Abstract
Lung morphogenesis is a well orchestrated, tightly regulated process through several molecular pathways, including TGF-β/bone morphogenetic protein (BMP) signaling. Alteration of these signaling pathways leads to lung malformation. We investigated the role of Follistatin-like 1 (Fstl1), a secreted follistatin-module–containing glycoprotein, in lung development. Deletion of Fstl1 in mice led to postnatal lethality as a result of respiratory failure. Analysis of the mutant phenotype showed that Fstl1 is essential for tracheal cartilage formation and alveolar maturation. Deletion of the Fstl1 gene resulted in malformed tracheal rings manifested as discontinued rings and reduced ring number. Fstl1-deficient mice displayed septal hypercellularity and end-expiratory atelectasis, which were associated with impaired differentiation of distal alveolar epithelial cells and insufficient production of mature surfactant proteins. Mechanistically, Fstl1 interacted directly with BMP4, negatively regulated BMP4/Smad1/5/8 signaling, and inhibited BMP4-induced surfactant gene expression. Reducing BMP signaling activity by Noggin rescued pulmonary atelectasis of Fstl1-deficient mice. Therefore, we provide in vivo and in vitro evidence to demonstrate that Fstl1 modulates lung development and alveolar maturation, in part, through BMP4 signaling.
Keywords: lung atelectasis, trachea formation, surfactant protein C, lung epithelial differentiation
Lung development is a well orchestrated process that is tightly regulated by transcription factors, hormones, growth factors, and other factors in temporal and spatial manners (1, 2). The mouse lung is derived from foregut endoderm in an embryonic day (E) 9.5 embryo. Trachea arises from the more proximal foregut tube, whereas the rest of the lung develops from two ventral buds that form at the distal end of the trachea, and undergoes branching morphogenesis to produce the pulmonary tree (3, 4). This branching morphogenesis is accompanied by differentiation of epithelial cell types along a proximal–distal axis, including bronchial Clara cells (proximal) and alveolar type I and type II epithelial cells (AEC-I and AEC-II, respectively; distal) (5). These highly specialized cell types render specific functions in the respiratory tract, such as the functional alveolar surface area formed by AEC-I and AEC-II cells for gas exchange (6).
TGF-β superfamily growth factors regulate organogenesis, including that of the lung (1, 7). For example, they exert an inhibitory effect on lung branching morphogenesis (8). Interference of TGF-β signaling with a dominant-negative TβRII (9) or anti-Smad2/3 oligos (10) in embryonic lung organ cultures stimulates branching morphogenesis. BMP4 is dynamically expressed in the distal epithelium; disruption of BMP4 signaling in lungs of Sp-C-Xnoggin or Sp-C-dnAlk6 transgenic mice abrogates the proximal–distal patterning in the lung where distal epithelial differentiation is inhibited while proximal differentiation is promoted (11, 12). BMP4 gain of function in the lung results in less extensive branching and decreased distal epithelial differentiation (11). The precise mechanism of TGF-β family members in regulating lung development is largely unclear.
Follistatin-like 1 (Fstl1), first identified as a TGF-β1–inducible gene (13), encodes a secreted extracellular glycoprotein belonging to the Fst-SPARC family, whose amino acid sequence contains a follistatin-like domain (14, 15). Its functions and the underlying mechanism are poorly understood. Studies in zebrafish (16, 17) suggest a developmental role of Fstl1 in early dorsoventral body axis establishment. In vitro studies have shown that Fstl1 is one of the mesenchymal factors determining oviductal epithelial cell fate (18). In this study, we generated Fstl1-deficient mice to examine the role of Fstl1 in lung development and found that Fstl1 is essential for normal tracheal formation as well as alveolar maturation. Furthermore, we demonstrated that Fstl1 regulates the differentiation of lung epithelial cells, in part, through negative regulation of BMP4 signaling.
Results
Generation of Fstl1-Deficient Mice.
To determine the biological function of Fstl1 in vivo, we first generated Fstl1+/− mice by intercrossing EIIa-Cre;Fstl1flox/+ mice (Fig. S1 A and B). Fstl1−/− mice were then generated by intercrossing Fstl1+/− mice. Western blotting confirmed the loss of Fstl1 protein expression (Fig. 1A). Fstl1−/− pups were born alive at the expected Mendelian ratio (27%, 127 of 472). However, all homozygous pups breathed irregularly and displayed a cyanotic skin color, then died shortly after birth (Fig. 1B). In addition, Fstl1−/− neonates displayed multiple defects, including abnormal dorsal–ventral pattern of the neural tube, hydroureter, and overall skeletal defects. Consistent with the pleiotropic developmental defects caused by the loss of Fstl1, in situ hybridization revealed widespread Fstl1 expression during mouse embryonic development, including in the lung (19).
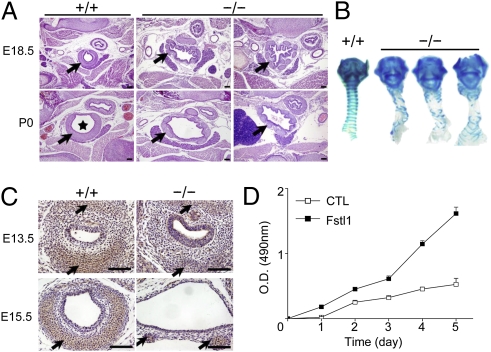
Fig. 1.
Generation of Fstl1−/− mice. (A) Western blot analysis of Fstl1 proteins from E15.5 embryos (Upper) or E18.5 lung tissues (Lower). (B) Examination of neonates after birth revealed that Fstl1−/− neonates were cyanotic. (C) Autopsy observation showed that WT lungs were expanded by inhalation of air, which can be seen as air bubbles in the distal regions, but Fstl1−/− lungs were collapsed and did not show evidence of air in the distal airways (tr, trachea).
Tracheal Malformation in Fstl1-Deficient Mice.
Fstl1−/− neonates had soft and unusually large tracheal tubes (Fig. 1C). Transverse sections of E18.5 homozygous trachea revealed a striking deformed lumen. Fstl1−/− tracheas were enlarged at the upper and lower levels but narrowed at the middle level, and their inner margins were irregular, with many small folds sometimes accompanied by protuberances in the tracheal aperture (Fig. 2A and Fig. S2A). At birth [postnatal day (P) 0], apart from a few instances of tracheal stenosis, most lumens of Fstl1−/− tracheas (>80%) were larger than their WT controls (Fig. 2A and Fig. S2A). These larger tracheas could be observed as early as E15.5 (Fig. S2B). Another consistent observation was a profound disorganization of the Fstl1−/− tracheal epithelium (Fig. S2C).
Fig. 2.
Tracheal malformation in Fstl1−/− mice. (A) Transverse sections of the middle portion of trachea from a WT and two homozygous mice before (E18.5) and after breath (P0) showed an increase in the lumen diameter (asterisk), dispersion and discontinuity of cartilage ring (arrow), and disorganization of the epithelial layer in the mutants. (Scale bars, 100 μm.) (B) Alcian blue staining revealed impaired banding pattern of tracheal C-ring cartilage in all mutant skeletal preparations (ventral views). (C). Sections stained with an antibody against type II collagen (arrows). (Scale bars, 100 μm.) (D). Effects of stable overexpression of Fstl1 on cell proliferation of ATDC5 cells with MTT assays.
E15.5 and E18.5 Fstl1−/− trachea also displayed interrupted or truncated cartilages, whereas their WT littermates showed C-shaped cartilage rings (Fig. 2A and Fig. S2 A and B). The larynx and the trachea were greatly disorganized in all homozygous fetuses (Fig. 2B). The number of tracheal rings formed was reduced (Fstl1+/+, 14 rings, n = 15; Fstl1−/−, four to eight rings, n = 10) and the rings did not grow and extend as dorsally as those in WT samples. These impaired cartilages failed to provide the airway with a rigid skeletal support, resulting in the soft and flabby tracheal tubes. Immunohistochemistry (IHC) analysis also revealed extremely attenuated type II collagen signals at E13.5 and E15.5 (Fig. 2C), showing the defective cartilaginous differentiation in Fstl1−/− mice.
It has been suggested that tracheal cartilage formation is a multistep process. The committed mesenchymal cells first condense and proliferate to form cartilage primordia that prefigure the overall shape of future cartilages (E10.5–E12.5). Expression of cartilage-specific proteins (such as type II collagen) is then initiated and cells differentiate into chondrocytes (E13.5–E15.5). After E15.5, well formed C-rings can be observed (20). To further examine the function of Fstl1 in cartilaginous development, we generated stable clones overexpressing Fstl1 using murine mesenchymal cells, ATDC5, which can differentiate into chondrocytes in the presence of insulin-transferrin-sodium selenite (ITS) (21). As determined by MTT assay, Fstl1 increased the proliferation of mesenchymal cells (Fig. 2D). Fstl1 also strongly promoted ITS-induced Col2a1 mRNA expression (6.7 fold) by using quantitative RT-PCR (qRT-PCR). Collectively, Fstl1 is essential for all chondrogenic steps in the development of the upper respiratory tract. Deletion of Fstl1 limits the proliferation and differentiation of cartilaginous precursors, resulting in malformed rings during tracheal development. However, Fstl1−/− trachea with impaired cartilage rings does not develop significant stenosis, indicating that tracheal defects may not be the main cause of respiratory failure in Fstl1−/− neonates.
Lung Atelectasis in Fstl1-Deficient Mice.
Gross phenotype of lung was largely normal in neonatal Fstl1−/− pups. Their lungs had similar size and the correct number of lobes (Fig. 1C), and showed similar lung/body dry weight ratio compared with their WT littermates (Fstl1−/−, 0.47%, n = 7; Fstl1+/+, 0.48%, n = 10; P > 0.05). The striking abnormality of Fstl1−/− lungs was their condensed appearance with a few big air bubbles in the distal airways (Fig. 1C). Histological examination of WT lungs displayed many small distal saccules with thin septa (E18.5) and showed normal saccular expansion at birth (P0). By contrast, E18.5 Fstl1−/− lungs had a 60% reduction in air sac spaces and thickened hypercellular intersaccular septa (Fstl1−/−, 30.5 ± 2.0 μm; Fstl1+/+, 15.5 ± 2.2 μm, n = 4; P < 0.05; Fig. 3A). At birth (P0), they were atelectatic and characterized by areas of poorly expanded sacs as well as some compensating overexpanding bronchioli (Fig. 3A and Fig. S3A). This condensed appearance started from the saccular stage (E17.5 to approximately P0; Fig. S3A).
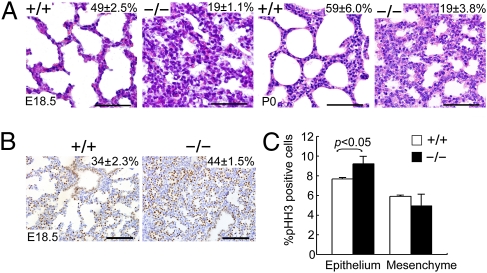
Fig. 3.
Abnormal lung morphogenesis and lung epithelial cell hyperplasia in Fstl1−/− mice. (A) Histological analysis of embryos (E18.5) and newborn pups (P0) revealed normal inflated alveoli with thinner septa in WT, but thickened hypercellular septa with reduced airspaces in Fstl1−/−. (Scale bars, 50 μm.) (B) Distribution of TTF1 by IHC. (Scale bars, 100 μm.) The percentage of the total examined sac area of the lung section or the percentage of the TTF1-positive cells in the total cells of the lung are shown in the top right corner of each diagram (P < 0.05). (C) Quantification of cell proliferation by p-HH3 immunostaining in E15.5 WT and Fstl1−/− lungs. The graph represents the mean of four independent experiments showing the comparison of the percentages of p-HH3–positive cells in the epithelium and mesenchyme between WT and Fstl1−/− lungs.
To determine whether the hypercellularity of Fstl1−/− lungs is caused by increased relative numbers of epithelial cells, we performed IHC with antibody to TTF1, which marks all lung epithelial cells. At E18.5, Fstl1−/− lungs had a 29% increase in the number of TTF1-positive epithelial cells (Fig. 3B) compared with WT controls. Sections were also stained with a phosphohistone H3 (p-HH3) monoclonal antibody to detect cells undergoing mitosis. At E15.5, Fstl1−/− lungs showed a 19% increase in the number of p-HH3–positive cells in the epithelial compartment but a slight reduction in the mesenchymal compartment (Fig. 3C and Fig. S3B). By E18.5, few stained cells were present in either Fstl1−/− or WT lungs (Fig. S3B). BrdU incorporation showed a similar result (Fig. S3 C and D). In addition, no significant cell death was observed by TUNEL staining in either Fstl1−/− or WT lungs. Thus, Fstl1 deficiency increases epithelial cell proliferation, resulting in a hypercellular phenotype of Fstl1−/− lungs.
Impaired Distal Epithelial Differentiation in Fstl1−/− Lung.
To determine whether the epithelial differentiation occurred properly in hypercellular septa of Fstl1−/− embryos, markers for proximal (CC10) and distal (SP-C) epithelium were used to immunostain sections of E18.5 lungs. Both markers were present in the Fstl1−/− lungs, suggesting that the absence of Fstl1 activity does not interfere with the differentiation of specialized lung epithelial cells along a proximal–distal axis. However, Fstl1−/− lungs had a higher percentage of SP-C–positive staining cells (Fstl1−/−, 24.98 ± 3.6%; Fstl1+/+, 12.65 ± 0.23%, n = 4; P < 0.01; Fig. 4A), which was consistent with the higher Sftpc mRNA level in Fstl1−/− lungs compared with WT lungs (Fig. 4B). The expression pattern and mRNA level of Cc10 were comparable between WT and Fstl1−/− lungs (Fig. 4 A and B).
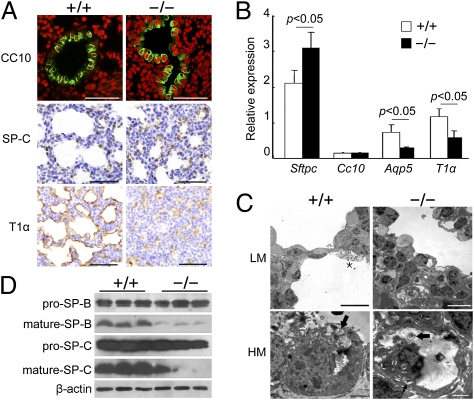
Fig. 4.
Impaired alveolar epithelial cell differentiation/maturation in Fstl1−/− lungs. (A) Expression of differentiation marker genes for lung epithelial cells in E18.5 embryos. (Scale bars, 50 μm.) (B) The relative expression levels of differentiation marker genes in E18.5 Fstl1−/− lungs as determined by qRT-PCR. Data represent the mean ± SEM in triplicates. (C) Transmission EM of the lung septa of E18.5 embryos shown at low magnification (LM) and high magnification (HM). Squamous AEC-I cell in WT was closely opposite to densely stained capillary endothelial cell creating thin blood–air barrier (Upper Left). Cuboidal AEC-II cells in WT lungs contained many lamellar bodies (arrows) and apical microvilli (Lower Left). Surfactants (asterisk) were visible in the saccular spaces (Upper Left). The blood–air barrier was significantly thicker with increased numbers of undifferentiated cuboidal epithelial cells in Fstl1−/− (Upper Right). These cells were immature with dispersed cytoplasmic glycogen and some small lamellar bodies (arrows; Lower Right). (Scale bars: Upper, 10 μm; Lower, 2 μm.) (D) Western blotting of pro–SP-C and pro–SP-B, mature SP-C, and mature SP-B proteins in extracts of whole lungs taken from WT and Fstl1−/− embryos at E18.5.
To analyze distal epithelial differentiation in Fstl1−/− lungs, we next performed IHC with antibodies specific for AEC-I cells (T1α) or AEC-II cells (SP-C). In contrast to the increased expression of SP-C, the percentage of T1α staining coverage was decreased in the lung saccules of Fstl1−/− mice (4.8 ± 1.7%, n = 4) compared with WT controls (12.2 ± 3.2%, n = 4; P < 0.05; Fig. 4A). qRT-PCR analyses showed a marked decrease in mRNA expression levels of both T1α and Aqp5 (AEC-I cell marker) in E18.5 Fstl1−/− lungs (Fig. 4B), confirming less differentiation of AEC-I cells.
We further examined the lung epithelium from E18.5 WT and Fstl1−/− mice at the ultrastructural level. Squamous AEC-I cells were clearly visible in WT lungs whereas it was lacking in Fstl1−/− saccules (Fig. 4C). Although the cuboidal AEC-II cells were observed in both WT and mutant lungs, the WT cells were mature with many apical microvilli and lamellar bodies. Surfactant materials were observed within the saccular spaces. By contrast, most cuboidal AEC-II cells lining Fstl1−/− saccules were immature with smaller apical microvilli, fewer developing lamellar bodies, and dispersed cytoplasmic glycogen (Fig. 4C). The glycogen-enriched immature AEC-II cells were further determined by increased periodic acid–Schiff (PAS) staining in the saccular epithelium (Fig. S3E), compared with WT controls. Taken together, deletion of Fstl1 is associated with impaired distal epithelial differentiation, as manifested by the promoted differentiation but delayed maturation of AEC-II cells and the less differentiated AEC-I cells. This structural immaturation of saccular epithelium causes respiratory failure of Fstl1−/− neonates.
Surfactant Dysfunction of Immature AEC-II Cells in Fstl1−/− Mice.
Mature AEC-II cells are responsible for the production and secretion of the lung surfactants that are crucial in lowering the surface tension in the lung and thereby preventing end-expiratory atelectasis (22). An insufficient production of surfactants, especially the two surfactant-associated proteins (SP-B and SP-C), in both infants and adults, has already been reported to be associated with respiratory distress syndrome (7, 23). We next analyzed the expression and secretion of SP-B and SP-C in Fstl1−/− lungs. Western blot analysis (Fig. 4D) showed that deletion of Fstl1 slightly affected the expression of pro-SP-B/SP-C from E18.5 lung tissues. However, production of mature SP-B/SP-C was strikingly decreased in Fstl1−/− lungs. These data suggest that absence of Fstl1 activity interferes with SP-B/SP-C mature processing in AEC-II. The reduced surfactant production of immature AEC-II cells led to Fstl1−/− lung atelectasis.
Fstl1 Binds to BMP4 and Negatively Regulates Smad Signaling in Vitro.
To determine the molecular mechanisms whereby the absence of Fstl1 activity results in the phenotypes described earlier, we examined the Smad-mediated TGF-β/activin/BMP signaling. Western blotting using lung extracts showed higher phosphorylation levels of Smad1/5/8 (Fig. 5A) from E18.5 Fstl1−/− lungs than those from WT littermates, whereas similar levels of phosphor-Smad2 were observed between the genotypes (Fig. S4A). These data indicate that the modulating effects of Fstl1 on BMP/Smad1/5/8 signaling are during the saccular lung development. We then examined the role of Fstl1 by using in vitro cultured Hep3B cells. As shown in Fig. 5B, phosphorylation of Smad1 induced by BMP4 was inhibited by recombinant Fstl1 protein or Fstl1 overexpression. In agreement with this, Fstl1 inhibited the BMP4-induced expression of the reporters BRE-luciferase (Fig. 5C) or GCCG-luciferase (Fig. S4B) in a dose-dependent manner. Meanwhile, Fstl1 featured a comparable activity to other BMP antagonists, such as Noggin and Follistatin (Fig. 5B and C), further supporting its negative role in regulating BMP4/Smad signaling.
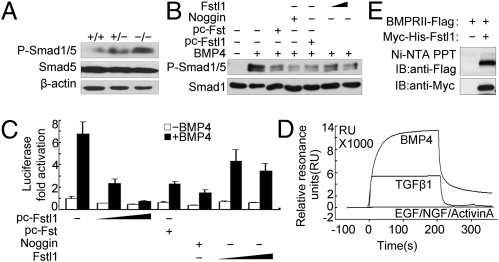
Fig. 5.
Fstl1 modulated BMP4/Smad1/5/8 signaling via binding to BMP4. (A) Phosphorylated Smad1/5 in lung tissues from WT and Fstl1−/− embryos at E18.5. (B) Fstl1 inhibited BMP4-induced phosphorylation of Smad1/5 in Hep3B cells. Recombinant Fstl1 protein (25, 100 ng/mL) or transient overexpression of Fstl1 (pc-Fstl1, 1 μg) inhibited Smad1/5 phosphorylation induced by BMP4 (20 ng/mL). Noggin (25 ng/mL) and pc-Follistatin (Fst) (1 μg) were used as positive controls because they are known BMP antagonists. After 1 h of BMP4 treatment, cells were harvested for immunoblotting. (C) Fstl1 inhibited BMP4-induced expression of the reporter BRE-luciferase activities in Hep3B cells. The construct of BRE-luciferase reporter (0.3 μg) was cotransfected with pc-Fstl1 (0.1 μg, 0.2 μg) or treated with recombinant Fstl1 protein (25 ng/mL or 100 ng/mL). After 16 h of BMP4 treatment, cells were harvested for luciferase assay. Pc-Fst (0.1 μg) or Noggin (25 ng/mL) were used as control. The data represent the mean ± SEM of three independent experiments after normalized to Renilla activity. (D) Sensorgrams of SPR analyses show the binding of Fstl1 to BMP4 or to TGF-β1, not to activin A; EGF and NGF were used as negative controls. (E) Fstl1 binds to type II receptor of BMP4 using a pull-down assay. The Ni-Fstl1-BMPRII protein complex was immunoblotted with anti-Flag antibody to confirm the presence of BMPRII (Upper). Each blot was further developed with anti-Myc antibody to confirm the presence of Fstl1 (Lower).
As Fstl1 is a secreted protein, we reasoned that it should function at the ligand/receptor level. To test this possibility, we used constitutively active BMP type I receptors (caALK1 and caALK6), which can activate Smad1/5/8 independently of ligands and type II receptors. Overexpression of Fstl1 did not inhibit caALK1/6-induced activation of GCCG-luciferase (Fig. S4C), suggesting that Fstl1 acts upstream of BMP type I receptors. We then determined whether Fstl1 could bind to either BMP4 or BMP4 receptors. The binding affinity of Myc-His-tagged mouse Fstl1 to BMP4 was determined by surface plasmon resonance (SPR) analysis. Kinetic measurements using different concentrations of Fstl1 yielded a Kd of 7.2 nM for BMP4 (Fig. 5D). There was weak binding of Fstl1 to TGF-β1 (Kd of 36 nM), but no binding to activin A, nerve growth factor, or epidermal growth factor. These data indicate that Fstl1 directly and specifically binds BMP4. We further examined whether Fstl1 could bind to BMP receptors. Fstl1 could pull down the BMP type II receptor (BMPRII; Fig. 5E), but not the type I receptor, ALK6 (Fig. S4D). As Fstl1 has the ability to interact with both BMP4 and its type II receptors, we deduced that there is competition between BMP and Fstl1 in receptor binding. Indeed, BMP4 competed with Fstl1 in binding with BMPRII (Fig. S4E). We therefore concluded that Fstl1 negatively regulates BMP4 signaling through interfering with the ligand–receptor interaction.
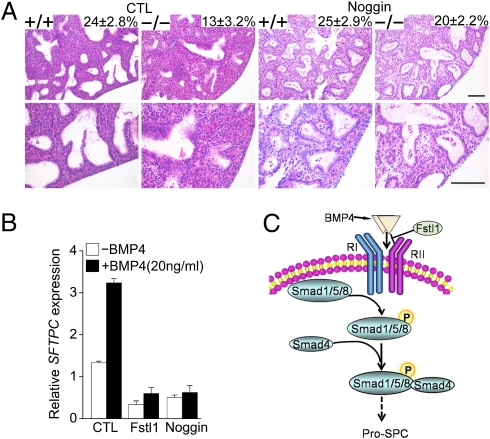
Reducing BMP Signaling Activity Rescues Pulmonary Atelectasis of Fstl1−/− Mice.
If increased BMP/Smad signaling in Fstl1−/− lung plays a causative role in the development of atelectasis, it is expected that reducing BMP signaling would ameliorate or even rescue the atelectasis in Fstl1−/− lungs. To test this possibility, we used an E15.5 embryonic mouse lung explant model of saccular stage development (24) and added Noggin, an antagonist of BMP ligand, to reduce BMP signaling activity. After 48 to 54 h of ex vivo culture, sections of Fstl1−/− explants showed condensed/atelectatic phenotype with 54% less dilation of saccular airways compared with WT controls (Fig. 6A), similar to the 60% reduction in the sac spaces of in vivo E18.5 Fstl1−/− lungs (Fig. 3A). Addition of Noggin increased the saccular spaces of Fstl1−/− explant, with 17% less dilation than that of WT control, indicating the atelectatic defect seen in Fstl1−/− lungs was partially rescued (Fig. 6A). Bright-field microscopy images of saccular airways at the explant periphery showed a similar result (Fig S5 A and B). Furthermore, we observed that Fstl1 had similar inhibitory effect of Noggin on BMP4-induced SFTPC mRNA expression in A549 cells (Fig. 6B). Additionally, Noggin reduced Sftpc mRNA expression in Fstl1−/− explants (Fig S5C). These data demonstrate a functional link between the Fstl1 and BMP4-dependent signaling pathways (Fig. 6C) during embryonic saccular lung development.
Fig. 6.
Reducing BMP4 signaling activity rescued atelectasis phenotype of Fstl1−/− embryonic lung explants. (A) Noggin increased saccular dilation of Fstl1−/− fetal lung explants. E15.5 lung explants from WT and Fstl1−/− littermates were cultured for 48 to 54 h in absence (CTL) or presence of Noggin (500 ng/mL). H&E staining of explant sections shown at low (Upper) and high (Lower) magnification. Saccular dilation was shown in Noggin treated-Fstl1−/− explants. The percentage of the total examined sac area of the lung section is shown in the top right corner of each diagram (Upper). (Scale bar, 100 μm.) (B) Fstl1 (100 ng/mL) inhibited BMP4-increased SFTPC expression in A549 cells. The data represent the mean ± SEM of three independent experiments. (C) Summary of the role of Fstl1 in embryonic lung development. Fstl1 modulates BMP4-induced Smad-1/5/8 activity and inhibits pro–SP-C expression.
Discussion
The lung is derived from an outpocketing of the foregut endoderm into the mesenchyme of the fetal thorax. Its formation depends upon complex interactions between epithelial–mesenchymal cells via paracrine factors. The present study provides evidence for the critical role of the secreted glycoprotein Fstl1 in mouse lung and trachea development. Targeted inactivation of the Fstl1 gene resulted in tracheal flaccidity, saccular septal hyperplasia, end-expiratory atelectasis, impairments of distal saccular epithelial cell differentiation and maturation, and, ultimately, failure of lung function. Mechanistically, Fstl1 executes its functions partially through interaction with BMP4 to negatively modulate BMP4/Smad signaling.
Mice lacking Fstl1 showed malformed tracheal cartilage rings. Malformations of laryngotracheal cartilage in human infants with congenital airway anomalies can cause death (25). However, to date, the genetic basis of this malformation has not been established. Our model suggests Fstl1 as a candidate gene for correct laryngotracheal cartilage formation. During cartilaginous development, Fstl1 might promote the proliferation of committed mesenchymal cells in cartilage primordia, or help these precursor cells to differentiate into chondrocytes. The role of Fstl1 in chondrogenesis is supported by the evidence of overall skeletal defects in Fstl1−/− mice. Interestingly, without the rigid skeleton support, Fstl1−/− mice did not develop tracheal stenosis, which has been seen in the neonatal lethality of mice deficient in Wnt5a (26) and Hoxa5 (27). Instead, Fstl1−/− mice had enlarged tracheal lumens. Similar observations were reported in Hoxa-3 mutants (28). It is unclear whether other tracheal tissue components are involved.
Target deletion of Fstl1 causes inhibition of saccular structural maturation. This abnormal phenotype is characterized by failure in progression of saccular septa thinning, which is necessary for gas exchange. In Fstl1−/− mice, saccules were lined by a cuboidal epithelium that lacked squamous AEC-I cells. Fstl1 inactivation appeared to increase lung cellular proliferation (29, 30), but these cuboidal cells were immature SP-C–positive AEC-II–like cells containing large glycogen droplets and some LB bodies. Our model is different from many genetically immature lung phenotypes, such as Foxa2-null mice (31) and Pten-null mice (32), in which numerous proliferative epithelial cells in the thickened saccular septa are often accompanied with the inhibition of AEC-II cell differentiation as reflected by the reduced Sftpc expression and few LB bodies. The exact molecular mechanisms responsible for this specific activity of Fstl1 remain to be elucidated. On the contrary, our model is in agreement with the hypothesis that AEC-I cells are differentiated from AEC-II cells (6) and further suggests that the requirement of mature AEC-II cells.
Structural immaturation of Fstl1−/− lungs was accompanied by biochemical immaturation. The productions of mature SP-B/C in Fstl1−/− lungs were significantly decreased. Clinically, respiratory failure of neonates mostly resulted from insufficient production of surfactant (7, 33). Infants born with congenital SP-B or SP-C deficiency (33) and mice with a targeted deletion of the SP-B gene (23) succumb to respiratory distress syndrome. The production of mature SP-B/SP-C requires specific multistep proteolytic cleavages as pro–SP-B/C are trafficked through the regulated secretory route (22). Unfortunately, the mechanisms underlying this are largely unknown. We postulate that Fstl1 may play a role in surfactant processing. The structural and biochemical immaturation of Fstl1−/− lungs causes saccular collapse (pulmonary atelectasis), which is the main cause for neonatal death.
In the present study, we provided several lines of evidence that Fstl1 may modulate BMP signaling. Although its orthologues in zebrafish, zfstl 1/2, have been observed to function redundantly to nog1 and chd to antagonize BMP activity during zebrafish development (16, 17), effects of Fstl1 on BMP signaling are still under discussion (18). No evidence of the interaction between Fstl1 and TGF-β superfamily proteins has been proposed. Our in vitro data showed that Fstl1 can directly bind BMP4 and exert its function by interfering with the BMP4/BMPRII complex and negatively regulate downstream Smad signaling. This is similar to the function of its paralogue Follistatin. Follistatin is a well known TGF-β superfamily antagonist protein, with a high binding affinity for activin (34). Although, Fstl1 does not bind activin, it binds BMP4 with a Kd similar to that of Follistatin. It is unclear whether Fstl1 binds BMP4 via FS domain in a manner similar to Follistatin (14, 34).
BMP4 signaling has been implicated in the regulation of morphologically correct development of lung, specifically distal epithelial differentiation (11, 12). In the current mouse model, we observed up-regulation of phospho-Smad1/5 together with the increased SP-C–positive staining AEC-II cells in atelectatic Fstl1−/− lungs. Reducing BMP signaling activity by Noggin partially rescued the collapsed saccules and reversed the increased Sftpc mRNA levels in Fstl1−/− lung explants. The defective phenotype is, at least in some respects, similar to those reports for impaired BMP activity (12), supporting our hypothesis that Fstl1 affects distal lung epithelial differentiation partially through modulating BMP signaling. Fstl1 may orchestrate with other BMP antagonists via their individual spatiotemporal expression patterns and control BMP4 signaling that is required for distal epithelial differentiation.
We have shown here that targeted deletion of the Fstl1 gene in mice caused neonatal death from tracheal impairment and saccular immaturity. Fstl1 is essential for tracheal cartilage formation and peripheral lung epithelial differentiation and maturation. We further demonstrated that Fstl1 interacted with BMP4 and regulated lung AEC-II cell differentiation by negatively regulating BMP4/Smad1/5/8 signaling. Taken together, Fstl1 acts as a BMP4 signaling antagonist to modulate saccular maturation. The precise mechanisms that Fstl1 regulates tracheal cartilage formation and alveolar epithelial cell differentiation, if and how Fstl1 regulates other signaling pathways, such as Wnt and TGF-β1, during lung morphogenesis, and the interplay among Fstl1-BMP4, Fstl1-Wnt, and Fstl1-TGF-β1, are actively pursued in our laboratories. This study and our continuing efforts will provide insight into the mechanism coordinating the TGF-β superfamily in regulating organogenesis and into the understanding of the molecular mechanisms of congenital tracheal and lung anomalies in human, and would provide new strategies for new therapeutic developments.
Experimental Procedures
Generation of Fstl1−/− Mice.
Fstl1flox/+ mice (129S4 × C57BL/6J) were generated by standard homologous recombination. In these mice, Fstl1 exon 2 encoding the signal peptide was flanked by loxP sequences. Fstl1flox/+ mice were then mated to EIIa-Cre transgenic mice (FVB/N; Jackson Laboratory). Deletion of exon 2 results in loss of its signal peptide and disrupts its ORF, leading to loss of Fstl1 expression. The chimeric offspring were backcrossed with C57BL/6J to generate Fstl1+/− mice, which then were intercrossed for production of Fstl1 deficient (Fstl1−/−) mice.
SI Experimental Procedures provides additional details on the generation of Fstl1−/− mice, as well as details on genotyping and Southern analysis, Western blotting, pull-down assay, qRT-PCR, morphological analysis, cell culture and saccular explant culture, luciferase assay, SPR analysis, and statistical analysis.
Supplementary Material
Acknowledgments
We thank Xu Zhang for providing critical reagent; and Zhengping Xu and Henry Y. Keutmann for providing human Fst eukaryotic expression plasmid (pc-Fst). This work was supported by Ministry of Science and Technology (China) Grants 2007CB947100, 2009CB522101, and 2010CB833706; and National Natural Science Foundation of China Grants 30671094, 30771130, 31071241, and 30930050.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007293108/-/DCSupplemental.
References
- 1.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 3.Rossant J, Tam PPL. Mouse Development: Patterning, Morphogenesis, and Organogenesis. Amsterdam: Elsevier; 2002. [Google Scholar]
- 4.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: Current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 5.Hogan BL, Yingling JM. Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr Opin Genet Dev. 1998;8:481–486. doi: 10.1016/s0959-437x(98)80121-4. [DOI] [PubMed] [Google Scholar]
- 6.Warburton D, et al. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 7.Mendelson CR. Role of transcription factors in fetal lung development and surfactant protein gene expression. Annu Rev Physiol. 2000;62:875–915. doi: 10.1146/annurev.physiol.62.1.875. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Dey CR, Wert SE, Whitsett JA. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-beta 1 chimeric gene. Dev Biol. 1996;175:227–238. doi: 10.1006/dbio.1996.0110. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, et al. Abrogation of transforming growth factor-beta type II receptor stimulates embryonic mouse lung branching morphogenesis in culture. Dev Biol. 1996;180:242–257. doi: 10.1006/dbio.1996.0298. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Lee M, Smith S, Warburton D. Abrogation of Smad3 and Smad2 or of Smad4 gene expression positively regulates murine embryonic lung branching morphogenesis in culture. Dev Biol. 1998;194:182–195. doi: 10.1006/dbio.1997.8825. [DOI] [PubMed] [Google Scholar]
- 11.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- 12.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–4015. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- 13.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 14.Hambrock HO, et al. Structural characterization of TSC-36/Flik: Analysis of two charge isoforms. J Biol Chem. 2004;279:11727–11735. doi: 10.1074/jbc.M309318200. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, et al. Identification of a follistatin-related protein from the tick Haemaphysalis longicornis and its effect on tick oviposition. Gene. 2006;372:191–198. doi: 10.1016/j.gene.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Dal-Pra S, Fürthauer M, Van-Celst J, Thisse B, Thisse C. Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev Biol. 2006;298:514–526. doi: 10.1016/j.ydbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Esterberg R, Delalande JM, Fritz A. Tailbud-derived Bmp4 drives proliferation and inhibits maturation of zebrafish chordamesoderm. Development. 2008;135:3891–3901. doi: 10.1242/dev.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umezu T, Yamanouchi H, Iida Y, Miura M, Tomooka Y. Follistatin-like-1, a diffusible mesenchymal factor determines the fate of epithelium. Proc Natl Acad Sci USA. 2010;107:4601–4606. doi: 10.1073/pnas.0909501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams D, Larman B, Oxburgh L. Developmental expression of mouse Follistatin-like 1 (Fstl1): Dynamic regulation during organogenesis of the kidney and lung. Gene Expr Patterns. 2007;7:491–500. doi: 10.1016/j.modgep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001;11:527–532. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 21.Shukunami C, et al. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol. 1996;133:457–468. doi: 10.1083/jcb.133.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulugeta S, Beers MF. Surfactant protein C: Its unique properties and emerging immunomodulatory role in the lung. Microbes Infect. 2006;8:2317–2323. doi: 10.1016/j.micinf.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Clark JC, et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci USA. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamin JT, et al. The role of integrin alpha8beta1 in fetal lung morphogenesis and injury. Dev Biol. 2009;335:407–417. doi: 10.1016/j.ydbio.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: An in-depth review. Chest. 2005;127:984–1005. doi: 10.1378/chest.127.3.984. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- 27.Aubin J, Lemieux M, Tremblay M, Bérard J, Jeannotte L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol. 1997;192:432–445. doi: 10.1006/dbio.1997.8746. [DOI] [PubMed] [Google Scholar]
- 28.Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development. 1995;121:1989–2003. doi: 10.1242/dev.121.7.1989. [DOI] [PubMed] [Google Scholar]
- 29.Sumitomo K, et al. Expression of a TGF-beta1 inducible gene, TSC-36, causes growth inhibition in human lung cancer cell lines. Cancer Lett. 2000;155:37–46. doi: 10.1016/s0304-3835(00)00407-9. [DOI] [PubMed] [Google Scholar]
- 30.Chan QK, et al. Tumor suppressor effect of follistatin-like 1 in ovarian and endometrial carcinogenesis: A differential expression and functional analysis. Carcinogenesis. 2009;30:114–121. doi: 10.1093/carcin/bgn215. [DOI] [PubMed] [Google Scholar]
- 31.Wan H, et al. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci USA. 2004;101:14449–14454. doi: 10.1073/pnas.0404424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanagi S, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 34.Rider CC, Mulloy B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J. 2010;429:1–12. doi: 10.1042/BJ20100305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.