must be hydrolyzed for every triskelion released. These results raised two important questions. (i) What features of the mechanism cause triskelion release after hydrolysis of three ATP molecules? (ii) How can a single auxilin molecule coordinate the release of one triskelion?
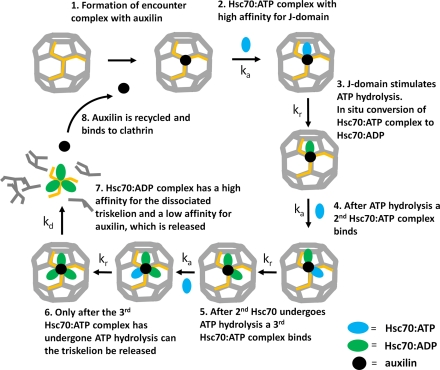
Here, we propose a model for the mechanism by which Hsc70 and auxilin act to disassemble clathrin cages based on analysis of our stopped-flow light-scattering data, which answers these questions and defines this mechanism more fully than has been previously possible. The model is illustrated in Fig. 5. Firstly, auxilin binds tightly to the clathrin cages, at a ratio of one auxilin per triskelion to achieve the optimum rate of disassembly. The Hsc70∶ATP complex then binds to the clathrin–auxilin cage, initially at a ratio of one Hsc70 per triskelion. The interaction of this Hsc70 with the J domain of auxilin stimulates the hydrolysis of ATP, causing a conformational change in the Hsc70 in which the Hsc70∶ADP complex is firmly attached to its binding site on clathrin. Following this, a second Hsc70∶ATP is recruited to the clathrin∶auxilin complex, a further ATP is hydrolyzed, and the second Hsc70 is attached. Finally, the third Hsc70∶ATP is recruited to the clathrin–auxilin complex, and upon ATP hydrolysis by this molecule the conformational strain imposed upon the clathrin by its interaction with Hsc70∶ADP leads to a concerted dismantling of the cage into component triskelia. The three Hsc70∶ADP molecules have a high affinity for the released triskelia and therefore remain tightly bound and are not recycled. In contrast, auxilin has a low affinity for the released triskelia and Hsc70-ADP and can therefore dissociate and be recycled.
Fig. 5.
An illustration of the serial Hsc70 binding and ATP hydrolysis model for the disassembly of clathrin cages highlighting the sequence of events on a single triskelion. 1: Auxilin has a high affinity for triskelion legs forming part of a clathrin cage and binds at a stoichiometry of one per triskelion. 2: An Hsc70∶ATP complex binds to the clathrin∶auxilin complex. 3: The interaction between auxilin’s J domain and Hsc70 stimulates ATP hydrolysis and induces a conformational change in Hsc70, increasing its affinity for clathrin. 4: Auxilin repositions, and a second Hsc70∶ATP is recruited to the clathrin∶auxilin complex. 5: The second Hsc70 interacts with auxilin’s J domain, ATP is hydrolyzed, and the resulting Hsc70∶ADP complex binds tightly to clathrin. Following hydrolysis, the auxilin again repositions, and a third Hsc70∶ATP binds to the clathrin∶auxilin complex. 6: Hydrolysis of the third ATP results in a weak interaction between the triskelion(Hsc70∶ADP)3 complex and the cage. The rate-limiting step is the disassembly of triskelia leading to cage collapse. 7: The Hsc70∶ADP complex has a high affinity for the released triskelia and remains bound, whereas the affinity of triskelia(Hsc70∶ADP)3 for auxilin is low; the previously bound auxilin is free now to rebind the cage in a catalytic manner (8).
Our suggestion that Hsc70-ADP remains tightly bound to the released triskelia comes from the amplitude data in Fig. 2C, which shows that disassembly does not proceed to completion when limiting concentrations of Hsc70∶ATP are used. The reaction will, however, proceed further when additional Hsc70∶ATP is added subsequently (Fig. S1). This agrees well with previous studies that have shown Hsc70 bound to the free triskelia (14, 15, 22) and supports the idea previously proposed that Hsc70 acts to chaperone the released clathrin triskelia back to the plasma membrane (25), preventing formation of empty cages within the cell. It should be noted that, in a similar previous study, Schuermann et al. (22) also observed a second, slower linear phase of disassembly following the initial fast exponential phase. We do not observe this second slower phase in our studies, but this may simply reflect small differences in assay conditions, which may affect the strength of the binding interaction between Hsc70∶ADP and clathrin. It should also be noted that, in vivo, nucleotide exchange factors interact with Hsc70, and it is likely that in the presence of such a nucleotide exchange factor we would observe recycling of Hsc70.
The requirement of a single auxilin per triskelion to achieve the maximum rate of disassembly is clearly shown from the stoichiometric point in our results in Fig. 2E. Interestingly, it has previously been shown that one auxilin per triskelion gives maximal stimulation of Hsc70 ATPase activity (9) and that a single auxilin bound per triskelion supported maximal Hsc70 binding (16). Thus, a ratio of one auxilin per clathrin triskelion appears to be of critical functional significance in the disassembly reaction. This is not to say that more auxilin cannot bind to clathrin—several reports have shown that much more auxilin than this can bind to clathrin (7, 18, 26), and three auxilins per triskelion are seen in a high-resolution EM structure (18). However these “extra” auxilin molecules are not required for the disassembly process, as we have shown that only a single auxilin per triskelion is required for optimum disassembly rates.
The time-resolved ATPase reactions (Fig. 3) show that three ATPs are hydrolyzed per triskelion released, a value in agreement with published data obtained for clathrin associated with AP180 (19). There are three possible explanations for this. Firstly, that three Hsc70 molecules bind per triskelion and each hydrolyzes one ATP in parallel reactions. Secondly, that a single Hsc70 binds per triskelion and hydrolyzes three ATPs in series. Thirdly, that three Hsc70s bind and hydrolyze ATP in series.
The second explanation, that a single Hsc70 molecule hydrolyzes three ATPs, contradicts extensive previous evidence that three Hsc70 molecules per triskelion are employed in the disassembly reaction. Previous binding studies have shown a maximal binding of three Hsc70s per triskelion at equilibrium (16, 24), and, like in our study, this was shown to occur with only a single auxilin per triskelion. It has also been shown that approximately three molecules of Hsc70 dissociate one triskelion when coated vesicles rather than empty cages were used (6) and it has been found that, following disassembly, three Hsc70s are bound to each free triskelion (14, 15). Identification of the Hsc70 binding motif and the location of this within the cage structure suggest that Hsc70 can bind to three potential binding sites on flexible regions protruding down from the hub (27).
This leaves us with an unusual stoichiometric situation, in which a single auxilin recruits three Hsc70 molecules, as has been suggested in previous models (6, 16). The interaction between the J domain of auxilin and Hsc70 is required for disassembly (16), but the binding of Hsc70 to auxilin occurs at a 1∶1 ratio (Table S1) (7, 10), so how does a single auxilin interact with three Hsc70 molecules? Our proposal that three Hsc70s are recruited and hydrolyze ATP in series would enable a single auxilin to stimulate the ATP hydrolysis by each Hsc70 in turn. In this model, auxilin and Hsc70 still interact at a 1∶1 ratio at any one time, with auxilin dissociating and moving from one Hsc70 to the next following ATP hydrolysis and attachment of Hsc70∶ADP to clathrin. Such a model is also consistent with the well-documented mechanism of other Hsp70/J domain systems (28). The evidence for the requirement of a series of steps to occur prior to triskelion release is provided by our stopped-flow scattering data (Fig. 4), which show a significant lag time before disassembly occurs. We tested the fit of our data to five related kinetic mechanisms (SI Text), all of which appear physically plausible. These include concerted, semiconcerted, and sequential mechanisms. The mechanism that fits optimally is an uncooperative, sequential, three-step process in which each Hsc70∶ATP binds to the clathrin∶auxilin cage and hydrolyzes the nucleotide with the same kinetic characteristics.
Where multiple binding events are proposed, it is logical to expect there to be cooperativity between binding sites, where sequential binding of ligand becomes tighter as the sites are occupied. For example, one might envisage that after the first Hsc70 binding and ATP hydrolysis step the corresponding triskelion leg becomes separated from the cage, causing a conformational change that makes binding of the second Hsc70 more likely, and so on. A possibility of such cooperative uncoating is contained within the molecular mechanism in Scheme S3, in which all microscopic rate constants are allowed to attain unique numerical values. However, this mechanism offered no statistical advantage over an otherwise identical model in which all three rate constants (either for Hsc70-ATP binding or for subsequent hydrolysis) kept the same value. We must therefore conclude that although cooperativity might in principle be present, it is weak to the point of being undetectable by our experimental method.
A recent cryo-EM structure (17) shows only around one Hsc70 bound per triskelion. In light of our model, this structure may represent an intermediate trapped in the first stage of this cycle at pH6, when only the first of the three Hsc70s has been recruited. The mechanism we propose also explains why three Hsc70s have been observed on released triskelions. Our proposed sequential mechanism is thus the simplest explanation that is consistent with both our results and previously published data.
Materials and Methods
Protein Expression and Purification.
Full-length Hsc70 was expressed in Sf9 cells by infection with baculovirus and subsequently purified from the soluble cell lysate by a three-step chromatographic process comprising hydroxyapatite, ATP-agarose, and gel filtration. Residues 401–910 of bovine auxilin were expressed as a GST-fusion protein in Escherichia coli BL21 cells. GST-auxilin401–910 was purified using a glutathione (GSH)-sepharose affinity column and is referred to elsewhere in this work as auxilin. The GST tag could optionally be cleaved off by incubation with thrombin and the GST removed by binding to GSH-sepharose beads. However, we observed no differences in the rates of clathrin disassembly or optimal stoichiometry with this cleaved auxilin compared to the GST-tagged version (Fig. S4). This is consistent with previous studies on the mechanism of auxilin function, which report no effect as a result of the presence of the GST tag (7, 8). Clathrin was purified from clathrin-coated vesicles that were extracted from pig brain by differential centrifugation and gel filtration. Clathrin cages were formed in vitro by dialyzing concentrated pure clathrin into buffer 7 [100 mM MES pH6.5, 15 mM MgCl2, 0.2 mM EGTA, 0.02% (wt/vol) sodium azide], and harvested by centrifugation (135,000 × g, 20 min, 4 °C). For complete details on all these expression and purification procedures, see SI Text.
Light-Scattering Assays.
Perpendicular light scattering was monitored using an LS50 fluorimeter (Perkin Elmer), at a wavelength of 390 nm (excitation and emission) and temperature of 25 °C. Unless otherwise stated in the figure legends, clathrin cages (0.09 μM triskelia), auxilin (0.015–0.36 μM), and ATP (500 μM) were premixed in a 60 μL total volume in buffer 2 (40 mM Hepes pH7, 75 mM KCl, 4.5 mM Mg acetate), and disassembly was initiated by addition of 6 μL Hsc70 (0.8–40 μM). Light scattering was monitored every 0.25 s for up to 3,000 s. Control experiments were carried out to ensure that the scattering signal obtained from both clathrin cages and disassembled clathrin triskelia were linearly dependent on the clathrin concentration (Fig. S5). It was also determined that the interaction between Hsc70 and ATP is very rapid and that it was not necessary to premix Hsc70 with ATP as is typical in the literature (Fig. S6, SI Text, and Table S3). The time taken for disassembly of half of the clathrin cages was obtained from the raw data traces as the time taken for the light-scattering signal to decrease below 0.55. The amplitude of cage disassembly was obtained from the scattering signal remaining after 300 s. Increasing the time beyond this made no significant difference to the level of the scattering signal.
Stopped-flow perpendicular light scattering was measured using a BioLogic MOS450 stopped-flow fluorimeter. Unless otherwise stated in the figure legends, syringe 1 contained clathrin (0.17 μM triskelia) and auxilin (0.025–1 μM), and syringe 2 contained Hsc70 (0.5–8 μM) plus ATP (1 mM), all in buffer 2. These two solutions were rapidly mixed in the stopped-flow at a 1∶1 ratio. Light scattering was monitored every 2 ms for up to 60 s. The excitation wavelength was 365 nm.
Electron Microscopy.
Clathrin cages (0.09 μM triskelia) were premixed with auxilin (0.1 μM) and ATP (500 μM) in buffer 2, and cage disassembly was initiated by addition of Hsc70 (final concentrations of 0.1, 0.2, or 0.5 μM). At specific time points (0–15 min), samples were removed and negative-stain EM grids prepared immediately. Grids were imaged using a Jeol 2011 transmission electron microscope with LaB6 filament. Multiple images (10–15) were obtained for each grid, each from a different grid section, at a magnification of 10,000×. The number of cages per image were counted and averaged. In counting cages, we made no specific judgment as to whether a cage was complete or partial but simply counted all objects that had elements of polyhedral cage structure characteristic of clathrin assemblies.
ATPase Assay During Cage Disassembly.
To measure the ATP hydrolysis during clathrin cage disassembly, Pi production was measured using the malachite green assay as previously described. Briefly, clathrin cages (0.33 μM triskelia) were mixed with auxilin (0.35 μM) and Hsc70 (8 μM) in buffer 2 at 25 °C. The reaction was initiated by addition of ATP (50 μM). At specific time points (5–480 s) samples were removed and mixed immediately with an equal volume of malachite green solution (0.3 mM malachite green oxalate, 10 mM sodium molybdate, 0.5% Triton X-100, 0.7 M HCl), which both quenched the reaction and provided the detection of Pi. The absorbance of each sample was measured at 680 nm after 10 min, and this was converted to [Pi] from a Pi standard curve. Data was fitted to a single exponential plus steady-state curve (Eq. S2) using GraphPad Prism
Numerical Modeling.
The stopped-flow light-scattering data shown in Fig. 4 were globally fitted to a system of simultaneous first-order differential equations corresponding to the reaction mechanism in Scheme 1, using the software DYNAFIT (29, 30). The data analyzed were obtained under pseudo first-order conditions, where the [Hsc70] is in significant excess over [clathrin]. Model discrimination analysis was performed using the second-order Akaike information criterion, AICc (23, 31). Nonsymmetrical confidence intervals for model parameters were estimated using the profile-t method (32, 33). Details of model selection are shown in the accompanying SI Text.
Supplementary Material
Acknowledgments.
We thank Yvonne Vallis, Harvey McMahon, and Helen Kent for helpful advice. We also thank Matthew Hicks and Robert Freedman for useful discussions. This work was supported by New Investigator Award G0601125 from the Medical Research Council. We thank the Electron Microscopy Facility, School of Life Sciences, University of Warwick (Wellcome Trust reference 055663/Z/98/Z) for technical support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018845108/-/DCSupplemental.
References
- 1.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: Formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–556. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhausen T. Clathrin. Annu Rev Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- 4.Schmid SL. Clathrin-coated vesicle formation and protein sorting: An integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 5.Pearse BM, Robinson MS. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg E, Greene LE. Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 7.Holstein SE, Ungewickell H, Ungewickell E. Mechanism of clathrin basket dissociation: Separate functions of protein domains of the DnaJ homologue auxilin. J Cell Biol. 1996;135:925–937. doi: 10.1083/jcb.135.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungewickell E, Ungewickell H, Holstein SE. Functional interaction of the auxilin J domain with the nucleotide- and substrate-binding modules of Hsc70. J Biol Chem. 1997;272:19594–19600. doi: 10.1074/jbc.272.31.19594. [DOI] [PubMed] [Google Scholar]
- 9.Barouch W, Prasad K, Greene LE, Eisenberg E. Auxilin-induced interaction of the molecular chaperone Hsc70 with clathrin baskets. Biochemistry. 1997;36:4303–4308. doi: 10.1021/bi962727z. [DOI] [PubMed] [Google Scholar]
- 10.Jiang RF, Greener T, Barouch W, Greene L, Eisenberg E. Interaction of auxilin with the molecular chaperone, Hsc70. J Biol Chem. 1997;272:6141–6145. doi: 10.1074/jbc.272.10.6141. [DOI] [PubMed] [Google Scholar]
- 11.Gao B, Greene L, Eisenberg E. Characterization of nucleotide-free uncoating ATPase and its binding to ATP, ADP, and ATP analogues. Biochemistry. 1994;33:2048–2054. doi: 10.1021/bi00174a010. [DOI] [PubMed] [Google Scholar]
- 12.Ha JH, McKay DB. ATPase kinetics of recombinant bovine 70 kDa heat shock cognate protein and its amino-terminal ATPase domain. Biochemistry. 1994;33:14625–14635. doi: 10.1021/bi00252a031. [DOI] [PubMed] [Google Scholar]
- 13.Greene LE, Eisenberg E. Dissociation of clathrin from coated vesicles by the uncoating ATPase. J Biol Chem. 1990;265:6682–6687. [PubMed] [Google Scholar]
- 14.Schlossman DM, Schmid SL, Braell WA, Rothman JE. An enzyme that removes clathrin coats: Purification of an uncoating ATPase. J Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad K, Heuser J, Eisenberg E, Greene L. Complex formation between clathrin and uncoating ATPase. J Biol Chem. 1994;269:6931–6939. [PubMed] [Google Scholar]
- 16.Ungewickell E, et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- 17.Xing Y, et al. Structure of clathrin coat with bound Hsc70 and auxilin: Mechanism of Hsc70-facilitated disassembly. EMBO J. 2010;29:655–665. doi: 10.1038/emboj.2009.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fotin A, et al. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature. 2004;432:649–653. doi: 10.1038/nature03078. [DOI] [PubMed] [Google Scholar]
- 19.Barouch W, Prasad K, Greene LE, Eisenberg E. ATPase activity associated with the uncoating of clathrin baskets by Hsp70. J Biol Chem. 1994;269:28563–28568. [PubMed] [Google Scholar]
- 20.Prasad K, Barouch W, Greene L, Eisenberg E. A protein cofactor is required for uncoating of clathrin baskets by uncoating ATPase. J Biol Chem. 1993;268:23758–23761. [PubMed] [Google Scholar]
- 21.Jiang J, Prasad K, Lafer EM, Sousa R. Structural basis of interdomain communication in the Hsc70 chaperone. Mol Cell. 2005;20:513–524. doi: 10.1016/j.molcel.2005.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuermann JP, et al. Structure of the Hsp110∶Hsc70 nucleotide exchange machine. Mol Cell. 2008;31:232–243. doi: 10.1016/j.molcel.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myung JI, Pitt MA. Model comparison methods. Method Enzymol. 2004;383:351–366. doi: 10.1016/S0076-6879(04)83014-3. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, et al. Identification of domain required for catalytic activity of auxilin in supporting clathrin uncoating by Hsc70. J Biol Chem. 2002;277:49267–49274. doi: 10.1074/jbc.M203695200. [DOI] [PubMed] [Google Scholar]
- 25.Jiang R, Gao B, Prasad K, Greene LE, Eisenberg E. Hsc70 chaperones clathrin and primes it to interact with vesicle membranes. J Biol Chem. 2000;275:8439–8447. doi: 10.1074/jbc.275.12.8439. [DOI] [PubMed] [Google Scholar]
- 26.Ahle S, Ungewickell E. Auxilin, a newly identified clathrin-associated protein in coated vesicles from bovine brain. J Cell Biol. 1990;111:19–29. doi: 10.1083/jcb.111.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapoport I, Boll W, Yu A, Bocking T, Kirchhausen T. A motif in the clathrin heavy chain required for the Hsc70/auxilin uncoating reaction. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-09-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer MP, Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: Application to HIV proteinase. Anal Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 30.Kuzmic P. DynaFit—A software package for enzymology. Method Enzymol. 2009;467:247–280. doi: 10.1016/S0076-6879(09)67010-5. [DOI] [PubMed] [Google Scholar]
- 31.Burnham KB, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer-Verlag; 2002. [Google Scholar]
- 32.Bates DM, Watts DG. Nonlinear Regression Analysis and Its Applications. New York: Wiley; 1988. [Google Scholar]
- 33.Brooks I, Watts DG, Soneson KK, Hensley P. Determining confidence intervals for parameters derived from analysis of equilibrium analytical ultracentrifugation data. Method Enzymol. 1994;240:459–478. doi: 10.1016/s0076-6879(94)40060-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.