Abstract
Structurally diverse libraries of novel small molecules represent important sources of biologically active agents. In this paper we report the development of a diversity-oriented synthesis strategy for the generation of diverse small molecules based around a common macrocyclic peptidomimetic framework, containing structural motifs present in many naturally occurring bioactive compounds. Macrocyclic peptidomimetics are largely underrepresented in current small-molecule screening collections owing primarily to synthetic intractability; thus novel molecules based around these structures represent targets of significant interest, both from a biological and a synthetic perspective. In a proof-of-concept study, the synthesis of a library of 14 such compounds was achieved. Analysis of chemical space coverage confirmed that the compound structures indeed occupy underrepresented areas of chemistry in screening collections. Crucial to the success of this approach was the development of novel methodologies for the macrocyclic ring closure of chiral α-azido acids and for the synthesis of diketopiperazines using solid-supported N methylmorpholine. Owing to their robust and flexible natures, it is envisaged that both new methodologies will prove to be valuable in a wider synthetic context.
Keywords: cheminformatics, macrocycles, natural products
The screening of libraries of small organic molecules (so-called small molecules) to identify useful modulators of biological systems is fundamental in the drug-discovery process and chemical biology studies in general (1–6). However, a crucial consideration is what compounds to use (6–9). In “unbiased” screening processes, where the precise nature of the biological target is unknown (for example, in a random drug-discovery screen), the selection criteria for small molecules is dramatically complicated by the absence of any existing structural guidelines (1, 6, 7, 9, 10). In such situations, it is not possible to define a priori the structural features that are required in the small molecules for them to be able to affect the biological system. This problem is also encountered when structurally unique modulators of a given biological system are desired (e.g., molecules that are structurally distinct from existing ligands).
It has been argued that the greater the area of total bioactive chemical space (i.e., the chemical space defined by all bioactive molecules) covered by a given small-molecule library, the higher the probability there is of identifying a compound with the desired properties in any screening experiment involving that library (6, 8, 9, 11, 12). Thus, in general, the identification of biologically active small molecules may be aided by screening functionally diverse compounds libraries (9).
Nature “sees” molecules as 3D surfaces of chemical information. Therefore the biological activity of any given molecule is intrinsically dependent upon its 3D shape (6, 13, 14). It has therefore been argued that the molecular shape diversity of a small-molecule library is the most fundamental indicator of overall functional diversity; indeed, substantial “shape space” coverage has been correlated with broad biological activity (12). In addition, conformational flexibility does contribute to shape diversity in this context, and this contribution is likely to be considerable in the flexible macrocycles discussed later in this work. Because the overall shape adopted by a given molecule is intrinsically linked to its structure, the overall functional diversity of a small-molecule library can be correlated (to some extent) with its overall structural diversity (which in turn is proportional to the amount of chemical space the library occupies) (6, 8, 9, 11). In the context of biological screening, the “ideal” library is one of such high structural (and thus functional) diversity that, for any aspect of any biological process, there exists a library compound that is able to modulate that aspect (8, 9, 15). Although the term “diversity” is somewhat subjective, there are four principle components of structural diversity that have been consistently identified in the literature (8, 9):
Appendage diversity (or building-block diversity)—variation in structural moieties around a common scaffold;
Functional group diversity—variation in the functional groups present;
Stereochemical diversity—variation in the orientation of potential macromolecule-interacting elements;
Scaffold diversity—presence of a range of distinct molecular scaffolds.
It has been demonstrated that the overall molecular shape diversity of a small-molecule library is mainly dependent upon the nature of molecular scaffolds present, with the peripheral substituents being of minor importance (12). Indeed, there is a widespread consensus that increasing the scaffold diversity in a small-molecule library is one of the most effective ways to increase its overall structural diversity (6, 8, 14, 16, 17). Scaffold diversity is thus intrinsically linked to shape, and thus functional, diversity. Structural complexity is another desirable feature in small-molecule libraries (6, 8, 18). It is generally considered that the more structurally complex a molecule, the more likely it is to interact with a biological macromolecule in a selective and specific manner (6–8, 19–22).
Traditionally, nature has served as a rich source of biologically active molecules (23–25). Natural products exhibit enormous structural diversity (26); however, there are well-documented problems associated with their use in screening experiments (6, 9). These difficulties have spurred the development of synthetic strategies for the de novo creation of small-molecule collections (6). However, the choice of what molecules to make is of paramount concern. Achieving a large coverage of chemical space simply by a random synthesis of compounds is not practicable or desirable; it is widely accepted that it is not synthetically feasible to produce all theoretically stable, small carbon-based molecules (6, 10, 20), and making and screening molecules cost, both in terms of time and money (6–8). The ideal synthesis of a diverse small-molecule library is one in which diversity is achieved in the most efficient manner possible; this presents a formidable challenge to the synthetic chemist (6). Diversity-oriented synthesis (DOS) is an approach toward small-molecule library synthesis, which seeks to achieve this goal (9, 10, 13, 27).
The overall aim of a DOS is to efficiently generate a library of small molecules with a high degree of structural, and thus functional, diversity that interrogates large areas of chemical space (9, 28). This includes known bioactive chemical space as, by definition, this is a fruitful region for the discovery of biologically active agents and regions of chemical space that are not accessed by existing compound collections; these areas may contain structurally unique molecules with exciting and unusual biological properties (8). In principle, the screening of such libraries should provide hits against a broad range of biological targets with increased frequency and decreased cost (9). This includes so-called “unduggable” targets and processes that have traditionally been seen as difficult or even impossible to modulate with small molecules (15). Indeed, molecules capable of modulating protein–protein interactions (29–31), transcription factor activity (32, 33), and multidrug resistance in pathogens (34, 35) have all been discovered using DOS libraries, whereas the typical compound libraries employed by pharmaceutical companies or commercial vendors have been largely unsuccessful in providing hits against such challenging and underexploited targets (9, 36, 37). This has been attributed to deficiencies in these compound libraries; the candidate small molecules seem to be well-suited to modulating “traditional” medicinal chemistry targets, but lack the necessary structural elements required to affect other processes (9, 15, 36–40).
It is important to emphasize that the ultimate goal of any small-molecule library synthesis and screening endeavor is the identification of biologically active compounds (6–8). If the library does not yield hits in a chosen screening experiment, it will be deemed a failure, no matter how structurally diverse it is (6, 8, 9). Thus a DOS should aim to efficiently and specifically access biologically relevant chemical space, rather than chemical space that cannot provide biologically useful molecules (8, 9). However, the boundaries of biologically relevant chemical space have yet to be defined (if indeed it is ever possible to do so) and thus the structural constraints that this consideration imposes upon molecules are not precisely known (8, 9). In terms of increasing the overall efficiency of the library synthesis and screening sequence (i.e., maximizing the number of hits), it can be argued that, because it is impossible to access all possible chemical space (vide supra), the incorporation into a compound set of some bias toward known biorelevant chemical space may be desirable. There are several synthetic strategies, such as biologically oriented synthesis (41), biology-inspired synthesis (1), privileged structure synthesis (42), and diverted-total synthesis (43), which seek to generate libraries of compounds which, while structurally unique, are each based around the core structures of known biologically active molecules, typically natural product templates (8, 9). It has been argued that evolutionary pressure has “prevalidated” natural products, and thus compounds that are structurally similar, to be able to modulate biological systems (1, 6, 8, 44). However, such methods inevitably generate compound collections with a relatively low degree of overall scaffold diversity and thus chemical space coverage (9), with an emphasis toward known bioactive regions; that is, the compounds generated would be expected to explore areas of chemical space within the boundaries of, or “close to,” those occupied by known bioactive small molecules. Although the biological relevance of compounds from such regions is expected to be high, it is likely that the low-hanging fruit have already been picked as a consequence of decades of focused investigation, principally by pharmaceutical companies (8, 9). In addition, molecules from such regions of chemical space have proven to be largely inadequate when it comes to modifying more challenging biological targets (vide supra). As outlined previously, there is undoubted value in generating molecules with atypical molecular scaffolds that access unexplored regions of chemical space as these areas may contain molecules with exciting, unique biological properties, which interact with previously undescribed target molecules or act via unique modes of action (8). It can be argued that small-molecule libraries that incorporate molecules based on both unique scaffolds and known scaffolds of proven biological relevance represent a prudent method of increasing “hit” frequency (by targeting areas of chemical space “close” to known bioactive molecules) without compromising too much on the exploration of uncharted regions of chemical space.
Peptide motifs are present in many biologically active molecules, and many peptides are drugs (45, 46). The biological properties of peptides can be tuned by introducing bioisosteric modifications on them. The resulting molecules are known as peptidomimetics, and they usually present improved stability, bioavailability, and selectivity toward a particular target. An important example of these bioisosteric modifications is the replacement of an amide bond by a triazole ring, which introduces rigidity to the molecule and mimics either the cis- or the trans-like configuration of the amide bond (47, 48).
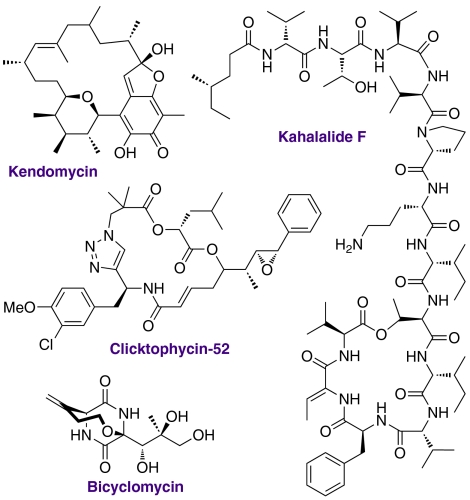
There are many biologically active molecules that contain cyclic peptide and peptidomimetic structural units including diketopiperazines (DKPs, the smallest possible cyclic peptides, Fig. 1) (49, 50). Many compounds incorporating macrocyclic peptides and peptidomimetics (51–54) are also known to be capable of modulating biological systems. Despite such valuable properties, macrocyclic peptides (indeed macrocyclic compounds in general) are arguably a poorly explored structural class within drug discovery (54), and such compounds are underrepresented in current small-molecule libraries. This relative paucity of compounds can be attributed primarily to synthetic intractability (54). Thus there is a need for the development of efficient methods of broad utility for the synthesis of a diverse range of macrocyclic peptides and peptidomimetics so that the biological usefulness of these structural moieties can be investigated and exploited further.
Fig. 1.
Chemical structures of some biologically active macrocycles (49–53).
Within our research group we are interested in the development of previously undescribed synthetic methodologies that can be applied in a DOS context for the construction of libraries of structurally unique compounds with increased biological relevance. Toward this end, we wanted to explore the incorporation of structural motifs from known bioactive molecules (or isosteres thereof) into macrocyclic ring structures. In this paper we report the development of a DOS strategy for the generation of structurally diverse small molecules based around biologically relevant macrocyclic peptidomimetic frameworks. In a proof-of-concept study this approach was applied to the synthesis of a library of 14 structurally diverse and complex compounds containing these motifs. The modular nature of the synthetic routes developed should enable their application in future DOS studies for the creation of larger libraries of these derivatives incorporating greater levels of structural diversity. In addition, we envisage that these chemistries will prove to be useful in a wide variety of other synthetic applications.
Results and Discussion
Outline of the Synthetic Strategy.
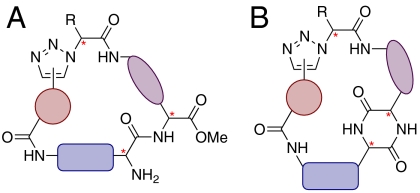
We targeted the synthesis of structurally unique and diverse small molecules based upon two general macrocyclic peptidomimetic structural types (A and B, Fig. 2). Both structures have a trizaole ring that replaces an amide bond, and structure B also incorporates a DKP in the macrocycle. Additionally, both A and B contain seven points where stereochemical and scaffold diversity can be easily introduced. The presence of free amine groups in the library compounds was desired as these would provide synthetic handles for further derivatization; this should allow a means of tuning the biological profile of any “hits” or enable labeling of the compounds for use as chemical probes.
Fig. 2.
General structures of the library compounds: (A) macrocyclic peptidomimetics; (B) macrocyclic DKPs. Diversity can be introduced in each structure by changing the building blocks and reaction conditions. Asterisks indicate chiral centers.
Initial proof-of-concept studies were directed toward the synthesis of a library of 12 compounds based around four different scaffolds: cis-DKPs, trans-DKPs, 1,4-, and 1,5-triazoles. Stereochemical diversity in this collection is high as every molecule contains three stereogenic centers that are changed throughout the library.
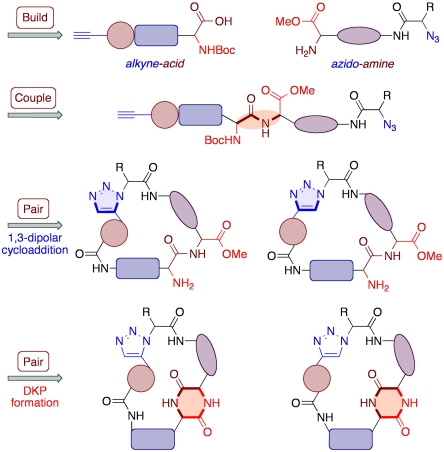
Our strategy for the synthesis was based around the elegant build/couple/pair (B/C/P) approach described by Nielsen and Schreiber (55). The outline of our B/C/P pathway is shown in Scheme 1. The build step involves the preparation of two types of chiral building blocks: The first contains a free amine and an azide (“azido-amine” building blocks), whereas the second contains a carboxylic acid and an alkyne (“alkyne-acid” building blocks). Coupling of three of these building blocks via amide bond formation would furnish a range of tripeptide derivatives; this process provides the basis for stereochemical diversity. The subsequent pair phase provides the basis for scaffold diversity and will comprise of two cyclization steps. First, a 1,3-dipolar cycloaddition will be used to selectively combine the azide and alkyne functionalities of these tripeptides, thus generating the desired macrocyclic peptidomimetic architecture. Intramolecular cyclization reactions between amine and carbonyl moieties would then introduce the DKP motif into the macrocyclic framework. There are several key synthetic challenges associated with the proposed route. Two are particularly worthy of note. First, epimerization of the chiral centers needs to be avoided at all stages of the synthesis in order to obtain the products as single stereoisomers (and thus access the full matrix of stereochemical isomers in the library, i.e., maximizing stereochemical diversity). In addition, generation of both 1,4- and 1,5-subsituted triazoles from each tripetide derivative is desired so as to be able to access the desired scaffold diversity in the library; thus the 1,3-dipolar cycloaddition needs to operate in a highly controllable and regioselective fashion.
Scheme 1.
Outline of the B/C/P strategy for the preparation of the target library.
Synthesis of the Proof-of-Concept Library.
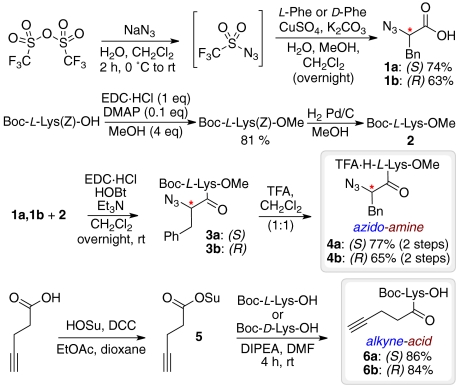
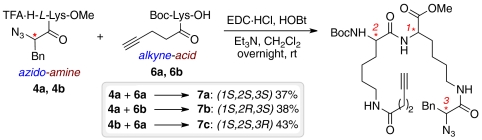
All building blocks were prepared starting from simple, commercially available and relatively cheap amino acid derivatives (Scheme 2). For the synthesis of the “azido-amine” building blocks, α-azido acids (S)-2-azido-3-phenylpropanoic acid 1a and the corresponding enantiomer 1b were obtained from L phenylalanine (L-Phe) and D phenylalanine (D-Phe) by azido transfer using in situ generated trifluoromethanesulfonyl azide (56). Boc-L-Lys(Z)-OH was methylated using EDC·HCl and 4-(N,N-dimethylamino)pyridine and catalytically hydrogenated to yield Boc-L-Lys-OMe 2 that was coupled to 1a and 1b. Subsequent Boc removal using TFA yielded the two azido-amine building blocks 4a,b. The necessary alkyne-acid building blocks Boc-L-Lys(N-4-pentynoic acid)-OH 6a and Boc-D-Lys(N-4-pentynoic acid)-OH 6b were obtained by acylation of Boc-L-Lys-OH and Boc-D-Lys-OH with 4-pentynoic acid succinimidyl ester (57).
Scheme 2.
Build step: Building-block synthesis.
In the couple step, stereochemical diversity was efficiently generated by combination of building blocks 4a,b with 6a,b. Three different tripeptide derivatives 7a–c were obtained as single stereoisomers (Scheme 3).
Scheme 3.
Couple step: Coupling of building blocks 4a,b and 6a,b via amide bond formation.
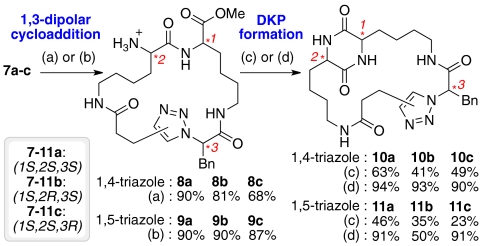
With tripeptide derivatives 7a–c in hand, we were ready to attempt the key 1,3-dipolar cycloaddition process to generate the 1,4- and 1,5-disubstituted triazoles with concomitant construction of the macrocyclic architecture. Though there are numerous reported examples of the use of regioselective 1,3-dipolar cycloadditions to furnish 1,4-triazole containing macrocycles (58), only a handful of these employed chiral α-azido acids as the substrates (48, 59, 60). The synthesis of 1,5-triazole containing macrocycles via 1,3-dipolar cycloadditions (61) is much less common, and in the very limited number of examples that use α-azido acid containing substrates, the yields of the isolated products are typically very low (47). A method where the formation of the macrocycle proceeded with good regioselectivity (1,5-triazole over 1,4-triazole) and moderate yields has been recently described, but there were no stereogenic centers adjacent to the azide present in the substrates (62, 63). We faced the challenge of performing the same reaction with chiral α-azido acids, desiring both high yields and no epimerization of the chiral center adjacent to the azide. Such a reaction would be expected to be of significant interest for the synthesis of natural product derivatives and for other applications in synthetic organic chemistry.
We envisaged that regioisomeric macrocycles containing 1,4- and 1,5-disubstituted triazoles could be obtained by reacting peptides 7a–c with Cu or Ru catalysts, respectively, in a “click” type 1,3-dipolar cycloaddition reaction (Scheme 4) (64, 65). In the final optimized conditions tripeptide derivatives 7a–c were refluxed overnight in the presence of CuI and N,N-diisopropylethylamine (DIPEA) in THF (“copper route”) or [Cp∗RuCl]4 in toluene (“ruthenium route”). The first six members of the library (8a–c and 9a–c) were obtained after subsequent Boc removal. Under these optimized conditions, the 1,3-dipolar cycloaddition reactions were completely regioselective and proceeded without epimerization of the chiral centers (a single stereoisomer was detected in the crude 1H NMR of each cyclization product). The purification was typically straightforward (flash chromatography on silica gel), and the products were obtained in very good yields (68–90%).
Scheme 4.
Pair Steps. 1,3-Dipolar cycloaddition followed by DKP formation. Conditions: (A) (i) CuI (2 eq), DIPEA (3 eq), THF, reflux, overnight; (ii) HCl-MeOH (1.25 M); (B) (i) [Cp∗RuCl]4, toluene, reflux, overnight; (ii) HCl-MeOH (1.25 M); (C) AcOH-NMM (1∶1.5), 2-butanol, overnight reflux (D) AcOH-NMM* (1∶1.5), 2-butanol, microwave 150 ºC, 3–6 h. NMM*: morpholinomethyl-polystyrene (3.51 mmol/g).
The next pair step involved introduction of the DKP unit into the macrocyclic framework of the cycloaddition products. DKP formation is a common side reaction in peptide synthesis, which is especially favored in basic conditions or under acetic acid catalysis (66). Preliminary experiments explored DKP formation under basic conditions. However, these reactions were found to be extremely sluggish, and attempts to accelerate the rate and increase conversion through the use of higher reaction temperatures led to epimerization of the chiral centers present. Therefore, during our optimization process, we decided to use slightly acidic buffered conditions [Scheme 4, conditions (c)] (67). Using these conditions we typically observed 100% conversion. However, separation of the N methylmorpholine (NMM) salts from the desired products proved to be difficult due to the high water solubility of the DKPs. Thus, the yields after separation either by precipitation or flash column chromatography on silica gel were relatively low. To solve this problem we decided to use solid-supported NMM (morpholinomethyl-polystyrene). Also thermal heating was replaced by microwave heating. When these conditions were used [Scheme 4, conditions (d)], the only necessary work up was filtration of the solid support and evaporation to dryness. Thus, the yields were typically higher than 90%. The only exception was compound 11b, which was obtained with 50% yield because an extra recrystallization was necessary to obtain the pure product. To the best of our knowledge the use of solid supported NMM in DKP formation is previously undescribed. It is envisaged that this will prove to be a valuable general method for the synthesis of DKPs that do not precipitate in the reaction media. In addition, the combination of solid-supported NMM with AcOH and microwave heating represents valuable previously undescribed methodology for the formation of difficult DKPs without epimerization of chiral centers.
Rigidifying the Macrocyclic Scaffold.
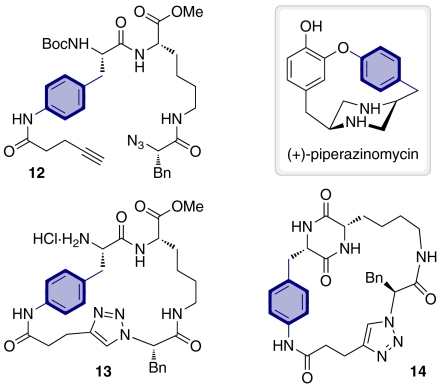
It is a widely accepted concept in medicinal chemistry that increasing the rigidity of a compound can increase its affinity for a target (68); more rigid molecules might also be expected to interact more selectively with a particular target. In addition, the introduction of extra rigidity into a given molecular framework may drastically affect its overall 3D structure and consequently provide an additional means of generating additional shape diversity. We were thus interested in generating previously undescribed macrocyclic peptidomimetics with increased structural rigidity as a means of probing areas of biologically relevant chemical space beyond those accessed by the library compounds described above. Toward this end we were inspired by the structure of (+)-piperazinomycin, a naturally occurring macrocyclic piperazine derivative with antimicrobial and antifungal activity (69). This molecule contains a biaryl ether ring linkage that is thought to confer rigidity upon the structure. We envisaged that extra rigidity in our general macrocyclic peptidomimetic scaffolds could be achieved by replacing a linear alkyl chain by an aromatic ring. Thus two previously undescribed macrocyclic peptidomimetics 13 and 14 were targeted (Fig. 3). Both compounds were prepared from linear peptide 12 using the same B/C/P strategy as for the rest of the library compounds. In this case, the best conditions for the 1,3-dipolar cycloaddition to obtain 13 involved refluxing in toluene in the presence of CuI and 1,8-diazabicyclo[5.4.0]undec-7-ene (45% yield). The DKP formation to obtain 14 was performed using condition (d) in Scheme 4, but in this case, a reaction time of 9 h of reaction was required to reach 100% conversion. This could possibly be attributed to the higher rigidity of the macrocycle that would be expected to make the additional ring closure more difficult. The synthesis of these two compounds demonstrates the versatility of the methodology employed; important structural modifications can be introduced into the macrocyclic derivatives in a simple way through variation in the building blocks employed.
Fig. 3.
Rigidified library compounds 13 and 14 inspired by the antibiotic (+)piperazinomycin and prepared from linear peptide 12. Compared to the other library compounds, a linear chain has been replaced by an aromatic ring (highlighted in blue).
Assessing the Diversity of the Library Compounds.
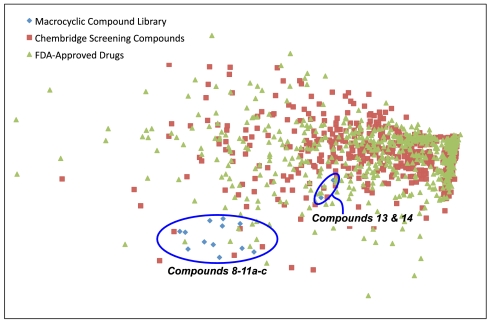
We have utilized a computational process for diversity assessment based around the calculation of various shape- and charge-based molecular surface descriptors followed by principal component analysis (PCA) (6, 9, 70, 71). Essentially this provides a measure of overall chemical space coverage (9). In brief, we analyzed various properties of compounds 8–11a–c, 13 and 14 with respect to their similarity to wider chemical space, as represented by US Food and Drug Administration (FDA)-approved drugs and a commercial small-molecule library (see SI Appendix for more detail). A set of 657 approved drugs was randomly selected from DrugBank (72), and 746 compounds were selected randomly from the ChemBridge screening library, as downloaded from ZINC (73). The distribution of these three compound sets in chemical space is illustrated in Fig. 4. It can be seen that FDA-approved drugs and the ChemBridge screening library compounds occupy similar regions of chemical space. On the other hand, 12 of the unique compounds (8–11a–c) occupy an area of chemical space away from this region defined by conventional medicinal chemistry (74). This result illustrates the value of our synthetic strategy for accessing previously undescribed areas of chemical space. Overall, a reasonable level of diversity (chemical space coverage) was achieved by the synthetic library. Interestingly, compounds 13 and 14 are very clearly separated from the other macrocyclic peptidomimetic derivatives, occupying a more populated area of chemical space due to a more “typical” aromatic ring replacing a previously linear chain in the molecules. Hence, by selecting a suitable synthesis scheme one can simultaneously decide which region of chemical space to explore, while at the same time ensuring sufficient diversity in the compounds being synthesized.
Fig. 4.
Location of novel macrocyclic peptidomimetics in chemical space defined by shape- and charge-based molecular surface properties. Randomly selected FDA-approved drugs and ChemBridge screening library compounds are also represented in the graphic. For all structures TAE/RECON electrostatic potential and electrostatic potential autocorrelation descriptors (75) were calculated as implemented in the molecular operating environment and the resulting variables were subject to principal component analysis in the same program. The first two principle components are displayed in this figure and the exploration of underrepresented chemical space by our DOS library described in this work can clearly be seen.
Conclusions
In conclusion, we have described a strategy for the DOS of a library of structurally unique and diverse macrocyclic peptidomimetics from simple, readily available amino acid starting materials. In a proof-of-concept study, a library of 14 such compounds displaying both scaffold and stereochemical diversity was generated. Computational analyses demonstrated that the library accesses both chemical space not explored by molecules from “traditional” medicinal chemistry collections and also more populated areas, showing the versatility of the developed synthetic methodology. In preliminary biological assays a number of library compounds showed interesting biological properties; in particular, compound 14 showed significant antibacterial activity against Staphylococcus aureus. Further biological screening is currently ongoing, and full results will be reported in due course. Because of the modular nature of our synthetic strategy, we anticipate that it holds significant potential for the DOS of libraries of macrocyclic peptidomimetics with greater levels of diversity. A wide variety of natural and unnatural α-, β-, and γ-amino acids could be used in the build step of the synthesis, which would allow the generation of considerable stereochemical and scaffold diversity. In addition, the final library compounds should prove amenable to further modification and diversification. The generation of the proof-of-concept library required the development of two previously undescribed pieces of synthetic methodology. First, we have described a protocol for the macrocyclic ring closure of chiral α-azido acids via regioselective and epimerization-free 1,5-disubstituted triazole formation. The macrocycle products were obtained in superior yields to those described in the literature (47). Second, we have reported the development of a unique method for the DKP synthesis using solid-supported N methylmorpholine in combination with microwave heating. The procedure allowed for the synthesis of structurally complex DKPs in excellent yields. Crucially, this method may represent a previously undescribed general strategy for the synthesis of DKP-based structures that could be difficult to isolate by other means. Owing to their robust and flexible natures, and given the presence of macrocyclic peptidomimetics and DKPs in many molecules of biological interest, it is envisaged that both previously undescribed methodologies will prove to be useful in a wider synthetic context.
Materials and Methods
General experimental remarks, complete experimental procedures for the synthesis of the building blocks, full characterization of all unique compounds, and details of the analysis of chemical space method are contained in SI Appendix.
Supplementary Material
Acknowledgments.
This work was supported by grants from the European Union, Engineering and Physical Sciences Research Council, Biotechnology and Biological Sciences Research Council, Medical Research Council, Royal Society, Frances and Augustus Newman Foundation, and Wellcome Trust (to D.R.S. and M.W.). A.I.-L. thanks the EU for a Marie-Curie Intra-European Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015267108/-/DCSupplemental.
References
- 1.Kaiser M, Wetzel S, Kumar K, Waldmann H. Biology-inspired synthesis of compound libraries. Cell Mol Life Sci. 2008;65:1186–1201. doi: 10.1007/s00018-007-7492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spring DR. Chemical genetics to chemical genomics: Small molecules offer big insights. Chem Soc Rev. 2005;34:472–482. doi: 10.1039/b312875j. [DOI] [PubMed] [Google Scholar]
- 3.Kawasumi M, Nghiem P. Chemical genetics: Elucidating biological systems with small-molecule compounds. J Invest Dermatol. 2007;127:1577–1584. doi: 10.1038/sj.jid.5700853. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel DA, Schroeder FC, Duvall JR, Schreiber SL. An oligomer-based approach to skeletal diversity in small-molecule synthesis. J Am Chem Soc. 2006;128:14766–14767. doi: 10.1021/ja065724a. [DOI] [PubMed] [Google Scholar]
- 5.Walsh DP, Chang YT. Chemical genetics. Chem Rev. 2006;106:2476–2530. doi: 10.1021/cr0404141. [DOI] [PubMed] [Google Scholar]
- 6.Galloway WRJD, Bender A, Welch M, Spring DR. The discovery of antibacterial agents using diversity-oriented synthesis. Chem Commun. 2009:2446–2462. doi: 10.1039/b816852k. [DOI] [PubMed] [Google Scholar]
- 7.Galloway WRJD, Diaz-Gavilan M, Isidro-Llobet A, Spring DR. Synthesis of unprecedented scaffold diversity. Angew Chem Int Edit. 2009;48:1194–1196. doi: 10.1002/anie.200805452. [DOI] [PubMed] [Google Scholar]
- 8.Galloway WRJD, Spring DR. Is synthesis the main hurdle for the generation of diversity in compound libraries for screening? Expert Opin Drug Dis. 2009;4:467–472. doi: 10.1517/17460440902916606. [DOI] [PubMed] [Google Scholar]
- 9.Galloway WRJD, Isidro-Llobet A, Spring DR. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat Commun. 2010;1:80. doi: 10.1038/ncomms1081. 10.1038/ncomms1081. [DOI] [PubMed] [Google Scholar]
- 10.Spring DR. Diversity-oriented synthesis; a challenge for synthetic chemists. Org Biomol Chem. 2003;1:3867–3870. doi: 10.1039/b310752n. [DOI] [PubMed] [Google Scholar]
- 11.Haggarty SJ. The principle of complementarity: Chemical versus biological space. Curr Opin Chem Biol. 2005;9:296–303. doi: 10.1016/j.cbpa.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz MK, Sauer WHB. Molecular shape diversity of combinatorial libraries: A prerequisite for broad bioactivity. J Chem Inf Comput Sci. 2003;43:987–1003. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
- 13.Burke MD, Schreiber SL. A planning strategy for diversity-oriented synthesis. Angew Chem Int Edit. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 14.Burke MD, Berger EM, Schreiber SL. Generating diverse skeletons of small molecules combinatorially. Science. 2003;302:613–618. doi: 10.1126/science.1089946. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber SL. Molecular diversity by design. Nature. 2009;457:153–154. doi: 10.1038/457153a. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy JP, et al. Application of combinatorial chemistry science on modern drug discovery. J Comb Chem. 2008;10:345–354. doi: 10.1021/cc700187t. [DOI] [PubMed] [Google Scholar]
- 17.Shelat AA, Guy RK. Scaffold composition and biological relevance of screening libraries. Nat Chem Biol. 2007;3:442–446. doi: 10.1038/nchembio0807-442. [DOI] [PubMed] [Google Scholar]
- 18.Lovering F, Bikker J, Humblet C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J Med Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 19.Spandl RJ, Bender A, Spring DR. Diversity-oriented synthesis; a spectrum of approaches and results. Org Biomol Chem. 2008;6:1149–1158. doi: 10.1039/b719372f. [DOI] [PubMed] [Google Scholar]
- 20.Lipinski C, Hopkins A. Navigating chemical space for biology and medicine. Nature. 2004;432:855–861. doi: 10.1038/nature03193. [DOI] [PubMed] [Google Scholar]
- 21.Bender A, et al. Diversity oriented synthesis: A challenge for synthetic chemists. In: Jaroch S, Weinmann H, editors. Chemical Genomics: Small Molecule Probes to Study Cellular Function. Berlin: Springer; 2006. pp. 47–60. [Google Scholar]
- 22.Hopkins AL, Mason JS, Overington JP. Can we rationally design promiscuous drugs? Curr Opin Struct Biol. 2006;16:127–136. doi: 10.1016/j.sbi.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Thomas GL, Wyatt EE, Spring DR. Enriching chemical space with diversity-oriented synthesis. Curr Opin Drug Disc Dev. 2006:700–712. [PubMed] [Google Scholar]
- 24.Clardy J, Walsh C. Lessons from natural molecules. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 25.Pucheault M. Natural products: Chemical instruments to apprehend biological symphony. Org Biomol Chem. 2008;6:424–432. doi: 10.1039/b713022h. [DOI] [PubMed] [Google Scholar]
- 26.Schneider G, Grabowski K. Properties and architecture of drugs and natural products revisited. Curr Chem Biol. 2007;1:115–127. [Google Scholar]
- 27.Schreiber SL. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 28.Comer E, Rohan E, Deng L, Porco JA., Jr An approach to skeletal diversity using functional group pairing of multifunctional scaffolds. Org Lett. 2007;9:2123–2126. doi: 10.1021/ol070606t. [DOI] [PubMed] [Google Scholar]
- 29.Di Micco S, et al. Identification of lead compounds as antagonists of protein Bcl xL with a diversity-oriented multidisciplinary approach. J Med Chem. 2009;52:7856–7867. doi: 10.1021/jm9010687. [DOI] [PubMed] [Google Scholar]
- 30.Kuruvilla FG, Shamji AF, Sternson SM, Hergenrother PJ, Schreiber SL. Dissecting glucose signalling with diversity-oriented synthesis and small-molecule microarrays. Nature. 2002;416:653–657. doi: 10.1038/416653a. [DOI] [PubMed] [Google Scholar]
- 31.Stanton BZ, et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5:154–156. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng PY, Tang YC, Knosp WM, Stadler HS, Shaw JT. Synthesis of diverse lactam carboxamides leading to the discovery of a new transcription-factor inhibitor. Angew Chem Int Edit. 2007;46:5352–5355. doi: 10.1002/anie.200700762. [DOI] [PubMed] [Google Scholar]
- 33.Koehler AN, Shamji AF, Schreiber SL. Discovery of an inhibitor of a transcription factor using small molecule microarrays and diversity-oriented synthesis. J Am Chem Soc. 2003;125:8420–8421. doi: 10.1021/ja0352698. [DOI] [PubMed] [Google Scholar]
- 34.Wyatt EE, et al. Identification of an anti-MRSA dihydrofolate reductase inhibitor from a diversity-oriented synthesis. Chem Commun. 2008:4962–4964. doi: 10.1039/b812901k. [DOI] [PubMed] [Google Scholar]
- 35.Thomas GL, et al. Anti-MRSA agent discovery using diversity-oriented synthesis. Angew Chem Int Edit. 2008;47:2808–2812. doi: 10.1002/anie.200705415. [DOI] [PubMed] [Google Scholar]
- 36.Dandapani S, Marcaurelle LA. Current strategies for diversity-oriented synthesis. Curr Opin Chem Biol. 2010;14:362–370. doi: 10.1016/j.cbpa.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 38.Pizzirani D, Kaya T, Clemons PA, Schreiber SL. Stereochemical and skeletal diversity arising from amino propargylic alcohols. Org Lett. 2010;12:2822–2825. doi: 10.1021/ol100914b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber SL. The state of the art of chemical biology. The gap between scientists’ aspirations and society’s expectations. ChemBioChem. 2009;10:16–29. [Google Scholar]
- 40.Taylor AM, Schreiber SL. Aziridines as intermediates in diversity-oriented syntheses of alkaloids. Tetrahedron Lett. 2009;50:3230–3233. doi: 10.1016/j.tetlet.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noren-Muller A, et al. Discovery of protein phosphatase inhibitor classes by biology-oriented synthesis. Proc Natl Acad Sci USA. 2006;103:10606–10611. doi: 10.1073/pnas.0601490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Privileged structures: Applications in drug discovery. Comb Chem High T Scr. 2004;7:473–493. doi: 10.2174/1386207043328544. [DOI] [PubMed] [Google Scholar]
- 43.Danishefsky S. On the potential of natural products in the discovery of pharma leads: A case for reassessment. Nat Prod Rep. 2010;27:1114–1116. doi: 10.1039/c003211p. [DOI] [PubMed] [Google Scholar]
- 44.Koch MA, et al. Charting biologically relevant chemical space: A structural classification of natural products (SCONP) Proc Natl Acad Sci USA. 2005;102:17272–17277. doi: 10.1073/pnas.0503647102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilby JM, et al. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 47.Horne W, Olsen C, Beierle J, Montero A, Ghadiri M. Probing the bioactive conformation of an archetypal natural product HDAC inhibitor with conformationally homogeneous triazole-modified cyclic tetrapeptides. Angew Chem Int Edit. 2009;48:4718–4724. doi: 10.1002/anie.200805900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bock VD, Speijer D, Hiemstra H, van Maarseveen JH. 1,2,3-Triazoles as peptide bond isosteres: Synthesis and biological evaluation of cyclotetrapeptide mimics. Org Biomol Chem. 2007;5:971–975. doi: 10.1039/b616751a. [DOI] [PubMed] [Google Scholar]
- 49.Williams RM, Durham CA. Bicyclomycin: Synthetic, mechanistic, and biological studies. Chem Rev. 1988;88:511–540. [Google Scholar]
- 50.Campbell J, Blackwell HE. Efficient construction of diketopiperazine macroarrays through a cyclative-cleavage strategy and their evaluation as luminescence inhibitors in the bacterial symbiont Vibrio fischeri. J Comb Chem. 2009;11:1094–1099. doi: 10.1021/cc900115x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamann MT. Technology evaluation: Kahalalide F (PharmaMar) Curr Opin Mol Ther. 2004;6:657–665. [PMC free article] [PubMed] [Google Scholar]
- 52.Magauer T, Martin H, Mulzer J. Total synthesis of the antibiotic kendomycin by macrocyclization using photo-fries rearrangement and ring-closing metathesis. Angew Chem Int Edit. 2009;48:6032–6036. doi: 10.1002/anie.200900522. [DOI] [PubMed] [Google Scholar]
- 53.Nahrwold M, Bogner T, Eissler S, Verma S, Sewald N. “Clicktophycin-52”: A bioactive cryptophycin-52 triazole analogue. Org Lett. 12:1064–1067. doi: 10.1021/ol1000473. [DOI] [PubMed] [Google Scholar]
- 54.Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat Rev Drug Discov. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen T, Schreiber S. Towards the optimal screening collection: A synthesis strategy. Angew Chem Int Edit. 2008;47:48–56. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lundquist JT, Pelletier JC. Improved solid-phase peptide synthesis method utilizing α-azide-protected amino acids. Org Lett. 2001;3:781–783. doi: 10.1021/ol0155485. [DOI] [PubMed] [Google Scholar]
- 57.Galibert M, Dumy P, Boturyn D. One-pot approach to well-defined biomolecular assemblies by orthogonal chemoselective ligations. Angew Chem Int Edit. 2009;48:2576–2579. doi: 10.1002/anie.200806223. [DOI] [PubMed] [Google Scholar]
- 58.Looper RE, Pizzirani D, Schreiber SL. Macrocycloadditions leading to conformationally restricted small molecules. Org Lett. 2006;8:2063–2066. doi: 10.1021/ol0604724. [DOI] [PubMed] [Google Scholar]
- 59.Bock VD, Perciaccante R, Jansen TP, Hiemstra H, van Maarseveen JH. Click chemistry as a route to cyclic tetrapeptide analogues: Synthesis of cyclo-[Pro-Val-Ψ (triazole)-Pro-Tyr] Org Lett. 2006;8:919–922. doi: 10.1021/ol053095o. [DOI] [PubMed] [Google Scholar]
- 60.Springer J, et al. Backbone amide linker strategy for the synthesis of 1,4-triazole-containing cyclic tetra- and pentapeptides. Eur J Org Chem. 2008;2008:2592–2600. [Google Scholar]
- 61.Zhang L, et al. Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J Am Chem Soc. 2005;127:15998–15999. doi: 10.1021/ja054114s. [DOI] [PubMed] [Google Scholar]
- 62.Kelly AR, et al. Accessing skeletal diversity using catalyst control: Formation of n and n + 1 macrocyclic triazole rings. Org Lett. 2009;11:2257–2260. doi: 10.1021/ol900562u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcaurelle LA, et al. An aldol-based build/couple/pair strategy for the synthesis of medium- and large-sized rings: Discovery of macrocyclic histone deacetylase inhibitor. J Am Chem Soc. 2010;132:16962–16976. doi: 10.1021/ja105119r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Edit. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 65.Huisgen R. 1,3-Dipolar cycloadditions—Introduction, survey, mechanism. In: Padwa A, editor. 1,3-Dipolar Cycloaddition Chemistry. Vol 1. New York: Wiley; 1984. pp. 1–176. [Google Scholar]
- 66.Gisin BF, Merrifield RB. Carboxyl-catalyzed intramolecular aminolysis. A side reaction in solid-phase peptide synthesis. J Am Chem Soc. 1972;94:3102–3106. doi: 10.1021/ja00764a036. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki K, Sasaki Y, Endo N, Mihara Y. Acetic acid-catalyzed diketopiperazine synthesis. Chem Pharm Bull. 1981;29:233–237. [Google Scholar]
- 68.Lumley JA. Compound selection and filtering in library design. QSAR Comb Sci. 2005;24:1066–1075. [Google Scholar]
- 69.Boger DL, Zhou JC. Total synthesis of (+)-piperazinomycin. J Am Chem Soc. 1993;115:11426–11433. [Google Scholar]
- 70.Ringner M. What is principal component analysis? Nat Biotechnol. 26:303–304. doi: 10.1038/nbt0308-303. [DOI] [PubMed] [Google Scholar]
- 71.Bauer RA, DiBlasi CM, Tan DS. The tert-butylsulfinamide lynchpin in transition-metal-mediated multiscaffold library synthesis. Org Lett. 2010;12:2084–2087. doi: 10.1021/ol100574y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wishart DS, et al. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–D906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irwin JJ, Shoichet BK. ZINC—A free database of commercially available compounds for virtual screening. J Chem Inf Model. 2005;45:177–182. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fergus S, Bender A, Spring DR. Assessment of structural diversity in combinatorial synthesis. Curr Opin Chem Biol. 2005;9:304–309. doi: 10.1016/j.cbpa.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Breneman CM, et al. New developments in PEST shape/property hybrid descriptors. J Comput Aid Mol Des. 2003;17:231–240. doi: 10.1023/a:1025334310107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.