Abstract
Dramatically up-regulated in the dorsal horn of the mammalian spinal cord following inflammation or nerve injury, neuropeptide Y (NPY) is poised to regulate the transmission of sensory signals. We found that doxycycline-induced conditional in vivo (Npytet/tet) knockdown of NPY produced rapid, reversible, and repeatable increases in the intensity and duration of tactile and thermal hypersensitivity. Remarkably, when allowed to resolve for several weeks, behavioral hypersensitivity could be dramatically reinstated with NPY knockdown or intrathecal administration of Y1 or Y2 receptor antagonists. In addition, Y2 antagonism increased dorsal horn expression of Fos and phosphorylated form of extracellular signal-related kinase. Taken together, these data establish spinal NPY receptor systems as an endogenous braking mechanism that exerts a tonic, long-lasting, broad-spectrum inhibitory control of spinal nociceptive transmission, thus impeding the transition from acute to chronic pain. NPY and its receptors appear to be part of a mechanism whereby mammals naturally recover from the hyperalgesia associated with inflammation or nerve injury.
Keywords: allodynia, neuropathic, BIBO3304, BIIE0246
Neuropeptide Y (NPY) is a 36-aa peptide that acts at G-protein-coupled Y receptors to initiate cellular signaling. NPY systems modulate a variety of physiological processes, including somatosensation (1). Expressed in neurons and terminals of the spinal cord dorsal horn (2), and up-regulated by peripheral inflammation or nerve injury, NPY is poised to inhibit the spinal transmission of sensory signals. For example, nerve injury elicits a profound de novo synthesis of NPY in large-diameter neurons of dorsal root ganglion (DRG) and their terminal regions in the dorsal horn (3–7), perhaps serving as an adaptive compensatory response to increased excitatory signaling (8). Increases in NPY last at least 24 wk, indicating that it is temporally available to confer long-lasting inhibition of pronociceptive neurotransmission (9), but this hypothesis has not been tested.
Of the five NPY receptors cloned in the mouse, only Y1 and Y2 are expressed in the adult DRG (they colocalize with peptides that are predominantly found in unmyelinated to thinly myelinated neurons) and dorsal horn (10–12). Y1-ir is found in cells and terminals of lamina IIi, whereas Y2-ir is found in terminals of I-IIo (13–15). Numerous studies using an intrathecal delivery approach have demonstrated that exogenous delivery of NPY reduces hypersensitivity to tactile and thermal stimulation in models of chronic pain, including the spared nerve injury (SNI) model of neuropathic pain; Y1 and Y2 receptor antagonists reverse these antiallodynic actions (16–20). However, the use of receptor-selective antagonists to evaluate the intrinsic actions of NPY is uncommon, perhaps because behavioral thresholds demonstrate a floor effect in major animal models. For example, although the Y1 receptor antagonist BIBO3304 slightly but significantly increased heat hyperalgesia when administered during the peak of complete Freund's adjuvant (CFA) inflammation (2 d), substantially low baseline thresholds (associated with powerful sensitization mechanisms) precluded a large effect (16).
To determine the contribution of endogenous NPY signaling to chronic pain, our studies used two complementary approaches. First, we used conditional deletion to down-regulate NPY expression. The Npytet/tet transgenic mouse contains a doxycycline (Dox)-regulated cassette at the Npy locus. Dox administration in drinking water dramatically decreased NPY in the brain of these adult Npytet/tet mice (21), but effects on pain behavior and NPY expression in ascending pain pathways have not been determined. Second, we used the intrathecal route of administration to deliver NPY receptor antagonists to the dorsal horn of the spinal cord. We avoided floor effects by using relatively mild models of inflammatory (low volumes of intraplantar CFA) and neuropathic pain (a modified spared nerve injury model involving transection of the common peroneal and sural nerves) that yield submaximal hyperalgesia, and by testing behavioral endpoints after hyperalgesia had largely subsided.
Results
Dox Depletes NPY in DRG and Spinal Cord of Npytet/tet Mice.
Original studies in the Npytet/tet mouse demonstrated that Dox dramatically reduced NPY expression in the paraventricular and arcuate nuclei of the hypothalamus, hippocampus, and amygdala (21). Here we extend these findings to spinal cord and sensory neurons. As expected, the DRG of Npytet/tet did not express NPY. When measured on the 14th day after SNI surgery, NPY-ir was up-regulated in ipsilateral but not contralateral dorsal horn (Fig. 1 A and B), and de novo expression of NPY-ir was induced in ipsilateral L4/L5 DRG (Fig. 1D). Such results have led to the hypothesis that inflammatory or sensory nerve injury increases NPY signaling at the dorsal horn, thereby inhibiting pronociceptive neurotransmission (20). Continuous exposure to Dox (in the drinking water), begun 14 d after SNI, essentially silenced NPY expression in dorsal horn (P < 0.01) (Fig. 1C) and DRG (Fig. 1E). Fig. 1F illustrates the time course and quantification of NPY depletion in DRG: NPY-ir was significantly decreased within 2 d, was barely detectable at 7 d, and was essentially silenced by 14 d [F(5,17) = 24.49, P < 0.0001, one-way ANOVA].
Fig. 1.
Dox reduces NPY-ir in DRG and dorsal horn of Npytet/tet mice and potentiates behavioral signs of neuropathic and inflammatory pain. Representative photomicrographs of NPY immunoreactivity in dorsal horn or DRG contralateral (A) or ipsilateral to SNI surgery in the absence (B and D) or presence of Dox (C and E). Yellow boxes denote the time periods when mice received Dox in the drinking water (see Methods for additional details). Out of a total of 471 profiles in the DRG water groups, 29 were found to be NPY-positive, and Dox time-dependently eliminated this NPY-ir in DRG (F) (n = 4–5 per time point). When initiated before SNI, conditional NPY knockdown increased cold allodynia (G), and extended the duration of tactile allodynia (H) (n = 4 per group).When initiated after SNI, conditional NPY knockdown increased cold (I) and mechanical (J) allodynia in a reversible and repeatable manner (n = 3–4 per group). When initiated after CFA, conditional NPY knockdown increased the magnitude and duration of tactile (K) and heat hypersensitivity (L) in a reversible and repeatable manner (Day of CFA injection = Day 0; n = 8 per group). When administered during the second period of NPY knockdown in K (52 d after induction of inflammation), intrathecal NPY but not vehicle attenuated mechanical hypersensitivity (K). NPY had no effect in Npytet/tet animals given regular water. Values represent mean ± SEM. ★P < 0.05 vs. controls given drinking water without Dox. See Fig. S1 for summary of other behaviors analyzed in NPYtet/tet mice, and Fig. S4 for summary of behaviors in wild-type mice during Dox and/or saccharin.
Conditional NPY Knockdown Increases Thermal and Tactile Hypersensitivity.
In control littermate Npytet/tet mice, SNI increased responsiveness to cold (response duration from ∼2–7 s) and mechanical stimulation (threshold from ∼2.5 to less than 0.01 g), the latter beginning to resolve within 6 wk. To test the hypothesis that endogenous NPY contributes to the development of cold and tactile hypersensitivity, Dox was initiated 10 d before SNI, and then continued for 110 d. Dox changed neither body weight [as observed previously (21)], baseline sensory thresholds (Fig. S1 A–C), nor motor coordination or ambulatory activity in an open field (Fig. S1 D–F). Importantly, NPY knockdown dramatically increased cold hyperresponsiveness [F(1,78) = 90, P < 0.0001] (Fig. 1G) and extended the duration of mechanical hypersensitivity by at least 8 wk [F(12,78) = 1.913, P < 0.05, group × time interaction] (Fig. 1H). Any potential actions of NPY depletion on tactile sensitivity during the first 42 d after SNI were likely masked by a floor effect.
To test the hypothesis that endogenous NPY tonically inhibits neuropathic pain, we depleted NPY after the establishment of nerve injury-induced hypersensitivity. Fig. 1 I and J illustrate that Dox dramatically reinstated cold and mechanical allodynia in a reversible and repeatable manner when delivered at 14 to 28 d after SNI [F(1,24) = 109.9, P < 0.0001 for cold; F(1,24) = 25.6, P < 0.0001 for mechanical], 66 to 71 d after SNI [F(1,25) = 35.3, P < 0.0001 for cold; F(1,25) = 54.8, P < 0.0001 for mechanical], or 95 to 105 d after SNI [F(1,25) = 51.3, P < 0.0001 for cold; F(1,25) = 188.8, P < 0.0001 for mechanical].
To determine the contribution of endogenous NPY signaling to inflammatory pain, we examined Npytet/tet mice in the CFA model. Fig. 1 K and L illustrate that Dox reinstated mechanical allodynia [F(1,224) = 113.8, P < 0.0001] and heat hyperalgesia [F(1,224) = 70.1, P < 0.0001]. This hypersensitivity dissipated upon cessation of Dox (P > 0.05), indicating reversibility. Reintroduction of Dox again increased mechanical allodynia [F(1,96) = 98.5, P < 0.0001] and heat hyperalgesia [F(1,96) = 31.4, P < 0.0001], indicating repeatability. To determine if this effect could be reversed, we administered NPY or vehicle to the intrathecal space during the second bout of Dox or regular water. Fig. 1K illustrates that intrathecal NPY reversed mechanical hypersensitivity [F(1,70) = 25, P < 0.0001], but did not change mechanical thresholds in control animals (P > 0.05).
NPY Receptor Antagonists Reinstate Neuropathic and Inflammatory Pain.
Our conditional knockdown approach provided target specificity but not spatial resolution. To test the hypothesis that the spinal cord is a site of endogenous NPY inhibition of chronic pain, we next delivered Y1 and Y2 receptor antagonists to the intrathecal space of C57BL/6 mice after nerve injury or CFA. To avoid the floor effect in tactile thresholds associated with the L5 spinal nerve ligation model of neuropathic pain, Raja and colleagues recently developed a partial ligation variant (22). This variant elicited shorter-lasting and less robust tactile hypersensitivity, thus allowing assessment of drug-induced increases in sensitivity. Along these lines, we cut only the two smaller primary branches of the sciatic nerve (common peroneal and sural transection, CpxSx). Compared with the SNI, CpxSx produced a relatively short-lasting decrease in tactile threshold that largely resolved within 28 d. This approach allowed us to bypass floor effects and administer receptor antagonists that we hypothesized would increase tactile hypersensitivity. Indeed, when administered 28 d after CpxSx, intrathecal administration of the Y1 antagonist BIBO3304 [F(3,133) = 19.74, P < 0.0001] (Fig. 2A) or the Y2 antagonist BIIE0246 [F(2,189) = 23.42, P < 0.0001] (Fig. 2B), but not vehicle, dose-dependently reinstated tactile hypersensitivity. Neither BIBO nor BIIE changed thresholds in sham-treated control mice without CpxSx, indicating that NPY antagonists do not elicit hypersensitivity in the absence of nerve injury (P > 0.05) (Fig. S2 A and C). When administered after the resolution of CFA-induced hypersensitivity, intrathecal BIBO3304 dose-dependently increased tactile [F(3,217) = 61.11, P < 0.0001] (Fig. 2C) and heat hypersensitivity [F(2,203) = 28.14, P < 0.0001] (Fig. 2E), respectively. Similarly, BIIE0246 dose-dependently increased tactile hypersensitivity [F(3,210) = 29.17, P < 0.0001] (Fig. 2D) and slightly increased heat hypersensitivity (P < 0.05) (Fig. 2F). Neither BIBO nor BIIE changed thresholds in sham-treated control mice, indicating that NPY antagonists do not elicit hypersensitivity in the absence of inflammation (P > 0.05) (Fig. S2 B and D).
Fig. 2.
NPY receptor antagonists reinstate neuropathic and inflammatory pain. Twenty-one to twenty-eight days after CPxSx surgery (A and B), or 9–14 d after intraplantar CFA (C, D, E, and F), the Y1 antagonist BIBO3304 (A, C, and E) or the Y2 antagonist BIIE0246 (B, D, and F) were intrathecally administered in C57BL/6 mice. Both compounds decreased tactile (A–D) and heat (E and F) thresholds. n = 5–22. Values represent mean ± SEM. ★P < 0.05 (for high dose compared with vehicle); *P < 0.05 (for medium dose compared with vehicle); +P < 0.05 (for low dose compared with vehicle). See Fig. S2 online for results in Sham control mice.
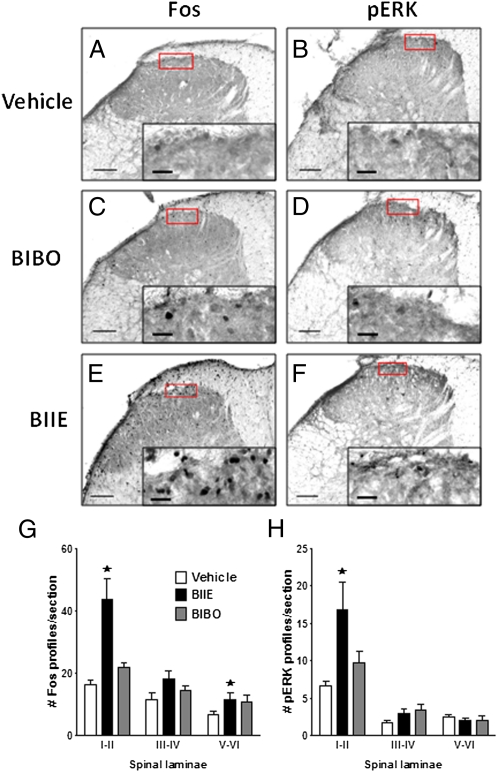
To test the hypothesis that the mechanism of NPY receptor antagonist-induced reinstatement of allodynia is associated with the activation of neuronal gene transcription and spinal cord central sensitization, we evaluated the protein expression of Fos and the phosphorylated form of extracellular signal-related kinase (p-ERK) in the dorsal horn (17, 23). Fig. 3 indicates that, compared with vehicle, BIIE0246 increased the stimulus-evoked expression of Fos-like (P < 0.05) and p-ERK (P < 0.05) immunoreactivity. This occurred in the absence of any changes in spinal p-p38 immunoreactivity (P > 0.05), suggesting that the increases in Fos and p-ERK are not secondary to a nonspecific global induction of gene expression.
Fig. 3.
The Y2 antagonist BIIE increased Fos and p-ERK in dorsal horn. Fourteen days after intraplantar CFA, vehicle (A and B), Y1 antagonist (C and D), or Y2 antagonist (E and F) was intrathecally-administered, and then dorsal horn sections of segments L4-L5 were stained with primary antibodies against Fos (A, C, E, and G) and p-ERK (B, D, F, and H). A to F are representative photomicrographs of the data summarized in panels G and H. The higher magnification image at the lower right inset of each image represents the area of the lower power image denoted by the red rectangle. (Scale bars: main image, 20 μm; Inset, 100 μm.) BIIE increased both Fos (in lamina I-II and V-VI) and p-ERK (lamina I-II) on the side ipsilateral to inflammation. n = 7–11 per group. Values represent mean ± SEM. ★P < 0.05 vs. vehicle.
Discussion
Despite the early discovery that injury dramatically up-regulates NPY expression along ascending pain pathways (24), and more recent studies indicating that ligand-induced activation of Y1 or Y2 receptors decreases behavioral signs of neuropathic and inflammatory pain (25), the contribution of endogenous NPY to pain inhibition has been difficult to establish. Although germ-line NPY deletion slightly increased autotomy behavior after complete sciatic nerve transection (26), we were unable to mimic such differences with partial nerve-injury models: germ-line NPY knockout mice exhibit normal signs of persistent pain (Fig. S3). One explanation is that compensatory changes during embryonic development are engaged in the chronic absence of NPY. For example, depletion of NPY in null mice could be associated with an up-regulation of NPY receptors (27) or other pain inhibitory circuits that do not depend on NPY. Such compensation during development may mask any effects of NPY deletion on pain behavior. To circumvent this, we used a conditional knockdown approach (21). When initiated before tissue or nerve injury, knockdown increased the intensity of thermal hypersensitivity and the duration of tactile hypersensitivity. Whether this reflects intrinsic NPY-mediated attenuation of the development of hypersensitivity is unclear, in part because NPY expression studies have not reported changes before 3 or 14 d after inflammation (7) or nerve injury (3, 8, 9, 28), respectively. When initiated after injury, knockdown produced a rapid, reversible, and repeatable increase in hypersensitivity (Fig. 1), indicating that NPY tonically inhibits the maintenance of persistent pain.
Our conditional knockdown approach decreases NPY expression in a global manner (21). This lack of spatial resolution prevents discrimination of pain-modulatory actions of NPY at the periphery, spinal cord, and various regions of the brain. To test the hypothesis that hypersensitivity after genetic knockdown results from loss of NPY receptor signaling in the dorsal horn, we used a pharmacological approach, based on a strategy used to establish the antiallodynic actions of exogenously administered NPY receptor agonists (17). To avoid floor effects associated with powerful sensitization during the early periods after CFA or nerve injury, we allowed behavioral signs of pain to regress and resolve. At this time, intrathecal administration of the receptor antagonists BIBO3304 and BIIE0246 dose-dependently reinstated tactile and thermal hypersensitivity in models of both neuropathic and inflammatory pain (Fig. 2), and the latter increased the expression of Fos and p-ERK, indicating activation of neurons and perhaps mechanisms of central sensitization in the dorsal horn (17, 23) (Fig. 3). Taken together, our findings suggest that NPY receptor signaling exerts a tonic, long-lasting, broad-spectrum inhibitory control of injury-induced allodynia and spinal gene expression, thus limiting the progression of chronic pain. We conclude that NPY and its receptors appear to be part of a mechanism whereby mammals naturally recover from the hyperalgesia associated with inflammation or nerve damage.
Microinjection of NPY into the rostral ventral medulla, a key site of descending inhibitory pain control, also reduces signs of allodynia and hyperalgesia following spared nerve injury (29). Indeed, NPY acts at numerous brain regions to inhibit nociception, including the periaqueductal gray, nucleus accumbens, and arcuate nucleus (30–33). In contrast, Ossipov et al. reported that microinjection of BIBO3304 into the nucleus gracilis reversed the mechanical allodynia associated with ligation of L5/L6 spinal nerves, and concluded that NPY is pronociceptive at the dorsal column nuclei of the somatosensory system (34). Thus, the direction of NPY action depends on the site of administration. Our results with conditional NPY mutants, however, suggest that the net global effect of NPY on neuropathic pain is inhibitory. We conclude that strategies designed to enhance spinal NPY receptor signaling could yield an important previously unexplored avenue for the treatment of inflammatory or neuropathic pain.
The majority of studies designed to understand the transition from acute to chronic pain focus on mechanisms of central sensitization, such as increases in the spinal cord expression and activity of voltage- and ligand-gated ion channels, peptide receptors, and neuro-immune factors that then drive dorsal horn neuron hyperexcitability (25). The intensity and duration of this central sensitization is determined by the net activity of local excitatory and inhibitory neurotransmitter systems, together with ongoing/evoked primary afferent activity and descending supraspinal control (20, 25). Spinal endogenous inhibitory systems serve as opposing compensatory influences, and the current study is indicative of the powerful capacity of the NPY receptor system to restrain allodynia and hyperalgesia for extended time periods. Our findings indicate that conditional NPY knockdown or spinal receptor blockade dramatically, repeatedly, and reversibly increases behavioral signs of neuropathic and inflammatory pain. Remarkably, if the original signs of hypersensitivity were allowed to resolve over several weeks or even months after the induction of a tissue or nerve injury, hypersensitivity could be rapidly reinstated by interrupting spinal NPY receptor function. Such reinstatement suggests that endogenous NPY masks a “silent sensitization” of excitatory systems that persists long after tissue or nerve injury, even in the absence of overt allodynia or hyperalgesia. Our approach, involving genetic or pharmacological manipulations performed long after behavioral manifestations of injury-induced pain have subsided, point to the importance of evaluating behavior for extended periods of time. A similar approach can be used to determine whether disruption of other pain inhibitory systems in the dorsal horn (e.g., μ- and δ-opioid, α2-adrenergic, purinergic A1, cannabinoid CB1 and CB2, muscarinic M2, GABAB, metabotropic glutamate type II-III, somatostatin) will reinstate signs of chronic pain. Such experiments will provide important clues as to whether defects in the compensatory up-regulation of inhibitory systems, including but not restricted to NPY, may contribute to the long-term maintenance of inflammatory or neuropathic pain. We conclude that our understanding of the maintenance of chronic pain will require that greater emphasis be placed on mechanisms involving an extended loss of inhibitory neurotransmission in the dorsal horn, thus leading to pharmacotherapeutic strategies that mimic and enhance these systems.
Methods
Animals.
Mice were housed four to five to a cage on a 12-h light/dark cycle (6:00 AM/6:00 PM) and were given food and water ad libitum. Richard Palmiter (University of Washington, Seattle, WA) kindly provided heterozygous breeding pairs of NPY null (35), and Npytet/tet mice (21). Speed congenics were used to generate Npytet/tet on a C57BL/6 background. Male homozygous and wild-type transgenic mice (bred in our facility) or commercial male C57BL/6 mice (Charles Rivers) weighed 20 to 30 g or 18 to 20 g at the time of nerve injury or inflammation, respectively. All animal use protocols were approved by the Institutional Animal Care and Use Committees of both Tulane University and the University of Kentucky.
Animal Models of Persistent Pain.
Peripheral nerve injury.
Anesthesia was induced and maintained throughout surgery with isoflurane (4% for induction, then 1.5% maintenance in oxygen). As previously described (36), the common peroneal and tibial nerves were ligated with 6–0 silk (Ethicon); the knot and adjacent nerve (1–2 mm) were then transected, sparing the sural nerve (SNI). Alternatively, as described by Shields et al. (37), the common peroneal and sural nerves were ligated and transected (CpxSx). Further methodological details are provided in SI Methods.
Persistent inflammation.
Five microliters of CFA was subcutaneously injected into the midplantar region of the left hindpaw. Sham CFA involved intraplantar placement of the 30-gauge needle for 10 s.
Dox Administration to Npytet/tet Mice.
Protocols.
Npytet/tet mice were provided drinking water with saccharin (5 mM; Sigma) or saccharin + Dox hyclate (2 mg/mL, prepared fresh every other day, D9891; Sigma) as follows: Dox begun 14 d after SNI, and then killed 1, 2, 5, 7, or 14 d later (Fig. 1 A–F); from 10 d before SNI until 100 d after SNI (Fig. 1 G and H); from 14 to 28, 67 to 71, and 96 to 105 d after SNI (Fig. 1 I and J); from 10 d before CFA until 28 d after CFA, and then again from 42 to 52 d after CFA (Fig. 1 K and L) administration.
Saccharin.
Because Dox is bitter, saccharin was added to enhance palatability of Dox. Because of several reports of sweet substance-induced analgesia in rats [although this appears to be limited to infant rats (38)], we determined the effect of saccharin on behavioral responses to tactile and thermal stimuli in wild-type mice after SNI. Delivery of Dox or saccharin in the drinking water did not change the development or intensity of behavioral signs of mechanical or cold allodynia following either SNI or CFA administration (P > 0.05) (Fig. S4).
Intrathecal Drug Administration.
As first described in lightly anesthetized mice (39), and then adapted to awake animals (40), drugs were administered by direct lumbar puncture in a total volume of 5 μL. Each animal was injected twice using a cross-over design, with 4 to 7 d separating the first injection of vehicle or drug from the second injection of drug or vehicle, respectively. Groups given a particular dose of a drug (or vehicle) on the first vs. the second injection did not differ, and so were combined for analysis. Antagonist injections of BIBO3304 (gift from Boehringer-Ingelheim) or BIIE0246 (Tocris) were performed 21 to 28 d after CpxSx surgery and 9 to 14 d after CFA injection. Further methodological details are provided in SI Methods.
Behavioral Testing.
All animals were acclimated to a stainless steel grid within individual Plexiglas tubes for 30 to 60 min. Next, a single experimenter (B.S.) evaluated mechanical allodynia, followed by cold allodynia (SNI) or heat hyperalgesia (CFA). This experimenter was blinded to Dox or drug treatment by another experimentor (R.D. or G.C.). Further methodological details of behavioral testing of mechanical threshold, responses to heat and cold, ataxia, and locomotor activity are provided in the SI Methods.
Mechanical threshold.
Tactile responsivity was assessed with an incremental series of eight von Frey monofilaments of logarithmic stiffness (Stoelting, Inc.; ∼0.008–6.0 g). The 50% withdrawal threshold was determined using the up-down method, modified by Chaplan et al. (41).
Response to cool stimulation.
Using a syringe connected to PE-90 tubing, flared at the tip to a diameter of 2 mm, a drop of acetone was carefully applied to the plantar paw. Surface tension maintained a drop volume of 10 to 12 μL. The duration of paw withdrawal was recorded with a 45-s cutoff.
Response to heat stimulation.
The thermal stimulus consisted of a radiant heat source (8 V, 50 W lamp; Ugo Basile) positioned under the glass floor directly beneath the hindpaw. The stimulus was applied to the ipsilateral hindpaw and the latency to paw withdrawal was recorded.
Immunohistochemistry.
Animals were deeply anesthetized with an intraperitoneal overdose of ketamine:xylazine injection [1 mL/kg of 88.9 mg/mL ketamine (Vedco); 11.1 mg/mL xylazine (Henry Schein)], and perfused with PBS followed by 10% buffered formalin. DRG and spinal cords (L4 and L5) were postfixed in 10% formalin, cryoprotected in 30% sucrose, and sectioned at 20 μm (DRG) or 40 μm (spinal cord).
Fluorescence.
Sections were washed, blocked in 3% NDST, and incubated overnight in primary NPY antiserum, rabbit polyclonal (Peninsula Laboratories) 1:5,000 (DRG) or 1:1,000 (spinal cord). Sections were washed and blocked again, incubated in Alexa 488-conjugated donkey anti-rabbit antibody (1:700; Invitrogen) for 2 h at room temperature, washed again, mounted, and cover-slipped.
Peroxidase.
Sections were washed, blocked, and incubated overnight with primary antiserum directed against Fos, 1:20,000 (Calbiochem), p-ERK, 1:500 (Cell Signaling), or p-p38, 1:150 (Cell Signaling). Tissue was washed, incubated in biotinylated goat anti-rabbit or goat anti-mouse 2° antibody (1:200; Jackson ImmunoResearch Laboratories), washed again, exposed to avidin-biotin-peroxidase complex for 1 h at room temperature, and then 0.05% diaminobenzidine, washed again, mounted, and cover-slipped. For further details of the immunohistochemistry, see SI Methods.
Image Acquisition and Quantification.
NPY.
Digital photomicrographs of at least five randomly selected sections were taken from both L4 and L5 DRG. To standardize exposure time on a Nikon Eclipse TE2000-E fitted with a 10× objective, we used the “autoexpose” function of NIH Image J on three stained photomicrographs; the average exposure time was applied to all further image captures. Only cells with a visible nucleus were analyzed. Background luminosity was determined by averaging the maximum luminosity of five clearly unlabeled cells. Only cells with luminosity at least twice the background luminosity were counted as labeled cells. To ensure that cells were not counted more than once, distance between each section was at least 144 μm. Quantitative values are given in terms of NPY-positive profiles per total number of profiles for any given slice and averaged for each group.
Fos, p-ERK, and p-p38.
Dorsal horn images of segments L4-L5 were captured with a 10× objective on a Nikon Eclipse TE2000-E microscope, using Elements 3.1 software. After selecting a rectangular region of interest within an unstained region of the dorsal horn, luminosity was adjusted to yield a normalized grayscale background intensity reading. Bezier regions of interests were drawn to outline lamina I-II, III-IV, or V-VI. For computer-assisted quantification of Object Counts, “perimeter” was set to 12 to 50, and “circularity” was set to 0.7 to 1.0, followed by binary thresholding. Pilot studies demonstrated that automated counting yielded equivalent estimates of staining compared with manual counting by a blinded observer. Counts from at least five randomly selected sections were averaged for each animal.
Statistics.
Behavior.
To determine dose-/treatment-response relationships, data were analyzed by the two-way ANOVA (GraphPad Prizm 5) with dose/treatment/strain as the between-subjects factor and time as the repeated measure, and the F-values reported in Results. If the resulting F-value was significant (P < 0.05), then post hoc t tests with Bonferroni correction were performed to obtain individual comparisons at each time point, and significant differences of each dose/treatment compared with control are denoted in figures. All data are presented as mean ± SEM.
Immunohistochemisty.
Data were analyzed by two-way ANOVA. If the effects of an individual variable proved significant, these analyses were followed by post hoc t tests with Bonferroni correction. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Richard Palmiter for the generous donation of Npytet/tet mouse breeders, both R. Denis Soignier and Cavin Ward-Caviness for help with removal of nervous system tissue, and Brian Eisenstadt for assistance with sectioning of the dorsal root ganglion. This work was supported by National Institutes of Health Grants R01NS45954 and K02DA19656 (to B.T.), and Tulane post-Katrina Research Enhancement Funds’ a Ruth Kirshstein National Research Service Award Fellowship F31 NS057928 (to B.S.).
Footnotes
The authors declare no conflict of interest.
Portions of the transgenic studies were presented in abstract form at the National Institutes of Health Pain Consortium 4th Annual Symposium on Advances in Pain Research, Natcher Conference Center, Bethesda, MD, May 26, 2009 and at the annual meeting of the American Pain Society, Washington, DC, May 5–7, 2007.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017719108/-/DCSupplemental.
References
- 1.Hökfelt T, et al. Neuropeptide Y: Some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26(2-3):154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 2.Gibson SJ, et al. The distribution and origin of a novel brain peptide, neuropeptide Y, in the spinal cord of several mammals. J Comp Neurol. 1984;227(1):78–91. doi: 10.1002/cne.902270109. [DOI] [PubMed] [Google Scholar]
- 3.Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Wiesenfeld-Hallin Z, Hökfelt T. Effect of peripheral axotomy on expression of neuropeptide Y receptor mRNA in rat lumbar dorsal root ganglia. Eur J Neurosci. 1994;6(1):43–57. doi: 10.1111/j.1460-9568.1994.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 5.Brumovsky PR, Bergman E, Liu HX, Hökfelt T, Villar MJ. Effect of a graded single constriction of the rat sciatic nerve on pain behavior and expression of immunoreactive NPY and NPY Y1 receptor in DRG neurons and spinal cord. Brain Res. 2004;1006(1):87–99. doi: 10.1016/j.brainres.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 6.Wakisaka S, Kajander KC, Bennett GJ. Effects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neurons. Brain Res. 1992;598:349–352. doi: 10.1016/0006-8993(92)90206-o. [DOI] [PubMed] [Google Scholar]
- 7.Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hökfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J Neurosci. 1994;14:6423–6434. doi: 10.1523/JNEUROSCI.14-11-06423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munglani R, et al. Changes in neuronal markers in a mononeuropathic rat model relationship between neuropeptide Y, pre-emptive drug treatment and long-term mechanical hyperalgesia. Pain. 1995;63(1):21–31. doi: 10.1016/0304-3959(95)00013-I. [DOI] [PubMed] [Google Scholar]
- 9.Intondi AB, Zadina JE, Zhang X, Taylor BK. Topography and time course of changes in spinal neuropeptide Y immunoreactivity after spared nerve injury. Neuroscience. 2010;165:914–922. doi: 10.1016/j.neuroscience.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hokfelt T, et al. Phenotypic regulation in dorsal root ganglion neurons after nerve injury: focus on peptides and their receptors. In: Borsook D, editor. Molecular Neurobiology of Pain. Vol. 9. Seattle: IASP Press; 1997. pp. 115–143. [Google Scholar]
- 11.Wahlestedt C, Reis DJ. Neuropeptide Y-related peptides and their receptors—Are the receptors potential therapeutic drug targets? Annu Rev Pharmacol Toxicol. 1993;33:309–352. doi: 10.1146/annurev.pa.33.040193.001521. [DOI] [PubMed] [Google Scholar]
- 12.Brumovsky P, Shi TS, Landry M, Villar MJ, Hökfelt T. Neuropeptide tyrosine and pain. Trends Pharmacol Sci. 2007;28(3):93–102. doi: 10.1016/j.tips.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Kopp J, et al. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 14.Brumovsky P, et al. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J Comp Neurol. 2005;489:328–348. doi: 10.1002/cne.20639. [DOI] [PubMed] [Google Scholar]
- 15.Brumovsky P, et al. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience. 2006;138:1361–1376. doi: 10.1016/j.neuroscience.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 16.Taiwo OB, Taylor BK. Antihyperalgesic effects of intrathecal neuropeptide Y during inflammation are mediated by Y1 receptors. Pain. 2002;96:353–363. doi: 10.1016/S0304-3959(01)00481-X. [DOI] [PubMed] [Google Scholar]
- 17.Intondi AB, Dahlgren MN, Eilers MA, Taylor BK. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain. 2008;137:352–365. doi: 10.1016/j.pain.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuphal KE, Solway B, Pedrazzini T, Taylor BK. Y1 receptor knockout increases nociception and prevents the anti-allodynic actions of NPY. Nutrition. 2008;24:885–891. doi: 10.1016/j.nut.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahinda TB, Taylor BK. Intrathecal neuropeptide Y inhibits behavioral and cardiovascular responses to noxious inflammatory stimuli in awake rats. Physiol Behav. 2004;80:703–711. doi: 10.1016/j.physbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides. 2007;28:464–474. doi: 10.1016/j.peptides.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Ste Marie L, Luquet S, Cole TB, Palmiter RD. Modulation of neuropeptide Y expression in adult mice does not affect feeding. Proc Natl Acad Sci USA. 2005;102:18632–18637. doi: 10.1073/pnas.0509240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan Y, Yuan F, Carteret AF, Raja SN. A partial L5 spinal nerve ligation induces a limited prolongation of mechanical allodynia in rats: An efficient model for studying mechanisms of neuropathic pain. Neurosci Lett. 2010;471(1):43–47. doi: 10.1016/j.neulet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–17. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hökfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;17(1):22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 25.Taylor BK. Spinal inhibitory neurotransmission in neuropathic pain. Curr Pain Headache Rep. 2009;13:208–214. doi: 10.1007/s11916-009-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi TJ, et al. Effect of peripheral axotomy on dorsal root ganglion neuron phenotype and autonomy behaviour in neuropeptide Y-deficient mice. Regul Pept. 1998;75-76:161–173. doi: 10.1016/s0167-0115(98)00064-0. [DOI] [PubMed] [Google Scholar]
- 27.Gehlert DR, Shaw JL. Increased brain neuropeptide Y1 and Y2 receptor binding in NPY knock out mice does not result in increased receptor function. Peptides. 2007;28:241–249. doi: 10.1016/j.peptides.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Ma W, Bisby MA. Partial and complete sciatic nerve injuries induce similar increases of neuropeptide Y and vasoactive intestinal peptide immunoreactivities in primary sensory neurons and their central projections. Neuroscience. 1998;86:1217–1234. doi: 10.1016/s0306-4522(98)00068-2. [DOI] [PubMed] [Google Scholar]
- 29.Taylor BK, et al. Neuropeptide Y acts at Y1 receptors in the rostral ventral medulla to inhibit neuropathic pain. Pain. 2007;131(1-2):83–95. doi: 10.1016/j.pain.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Li JJ, Yu LC. Anti-nociceptive effect of neuropeptide Y in the nucleus accumbens of rats: An involvement of opioid receptors in the effect. Brain Res. 2002;940:69–78. doi: 10.1016/s0006-8993(02)02594-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang JZ, Lundeberg T, Yu L. Antinociceptive effects induced by intra-periaqueductal grey administration of neuropeptide Y in rats. Brain Res. 2000;859:361–363. doi: 10.1016/s0006-8993(99)02408-7. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Lundeberg T, Yu L. Involvement of neuropeptide Y and Y1 receptor in antinociception in nucleus raphe magnus of rats. Regul Pept. 2000;95(1-3):109–113. doi: 10.1016/s0167-0115(00)00165-8. [DOI] [PubMed] [Google Scholar]
- 33.Li JJ, Zhou X, Yu LC. Involvement of neuropeptide Y and Y1 receptor in antinociception in the arcuate nucleus of hypothalamus, an immunohistochemical and pharmacological study in intact rats and rats with inflammation. Pain. 2005;118:232–242. doi: 10.1016/j.pain.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Ossipov MH, et al. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22:9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 36.Bourquin AF, et al. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006 May;122(1-2):14e1–e14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 37.Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: A behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 38.Anseloni VC, et al. Age-dependency of analgesia elicited by intraoral sucrose in acute and persistent pain models. Pain. 2002;97(1-2):93–103. doi: 10.1016/s0304-3959(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 39.Hylden JL, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 40.Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 41.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.