Abstract
Reported biological activities of Stemona natural products, such as antitussive activity, inspired the development of synthetic methods to access several alkaloids within this family and in so doing develop a general route to the core skeleta shared by the class of natural products. The chemistry was subsequently adapted to afford a series of analogue sets bearing simplified, diverse Stemona-inspired skeleta. Over 100 of these analogues were subjected to general G protein-coupled receptor profiling along with the known antitussive compound, neostenine; this led to the identification of hit compounds targeting several receptor types. The particularly rich hit subset for sigma receptors was expanded with two focused library sets, which resulted in the discovery of a fully synthetic, potent chemotype of sigma ligands. This collaborative effort combined the development of synthetic methods with extensive, flexible screening resources and exemplifies the role of natural products in bioactivity mining.
Keywords: diversity-oriented synthesis, probe discovery
Nature continues to provide a wealth of new leads for potential therapeutic agents or pharmacological probes (1). The role of natural products in traditional medicine is well appreciated and has led to many important drugs that either retain the entire natural product structure or that incorporate (usually straightforward) chemical modifications. Today, biologically useful natural products are most commonly discovered through target-based or phenotypic screening, even in the absence of detailed information about the purpose of the natural product in its original biological setting. The success of these efforts has led some researchers to suggest that scaffolds related to natural product substructures may lead to useful chemical libraries due to the presumed preoptimization of these structures by natural selection (2–4). This argument loses force as compounds become less similar to the natural products that inspired them, but the use of even highly truncated natural product structures in screening can bear fruit if care is taken to retain features consistent with macromolecular binding in the process (5–9). Moreover, it is reasonable to expect that one’s chances for success in identifying structures having activities unrelated to the (possibly unknown) original impetus for evolutionary structure optimization increase if (i) the natural product has multiple bioactivities and (ii) the structure is sufficiently malleable for analogue construction.
The practical pursuit of such an approach presupposes efficient synthetic access to the desired scaffold, a prerequisite that might not be as straightforward as using the natural product itself as starting material. An attractive alternative is to build the proposed scaffold de novo, an activity that can be considerably informed by knowledge gained in natural products total synthesis [i.e., the “diverted chemical synthesis” concept elucidated by Wilson and Danishefsky (10)]. In this paper, we describe the use of such a strategy to identify several potent sigma receptor (Sig-R) ligands. The two key components of success were (i) efficient access to the lead natural products via total synthesis and (ii) the subsequent development of general synthetic methodology that allowed for systematic and flexible chemical optimization of a core moiety.
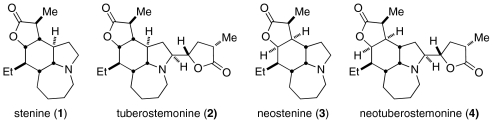
The extracts of stemonaceous flora have been utilized for centuries as treatments for respiratory ailments in established Asian traditions. Additional effects associated with these plants (or discrete compounds isolated from them) include insecticidal, anthelmintic, antitussive, and various neurochemical actions (11–17), although the molecular mechanisms responsible for their activities have rarely been identified (18). Four structurally related isolated Stemona alkaloid molecules are depicted in Fig. 1. The reported antitussive activities of two isolated Stemona alkaloid compounds, neostenine 3 and neotuberostemonine 4 against citric acid-induced cough in guinea pig animal models have validated the traditional medicine uses for these plants (19, 20). More recently, antitussive activity has been confirmed for additional isolated Stemona alkaloid compounds (21–23). The diversity of reported traditional uses combined with demonstrated in vivo activity of several members supported the pursuit of an appropriately simplified Stemona alkaloid structure for speculative screening.
Fig. 1.
Structures of selected Stemona alkaloids.
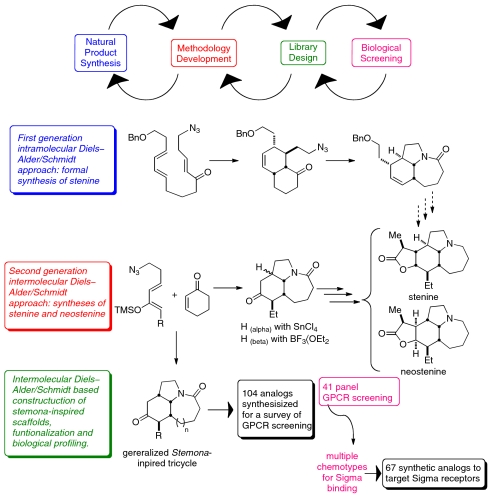
Stemona alkaloids 1–3 have been the targets of successful synthetic campaigns by this (24–26) and other groups (27–37), although the synthesis of neotuberostemonine 4 has yet to be realized. Our own interest in this class of natural products was driven initially by the challenges presented by these alkaloids in such a total synthesis context. To that end, we carried out a first-generation formal synthesis of stenine (26). In the course of this work, we discovered that it was possible to streamline the synthesis by combining two Lewis acid-promoted reactions, a Diels–Alder and an intramolecular Schmidt reaction, into a tandem reaction sequence (Scheme 1). Realizing the general value of this process for heterocyclic synthesis, this methodology was further developed into a general intermolecular variant (38) that was in turn reapplied to the problem of Stemona alkaloid synthesis. This methodology enabled efficient and practical total syntheses of both stenine (25) and neostenine (24) that yielded ample quantities for biological study (9 and 13 steps from commercially available starting materials, respectively). This Diels–Alder/Schmidt reaction sequence also provided straightforward access to Stemona alkaloid-like scaffolds for the parallel synthesis of a diverse compound collection (39).
Scheme 1.
The relationship between chemical methodology and other design elements of a natural product–inspired library program.
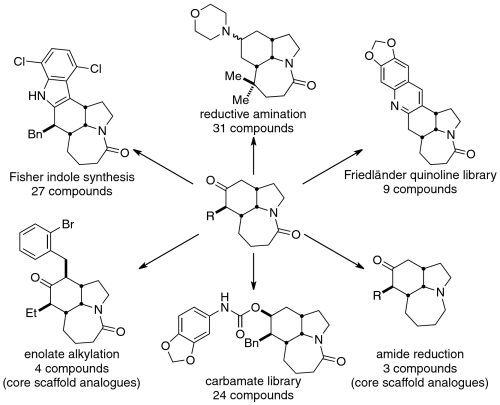
In addition to the scaffold bearing the ethyl side chain of stenine and neostenine, a collection of nonnatural Stemona-like ketoamide scaffolds were constructed via this route, which then served as the substrates for the preparation of six sets of functionalized analogues. These bore core changes that could not be readily accomplished by modification of the natural product itself. Representative examples of the various pathways of these initial efforts are shown in Scheme 2. Almost exclusively, these analogues displayed ring systems or functional groups substantially different from those of the natural products, including carbamates, amides, aryl groups, and heterocyclic moieties (e.g., indole and quinoline) (39). At the outset, it was unknown whether these analogues would possess any significant binding activity. We therefore did not feel constrained in the range of chemical manipulations we could explore, and in many cases the final compounds bear little resemblance to any known Stemona alkaloid. Herein, we report the initial evaluation of these compounds against a panel of G protein-coupled receptor (GPCR) targets relevant to central nervous system function and how those results have inspired additional library design, ultimately resulting in a series of highly potent Sig-R ligands.
Scheme 2.
Representative examples of the five classes of Stemona-inspired synthetic analogues.
Results and Discussion
Binding Studies of the Initial Library Against a Panel of CNS Targets.
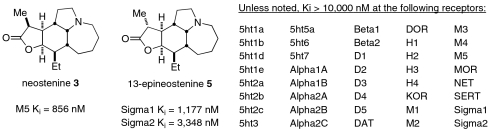
Because other naturally occurring antitussive agents have potent function at one or more CNS receptors (i.e., codeine, along with numerous related agents) (40), we sought to determine if neostenine bound to such targets. Thus, synthetic (±)-neostenine and (±)-13-epineostenine were screened against 40 individual GPCR and other molecular targets (Fig. 2). The compounds were initially screened at a constant concentration (10 μM) to identify possible activity of the compound. Results showing significant activity in the initial screen were selected for κi determinations using radioligand binding assays. It was determined that (±)-neostenine and (±)-13-epineostenine only showed binding at the muscarinic M5 receptor and Sig-Rs, respectively, as shown. The somewhat surprising binding differences observed between the epimers suggest a key role for the C-13 substituent. Notably, neither of the naturally occurring alkaloids tested had activity at any of the opioid receptors. Although Sig-R binding mediates the activity of some nonnarcotic antitussive agents, such as dextrophan (41), the lack of Sig-R binding in neostenine indicates that the source of this antitussive activity of this compound is still unknown. Target identification studies, along with the independent validation of the in vivo literature results, are called for but beyond the scope of the present paper.
Fig. 2.
Secondary binding results for (±)-neostenine and (±)-13-epineostenine. Secondary binding results are Ki values from radioligand binding assays (see SI Appendix for details).
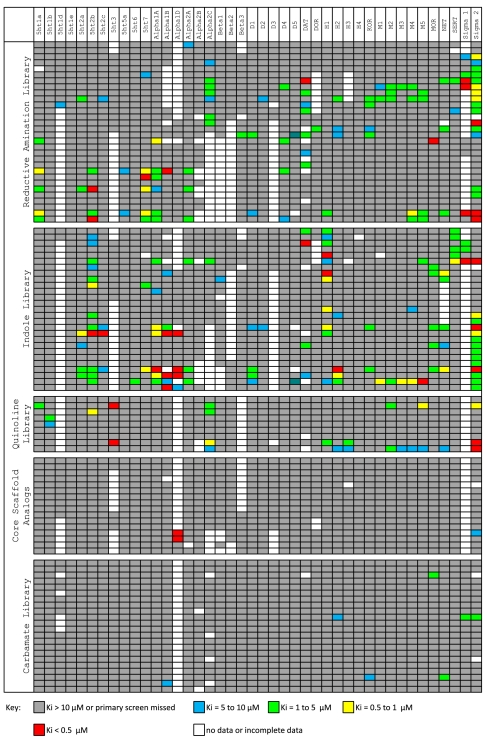
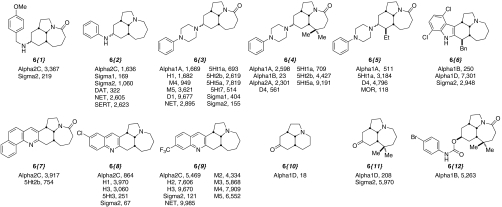
Next, a total of 104 synthetic Stemona analogues were selected for screening as described above [Fig. 3; for the complete binding data (numeric κi values) and compound identity, see Dataset S1]. The entries in Fig. 3 are grouped according to compound class as indicated in Scheme 2. Several patterns become discernable when analyzed from this perspective. The reductive amination compounds, derived primarily from aryl-containing amines, tended to possess hit profiles against the GPCR panel of greater potency and lower selectivity than most other compound classes. Similar trends of potency and selectivity were observed for the indole compound set. The Friedländer quinoline analogues displayed a more balanced activity/selectivity profile, whereas the carbamate analogue compound set, which lacks any basic nitrogen, demonstrated more modest binding activity. The results obtained for the twelve representative analogues displayed in Fig. 4 demonstrate these trends. Although compounds possessing submicromolar affinities for multiple classes of GPCR targets (e.g., adrenergic, muscarinic, serotonin, dopaminergic and Sig-R classes) emerged as possible hits for further investigation, we were most interested in a clustering of basic, nitrogen-containing compounds displaying potent affinity for the Sig-Rs (compounds 6{1–3, 8, and 9}). Of secondary interest was the adrenergic binding of the tertiary amines derived from the amide reduction of the core scaffolds. Thus, 6{10} possessed an alpha1D Ki of 18 nM with no additional binding of < 10,000 nM, and 6{11} possessed an alpha1D Ki of 208 nM and a sigma2 Ki of 5,970 nM.
Fig. 3.
Overview of binding for 104 Stemona analogues against 41 GPCR targets. Secondary binding results are Ki values from radioligand binding assays (see Dataset S1 for details). Examples of the five compound classes are shown in Scheme 2.
Fig. 4.
Structures and significant binding (Ki < 10 μM) of selected synthetic analogues. For structures and numerical Ki values of all synthetic analogues, see Dataset S1.
Construction of a Follow-Up Library and Binding Studies.
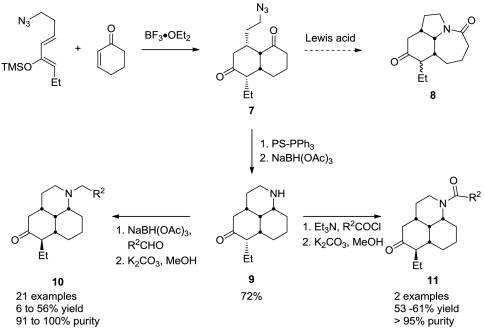
The observation that the majority of potent binding compounds possessed basic nitrogen prompted us to consider the construction of unique analogues containing alternative basic nitrogen heterocycles. The ideal method would furnish the heterocyclic products diastereoselectively with a minimum of manipulations and allow for independent functionalization at both the left- and right-hand hemispheres on the core scaffold. During the scale-up of Stemona scaffolds for library synthesis, we noted that the tandem Diels–Alder/Schmidt reaction could be halted at the Diels–Alder stage and the azido diketone product isolated by judicious choice of Lewis acid and by limiting the number of equivalents added. This observation permitted us to investigate an alternative Diels–Alder/aza–Wittig reaction sequence that afforded the tricyclic skeleton 9, which contained an unmasked basic nitrogen amenable to alkylation or amidation as desired (Scheme 3). The scaffold readily participated in reductive amination with a diverse range of aldehydes to provide a collection of tertiary amines in this series. Given the precedent of diverse GPCR binding observed for the basic nitrogen-containing compounds previously profiled (see Figs. 3 and 4), we reasoned that this collection of compounds would afford interesting screening results. We were inclined toward screening against the opioid receptors and Sig-Rs based on the association of these receptors with antitussive activity (40, 41), and we began our profiling investigations with these. In addition to their potential as an antitussive target, Sig-Rs have been implicated in a wide range of physiological functions and thus represent a unique therapeutic target for disorders such as depression, schizophrenia, and Parkinson’s disease (41–44). Although the precise role of Sig-Rs is not well understood, it is widely believed that the effects of Sig-Rs can be attributed to their modulation of other receptor types. This neuromodulatory role has complicated research on Sig-Rs and underscores the benefit of finding new compounds to modulate these receptors and probe their function (43, 44). Table 1 summarizes the overall yields for the initial library efforts as well as exploratory screening for κ opioid receptor (KOR), sigma 1, and sigma 2 receptor binding (Ki values) for these compounds.
Scheme 3.
Second-generation library obtained using a sequential Diels–Alder/aza–Wittig sequence.
Table 1.
Diels–Alder/aza–Wittig reaction sequence products and KOR/Sig-R binding values
| Entry | Product type | R2 = | Yield, % | KOR Ki, nM | Sigma 1 Ki, nM | Sigma 2 Ki, nM | Additional binding |
| 1 | 10 | CH2OBn | 44 | 3,604 | 59 | 124 | |
| 2 | 10 | CH2Ph | 32 | > 10,000 | 8 | 29 | D4, 8,856 |
| 3 | 10 | CH2CH2Ph | 55 | 3,630 | 8 | 11 | D4, 9,082 |
| 4 | 10 | 4-methoxyPh | 27 | no data | no data | no data | |
| 5 | 10 | 4-butoxyPh | 44 | > 10,000 | 16 | 17 | D4, 1,805 |
| 6 | 10 | 3-cyanoPh | 42 | 1,747 | 47 | 388 | |
| 7 | 10 | 4-dimethylaminoPh | 26 | > 10,000 | 6 | 124 | Alpha2A, 1,271; Alpha2B, 6,433; Alpha2C, 978; D4, 1,103; H1, 1,250; H2, 7,971; M3, 2,686; MOR 517 |
| 8 | 10 | 4-acetamidePh | 25 | 1,428 | 88 | 1,650 | |
| 9 | 10 | 3-benzyloxyPh | 41 | 2,709 | 63 | 90 | |
| 10 | 10 | 2,4-dichloroPh | 42 | 6,492 | 14 | 40 | |
| 11 | 10 | 4-chloro-3-nitroPh | 44 | 438 | 11 | 188 | |
| 12 | 10 | 3-bromo-5,6-dimethoxyPh | 27 | 6,651 | 37 | 97 | |
| 13 | 10 | 3-bromo-4-hydroxy-5-methoxyPh | 5 | > 10,000 | 1,358 | 1,937 | |
| 14 | 10 | 3-pyridine | 51 | > 10,000 | 18 | 840 | D4 8,840 |
| 15 | 10 | 2-naphthalene | 29 | 2,536 | 9 | 109 | |
| 16 | 10 | 4-quinoline | 48 | > 10,000 | 308 | no data | |
| 17 | 10 | 1-methyl-2-imidazole | 41 | > 10,000 | 1,463 | 397 | |
| 18 | 10 | 2-benzo[b]thiophene | 35 | > 10,000 | 14 | 515 | |
| 19 | 11 | Ph | 25 | 5,812 | > 10,000 | 757 | |
| 20 * | 10 | Ph | 32 | 1,115 | 2 | 175 | 5HT1A, 3,503; Alpha2A, 907; Alpha2B, 8,222; Alpha2C, 1,060; D4, 1,274; H1, 1,574; M3, 6,492; MOR, 737 |
| 21 * | 10 | 2,4,6-trifluoroPh | 51 | > 10,000 | 530 | 173 | |
| 22 | 10 | CH2CH3 | 34 | > 10,000 | 12 | 401 | 5HT1A, 4,500 |
*Epimeric at the ethyl side chain.
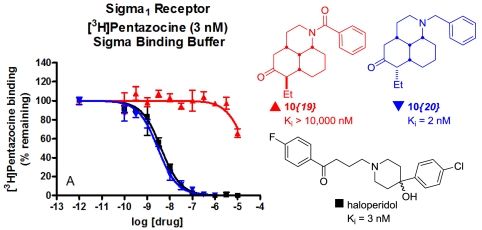
Following selective secondary binding evaluation for κ opioid and sigma binding, we noted that many compounds of this chemotype possessed potent sigma 1 receptor binding. Although full panel GPCR screening was not completed for this chemotype, six compounds (entries 1–3, 5, 6, and 14 in Table 1) were found to lack submicromolar binding against 5HT6 and D4 (arbitrarily chosen as representative countertargets). Five individual examples of compounds with a single digit nanomolar sigma 1 Ki value were found (entries 2, 3, 7, 15, and 20). In particular, entry 20 was an exciting result, due to both potency (2 nM Ki) and selectivity (sigma 1:sigma 2:KOR binding ratio of 1∶88∶558). Also of note was that the stereochemistry of the ethyl side chain has little effect on this potent sigma binding because derivatives of both epimers were similarly tight-binding. The sigma 1 secondary binding curves for the compounds in entries 19, 20, and the control compound, haloperidol, are shown in Fig. 5. The binding curves illustrate the dramatic link between activity and basic nitrogen incorporation with compound 10{20}, found to be of comparable potency to haloperidol, and compound 10{19}, found to be inactive. An analogous trend was observed for the sigma 2 binding between these compounds, although with a less drastic dependence on basic nitrogen incorporation. Although the structures of 10{20} and haloperidol differ substantially in connectivity, both contain a phenyl group tethered to the basic nitrogen. To assess whether the tricyclic scaffold is contributing significantly to the observed binding activity, the alkyl chain analogue was synthesized (entry 22). Gratifyingly, this analogue retained marked sigma binding affinity, thus dispelling any speculation that an aromatic moiety tethered amine is responsible for the activity.
Fig. 5.
Comparison of sigma 1 binding curves for select compounds. Figure comparing the binding potency of Haloperidol (black) and phenyl analogues as the free amine (blue) and where the nitrogen lone pair is tied up in amide resonance (red).
Three potent Sig-R binding compounds—10{20}, 10{22}, and 10{7} (which contains two basic nitrogen atoms)—were evaluated for their GPCR selectivity against 38 targets, and the results were incorporated in Table 1 (see SI Appendix for a heat map–formatted selectivity table). The three compounds displayed > 86∶1 selectivities for the limited number of receptors for which binding was observed at all; an impressive result for compounds obtained in such an abbreviated structure-activity optimization exercise. The N-propyl derivative 10{22}, designed to eliminate binding arising from a pendant aryl-containing side chain, was particularly selective. Here, the only non-Sig-R binding was detected against the 5HT1A receptor (Ki = 4,500 nM) as opposed to Sig-R 1 and Sig-R 2 (Ki values of 12 and 288 nM, respectively),
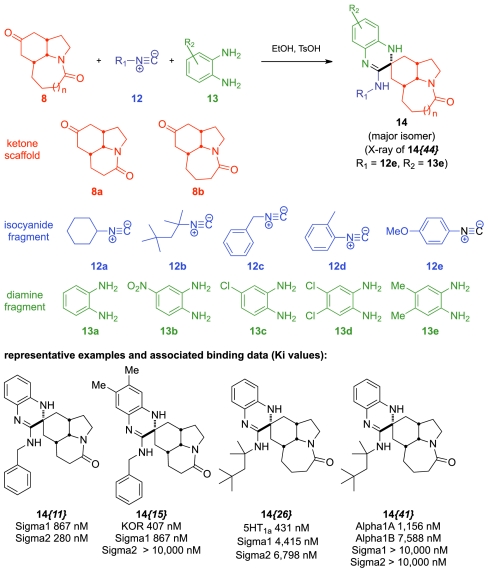
As part of our efforts toward greater skeletal diversity, additional ring systems were introduced through modifications of the ketone carbonyl on scaffolds constructed by a Diels–Alder/Schmidt reaction sequence. We applied the isocyanide multicomponent reaction (MCR) methodology developed by Shaabani et al. to transform the ketone carbonyl to the spirocyclic nitrogen heterocycles shown (Scheme 4) (45). This protocol smoothly incorporated three elements of diversity in a single operation to afford 44 heterocyclic analogues synthesized from the fragment pool in Scheme 4. This chemotype possesses an intriguing, complex spirocyclic molecular architecture not reported from any natural sources and incorporating three additional basic nitrogen sites. Although the MCR was not completely diastereoselective, a trend we had previously observed in the reductive amination at the same ketone carbonyl (39), the reaction did afford a dominant diastereomer that was assigned based on the X-ray crystal structure of the isolated compound 14{44}. Screening the full compound set for κ opioid, sigma 1, and sigma 2 receptor binding (Ki values) revealed a number of compounds with modest binding for these targets. Thus, compound 14{11} emerged as the most potent Sig-R binding candidate (Ki = 867, 280 nM for sigma 1 and sigma 2, respectively) and compound 14{15} was found to possess the most potent κ opioid binding (Ki = 407 nM) along with modest sigma 1 binding (Ki = 1,158 nM). Sixteen of the 44 compounds were also evaluated for 5HT1A binding, with only compound 14{26} found to possess significant 5HT1A binding activity (Ki = 431 nM). In addition, four compounds were screened against the full 43 GPCR assay panel as described previously for neostenine. The results showed this compound subset to be free from promiscuous binding, and compound 14{41} was discovered to possess selective adrenergic binding affinity (Ki = 1,156, 7,588 nM for Alpha1A and Alpha1B, respectively). Representative structures of this chemotype and associated binding activity are shown in Scheme 4 (for complete data on all compounds, see SI Appendix).
Scheme 4.
Construction of a novel nitrogen heterocyclic library.
Summary and Final Comments.
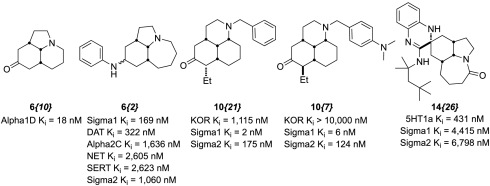
In this study, we have identified three sets of biaoctive compounds based on the tricyclic core of the Stemona alkaloid skeleton shown in Fig. 6. These compounds vary in their degrees of added structural complexity from a simple amide reduction (6{10}) to a greater than twofold increase of the molecular weight via an isocyanide MCR (14{26}). Each of these compounds has provided potent binding compounds that may be viewed as lead structures for further development. Moreover, we identified a particular chemical feature consistent with activity (a basic nitrogen atom), which suggested a scaffold-hopping maneuver that resulted in unique compounds with exquisite potency and selectivity against the Sig-Rs.
Fig. 6.
Summary of potent ligands discovered during the course of this work. Structures and significant (< 10,000 nM) binding of selected compounds highlighted in this work.
We note two strategic attributes of this work. First, the choice of testing venue for these libraries, although broad, was nonetheless informed by the reported activities of the naturally occurring alkaloids (i.e., it was reasonable to expect that purported antitussive compounds might exert effects on GPCRs found in the CNS). Second, the presence of total syntheses of the natural products themselves as well as versatile, flexible synthetic methods that would allow preparation of a diverse set of analogues was extremely helpful. In the present example, strongly potent ligands ultimately depended on the availability of a second-generation synthetic method able to provide a second major scaffold. Overall, this work demonstrates the positive synergy between the activities of natural product synthesis, synthetic methodology, and biological evaluation.
Materials and Methods
Primary and Secondary Binding Experiments.
Radioligand binding assays using cloned GPCRs, ion channels, and transporters were performed using membranes from transiently transfected or stable cell lines as previously detailed (1, 46) through the resources of the National Institute of Mental Health Psychoactive Drug Screening Program. Detailed protocols (including cell handling, buffer composition, assay conditions, etc.) for all assays are available online (http://pdsp.med.unc.edu/UNC-CH%20Protocol%20Book.pdf). Initial screening assays were performed using a 10-μM (final concentration) test compound, and the percent inhibition of specific binding by the test compound was determined. When the test compound inhibited > 50% of radioligand specific binding, Ki determinations were performed by measuring the inhibition of radioligand binding by various concentrations of test drug (11 concentrations spanning six orders of magnitude). Radioligand binding isotherms were regressed using the One Site Competition Binding function built into Prism 4.0 (GraphPad) to estimate compound IC50 values. Affinity constants (Ki values) were calculated from IC50 values using the Cheng–Prusoff approximation.
Representative Procedures.
6-Ethyl-1-propyldecahydro-1H-benzo[de]quinolin-5(3a1H)-one 10{22}.
To a solution of amine 9 (30.6 mg, 0.138 mmol) and propionaldehyde (20.0 mg, 0.345 mmol, 2.5 equiv) in dichloroethane (3 mL) was added solid sodium triacetoxyborohydride (117 mg, 0.552 mmol, 4 equiv). The reaction was stirred at room temperature (rt) for 14 h, diluted with aqueous sodium hydroxide (1 N, 6 mL), extracted with CH2Cl2 (2 × 3 mL), and evaporated to afford the crude tertiary amine products. The crude tertiary amine was dissolved in MeOH (4 mL) and K2CO3 (86 mg, 0.621 mmol, 4.5 equiv) was added. The reactions were stirred at rt for 16 h, filtered, and evaporated. Silica gel chromatography of the residue afforded 10{22} (12.4 mg, 0.047 mmol, 34% yield) as a viscous yellow oil. 1H NMR (400 MHz, CDCl3) δ 0.83 (t, J = 7.2 Hz, 3 H), 0.84 (t, J = 7.2 Hz, 3 H), 1.03 (m, 1 H), 1.10–1.34 (complex, 4 H), 1.39–1.56 (complex, 5 H), 1.60–1.67 (m, 2 H), 1.84–1.94 (m, 3 H), 2.01 (m, 1 H), 2.11 (m, 1 H), 2.31 (m, 2 H), 2.47 (m, 1 H), 2.65 (m, 1 H), 2.94 (m, 1 H); 13C NMR (100 MHz, CDCl3) δ u: 17.4, 17.9, 24.6, 29.9, 31.3, 33.3, 48.8, 52.5, 55.1, 210.8; d: 11.1, 12.0, 41.5, 44.7, 50.8, 56.1, 63.9; IR (neat) 2960, 2933, 2869, 1709 cm-1; HRMS (ESI) m/z calculated for C17H30NO ([M + H]+), 264.2327, found 264.2327.
3′-((4-Methoxyphenyl)amino)-6′,7′-dimethyl-31,5,6,7,7a,8,10,10a-octahydro-1H,1′H-spiro[azepino[3,2,1-hi]indole-9,2′-quinoxalin]-4(2H)-one 14{44}.
A reaction vial was charged with a magnetic stir bar, ketoamide 8b (50 mg, 0.24 mmol), diamine fragment 13e (39 mg, 0.29 mmol, 1.2 equiv), and p-toluenesulfonic acid (5 mg, 0.024 mmol, 0.1 equiv). A solution of isonitrile 12e (38 mg, 0.29 mmol, 1.2 equiv.) in EtOH (2.0 mL) was added via syringe through the septum cap, and the reaction was stirred for 14 h at rt. Water (7 mL) was added to precipitate the products. The reaction mixture was filtered, and the collected precipitate was washed with NaOH (0.5 N, 2 mL) and water (3 mL). The crude product was purified by chromatography on silica gel to afford the spiroheterocycle 14{44} (78 mg, 0.170 mmol, 71% yield) as a light brown solid. TLC Rf = 0.54 (50% acetone in CH2Cl2); mp > 260 °C; 1H NMR (CDCl3) δ 1.08 (t, J = 11.6 Hz, 1 H), 1.36 (m, 1 H), 1.55 (m, 2 H), 1.70–1.82 (m, 3 H), 2.09 (s, 3 H), 2.14 (s, 3 H), 2.32 (m, 2 H), 2.62 (m, 2 H), 3.23(m, 1 H), 3.50 (m, 1 H), 3.56 (m, 1 H), 3.81 (s, 3 H), 3.83 (m, 1 H), 5.30 (s, 1 H), 6.29 (s, 1 H), 6.49 (m, 2 H), 6.82 (d, J = 8.8 Hz, 1 H), 6.92 (d, J = 8.8 Hz, 2 H); 13C NMR δ d 18.9, 19.2, 33.4, 39.9, 55.5, 61.0, 115.0, 115.1 (× 2), 116.4, 122.3 (× 2); u 18.3, 28.8, 34.0, 35.7, 36.5, 39.3, 47.3, 55.6, 124.5, 127.5, 130.0, 130.2, 141.3, 152.3, 155.7, 175.3; IR 3294, 2927, 2857, 1640, 1611, 1500 cm-1; HRMS calculated for C28H35N4O2 [M + H+] 459.2760, found 459.2728. Crystals suitable for X-ray crystallographic analysis were obtained from slow evaporation of a hexanes/ethyl acetate solution.
For further synthetic details, experimental details, characterization data for all intermediates and representative library compounds, HPLC purity assessment for all library compounds, structures and Ki values for each compound in all active assays shown in Fig. 3, and additional binding assay results for chemotypes 10 and 14, see SI Appendix.
Supplementary Material
Acknowledgments.
We are grateful to Victor Day for X-ray crystallography services. We thank the National Institute of General Medical Sciences (GM-49093 and P050-GM069663), the National Institute of Mental Health’s Psychoactive Drug Screening Program [Contract HHSN-271-2008-000025-C (NIMH-PDSP)] and the National Institutes of Health (R01DA017204) for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference no. 806303).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016558108/-/DCSupplemental.
References
- 1.Roth BL, et al. Salvinorin A: A potent naturally occurring nonnitrogenous κ opioid selective agonist. Proc Natl Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonchick AP, et al. Highly enantioselective synthesis and cellular evaluation of spirooxindoles inspired by natural products. Nat Chem. 2010;2:735–740. doi: 10.1038/nchem.730. [DOI] [PubMed] [Google Scholar]
- 3.Kumar K, Waldmann H. Synthesis of natural product inspired compound collections. Angew Chem Int Ed. 2009;48:3224–3242. doi: 10.1002/anie.200803437. [DOI] [PubMed] [Google Scholar]
- 4.Nören-Müller A, et al. Discovery of protein phosphatase inhibitor classes by biology-oriented synthesis. Proc Natl Acad Sci USA. 2006;103:10606–10611. doi: 10.1073/pnas.0601490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kensuke I, Ichikawa S, Al-Dabbagh B, Bouhss A, Matsuda A. Function-oriented synthesis of simplified caprazamycins: Discovery of oxazolidine-containing uridine derivatives as antibacterial agents against drug-resistant bacteria. J Med Chem. 2010;53:3793–3813. doi: 10.1021/jm100243n. [DOI] [PubMed] [Google Scholar]
- 6.Shaw J, Menzella HG, Myles DC, Xian M, Smith AB., III Coumarin-derived discodermolide analogues possessing equivalent antiproliferative activity to the natural product-a further simplification of the lactone region. Org Biomol Chem. 2007;5:2753–2755. doi: 10.1039/b708884c. [DOI] [PubMed] [Google Scholar]
- 7.Wender PA, Verma VA, Paxton TJ, Pillow TH. Function-oriented synthesis, step economy, and drug design. Acc Chem Res. 2007;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 8.Romo D, et al. Evidence for separate binding and scaffolding domains in the immunosuppressive and antitumor marine natural product, pateamine A: Design, synthesis, and activity studies leading to a potent simplified derivative. J Am Chem Soc. 2004;126:10582–10588. doi: 10.1021/ja040065s. [DOI] [PubMed] [Google Scholar]
- 9.Wipf P, Reeves JT, Balachandran R, Day BW. Synthesis and biological evaluation of structurally highly modified analogues of the antimitotic natural product curacin A. J Med Chem. 2002;45:1901–1917. doi: 10.1021/jm0105171. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RM, Danishefsky SJ. Applications of total synthesis toward the discovery of clinically useful anticancer agents. Chem Soc Rev. 2007;36:1207–1226. doi: 10.1039/b611967k. [DOI] [PubMed] [Google Scholar]
- 11.Greger H. Structural relationships, distribution and biological activities of Stemona alkaloids. Planta Med. 2006;72:99–113. doi: 10.1055/s-2005-916258. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y-T, et al. Antitussive effects of Stemona tuberosa with different chemical profiles. J Ethnopharmacol. 2006;108:46–53. doi: 10.1016/j.jep.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Pilli RA, Rosso GB, Ferreira de Oliveira MdC. The Alkaloids: Chemistry and Biology. Vol 62. Amsterdam: Elsevier; 2005. The Stemona alkaloids; pp. 77–173. [DOI] [PubMed] [Google Scholar]
- 14.Pilli RA, Ferreira de Oliveira MdC. Recent progress in the chemistry of the Stemona alkaloids. Nat Prod Rep. 2000;17:117–127. doi: 10.1039/a902437i. [DOI] [PubMed] [Google Scholar]
- 15.Xu RS. Some bioactive natural products from Chinese medicinal plants. Stud Nat Prod Chem. 2000;21:729–772. [Google Scholar]
- 16.Lin W-H, Ye Y, Xu R-S. Chemical studies of new Stemona alkaloids, IV. Studies on new alkaloids from Stemona tuberosa. J Nat Prod. 1992;55:571–576. [Google Scholar]
- 17.Edwards OE. The Alkaloids: Chemistry and Physiology. Vol 9. New York: Academic; 1967. The Stemona alkaloids; pp. 545–551. [Google Scholar]
- 18.Shinozaki H, Ishida M. Inhibitory actions of tuberostemonine on the excitatory transmission at the crayfish neuromuscular junction. Brain Res. 1985;334:33–40. doi: 10.1016/0006-8993(85)90564-5. [DOI] [PubMed] [Google Scholar]
- 19.Leung PHH, Zhang L, Zuo Z, Lin G. Intestinal absorbtion of Stemona alkaloids in a Caco-2 cell model. Planta Med. 2006;72:211–216. doi: 10.1055/s-2005-916195. [DOI] [PubMed] [Google Scholar]
- 20.Chung H-S, Hon P-M, Lin G, But PP-H, Dong H. Antitussive activity of Stemona alkaloids from Stemona tuberosa. Planta Med. 2003;69:914–920. doi: 10.1055/s-2003-45100. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, et al. Oral absorption and antitussive activity of tuberostemonine alkaloids from the roots of Stemona tuberosa. Planta Med. 2009;75:575–580. doi: 10.1055/s-0029-1185363. [DOI] [PubMed] [Google Scholar]
- 22.Yang X-Z, et al. Alkaloids from roots of Stemona sessilifolia and their antitussive activities. Planta Med. 2009;75:174–177. doi: 10.1055/s-0028-1088345. [DOI] [PubMed] [Google Scholar]
- 23.Lin L-G, et al. Croomine- and tuberostemonine-type alkaloids from roots of Stemona tuberosa and their anittussive activity. Tetrahedron. 2008;64:10155–10161. [Google Scholar]
- 24.Frankowski KJ, Golden JE, Zeng Y, Lei Y, Aubé J. Syntheses of the Stemona alkaloids (±)-stenine, (±)-neostenine, and (±)-13-epineostenine using a stereodivergent Diels–Alder/azido-Schmidt reaction. J Am Chem Soc. 2008;130:6018–6024. doi: 10.1021/ja800574m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y, Aubé J. An expeditious synthesis of (±)-stenine. J Am Chem Soc. 2005;127:15712–15713. doi: 10.1021/ja055629m. [DOI] [PubMed] [Google Scholar]
- 26.Golden JE, Aubé J. A combined intramolecular Diels–Alder/intramolecular Schmidt reaction: Formal synthesis of (±)-stenine. Angew Chem Int Ed Engl. 2002;41:4316–4318. doi: 10.1002/1521-3773(20021115)41:22<4316::AID-ANIE4316>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 27.Lainchbury MD, et al. A protecting group free synthesis of (±)-neostenine via the [5 + 2] photocycloaddition of maleimides. J Org Chem. 2008;73:6497–6505. doi: 10.1021/jo801108h. [DOI] [PubMed] [Google Scholar]
- 28.Wipf P, Spencer SR. Asymmetric total syntheses of tuberostemonine, didehydrotuberostemonine, and 13-epituberostemonine. J Am Chem Soc. 2005;127:225–235. doi: 10.1021/ja044280k. [DOI] [PubMed] [Google Scholar]
- 29.Padwa A, Ginn JD. Studies on the synthesis of (±)-stenine: A combined intramolecular [4 + 2]-cycloaddition/rearrangement cascade. J Org Chem. 2005;70:5197–5206. doi: 10.1021/jo050515e. [DOI] [PubMed] [Google Scholar]
- 30.Ginn JD, Padwa A. Total synthesis of (±)-stenine using the IMDAF cycloaddition of a 2-methylthio-5-amido-substituted furan. Org Lett. 2002;4:1515–1517. doi: 10.1021/ol025746b. [DOI] [PubMed] [Google Scholar]
- 31.Wipf P, Spencer SR, Takahashi H. Total synthesis of (-)-tuberostemonine. J Am Chem Soc. 2002;124:14848–14849. doi: 10.1021/ja028603t. [DOI] [PubMed] [Google Scholar]
- 32.Morimoto Y, Iwahashi M, Kinoshita T, Nishida K. Stereocontrolled total synthesis of the Stemona alkaloid (-)-stenine. Chem Eur J. 2001;7:4107–4116. doi: 10.1002/1521-3765(20011001)7:19<4107::aid-chem4107>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto Y, Iwahashi M, Nishida K, Hayashi Y, Shirahama H. Studies on the asymmetric synthesis of Stemona alkaloids: Total synthesis of (-)-stenine. Angew Chem Int Ed Engl. 1996;35:904–906. [Google Scholar]
- 34.Morimoto Y, Iwahashi M, Nishida K, Hayashi Y, Shirahama H. Studies on the asymmetric synthesis of Stemona alkaloids: Total synthesis of (-)-stenine. Angew Chem Int Ed Engl. 1996;108:968–970. [Google Scholar]
- 35.Wipf P, Kim Y, Goldstein DM. Asymmetric total Synthesis of the Stemona alkaloid (-)-stenine. J Am Chem Soc. 1995;117:11106–11112. [Google Scholar]
- 36.Chen C-Y, Hart D. A Diels-Alder approach to Stemona alkaloids: Total synthesis of stenine. J Org Chem. 1993;58:3840–3849. [Google Scholar]
- 37.Chen C-Y, Hart D. Total synthesis of dl-stenine. J Org Chem. 1990;55:6236–6240. [Google Scholar]
- 38.Zeng Y, Reddy S, Hirt E, Aubé J. Domino reactions that combine an azido-Schmidt ring expansion with the Diels–Alder reaction. Org Lett. 2004;6:4993–4995. doi: 10.1021/ol047809r. [DOI] [PubMed] [Google Scholar]
- 39.Frankowski KJ, Neuenswander B, Aubé J. Explorations of stemona alkaloid-inspired analogues: Skeletal modification and functional group diversification. J Comb Chem. 2008;10:721–725. doi: 10.1021/cc800078h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahama K, Shirasaki T, Soeda F. Handbook of Experimental Pharmacology. Vol 187. Berlin: Springer; 2009. Central mechanisms III: Neuronal mechanisms of action of centrally acting antitussives using electrophysiological and neurochemical study approaches; pp. 219–240. [DOI] [PubMed] [Google Scholar]
- 41.Brown C, Fezoui M, Selig WM, Schwartz CE, Ellis JL. Antitussive activity of sigma-1 receptor agonists in the guinea-pig. Brit J Pharmacol. 2004;141:233–240. doi: 10.1038/sj.bjp.0705605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pubill D, et al. Effect of PCP and sigma ligands on both noradrenaline- and electrically-induced contractions and on [3H]-noradrenaline uptake in rat vas deferens. J Auton Pharmacol. 1998;18:239–244. doi: 10.1046/j.1365-2680.1998.18491.x. [DOI] [PubMed] [Google Scholar]
- 43.Leonard BE. Sigma receptors and sigma ligands: Background to a pharmacological enigma. Pharmacopsychiatry. 2004;37:S166–S170. doi: 10.1055/s-2004-832674. [DOI] [PubMed] [Google Scholar]
- 44.Walker JM, et al. Sigma receptors: Biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- 45.Shaabani A, Maleki A, Mofakham H, Khavasi HR. Novel isocyanide-based three-component synthesis of 3,4-dihydroquinoxalin-2-amine derivatives. J Comb Chem. 2008;10:323–326. doi: 10.1021/cc7001777. [DOI] [PubMed] [Google Scholar]
- 46.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.