Abstract
Background
Individuals who have undergone splenectomy may be at an increased risk of arterial and venous thrombosis. We sought to determine if splenectomy affects surrogate laboratory measures of cardiovascular risk in persons with hereditary spherocytosis (HS).
Procedure
We conducted a prospective cross-sectional study of 21 children and 36 adults with HS. Fasting blood samples were collected for complete blood count and plasma lipid panel, homocysteine, lipoprotein (a), C-reactive protein, and fibrinogen. The variables were compared between the groups with and without prior splenectomy by Mann–Whitney tests.
Results
Subjects with prior splenectomy had higher hemoglobin, white blood cell and platelet counts and lower reticulocyte counts and total serum bilirubin concentrations (P < 0.001). Subjects not having had splenectomy had lower than normal levels of total and LDL-cholesterol (LDL-C). Total and LDL-C values were significantly higher, as were fibrinogen and homocysteine concentrations, in the post-splenectomy subjects than in individuals with intact spleen.
Conclusion
Various lipid levels and other measures of cardiovascular risk are affected by splenectomy in persons with HS. Further investigations are indicated to more clearly define the balance of the potential benefits of hemolysis and anemia versus the deleterious effects of splenectomy in HS.
Keywords: adverse events, cardiovascular disease, hereditary spherocytosis, splenectomy
INTRODUCTION
Hereditary spherocytosis (HS) is the most common inherited hemolytic anemia, occurring in approximately 1 in 2,000–5,000 persons of Northern European descent. It has generally been recommended that patients with moderate to severe HS undergo surgical splenectomy since it results in near complete resolution of hemolysis [1]. Although it has long been recognized that persons with HS and other conditions without a functioning spleen are at an increased risk of bacterial sepsis [2], it has only recently been proposed that hyposplenism may result in other long-term sequelae [3], including arteriothrombosis (specifically atherothrombosis) [4,5], deep venous thrombosis (especially involving the portal venous system) [6], and pulmonary arterial hypertension (PAH) [7-9].
Vascular complications associated with asplenia have been described primarily in conditions characterized by ongoing intravascular hemolysis, such as thalassemia intermedia and sickle cell anemia. Nitric oxide scavenging by cell-free hemoglobin as well as arginine depletion resulting from arginase derived from erythrocytes is postulated to play a key role in the vasculopathy seen in these conditions [10]. However, an apparent increased risk of pulmonary hypertension and other vascular complications in disorders such as HS, where hemolysis is virtually eliminated following splenectomy, suggests that lack of the spleen, rather than or in addition to intravascular hemolysis, may be a contributing or even primary cause.
To date, the independent roles of intravascular hemolysis and asplenia in the genesis of vascular complications are undefined. Previous observations are conflicting. Whereas one study has suggested that HS patients with prior splenectomy are at increased risk of arteriosclerotic events, [11] another report from the same investigative group indicates that persons with HS who have not had splenectomy may actually be protected from arteriosclerotic complications [4,12].
In order to better evaluate this relationship, additional study of individuals with HS is indicated to assess the risk of vascular complications in two distinct populations—that is, persons with hemolysis (HS and intact spleen) and those without hemolysis (HS after splenectomy). We have previously shown that children with HS following splenectomy had elevated platelet counts and higher than normal hemoglobin levels, which persisted for many years, and that children with HS prior to splenectomy had hypocholesterolemia [13]. The aim of this current study was to prospectively determine if splenectomy affects selected laboratory markers of cardiovascular risk in individuals with HS in order to better define the relative roles of hemolysis and splenectomy in maintenance of vascular homeostasis.
MATERIALS AND METHODS
Patients
We conducted a prospective cross-sectional study of 57 patients, representing 28 kindred with HS, which included 21 children (age 5–17 years) and 36 adults (age ≥18 years). The median age of the subjects was 29 years (range 6–67 years). Most (29/34) of the adults had undergone splenectomy, whereas most (16/23) of the children had not. The median interval from splenectomy to study participation was 25.5 years (range 1–55 years). Table I displays the patient characteristics by splenectomy status.
TABLE I.
Characteristics of Patients With Hereditary Spherocytosis by Splenectomy Status
| Group 1: no splenectomy (n = 23) | Group 2: splenectomy (n = 34) | P-value | |
|---|---|---|---|
| Males, n (%) | 8 (35) | 11 (32) | 0.85 |
| Age (years) | <0.001 | ||
| Median | 13 | 40 | |
| Range | 6–53 | 10–67 | |
| Number by age and status | <0.001 | ||
| Age <18 years | 16 | 5 | |
| Age >18 years | 7 | 29 | |
| Race/ethnicity | 0.34 | ||
| Caucasian, n (%) | 16 (69.6) | 29 (85.3) | |
| African-American, n (%) | 2 (8.7) | 1 (2.9) | |
| Hispanic, n (%) | 5 (21.7) | 4 (11.8) | |
| Family history of HS, n (%) | 21 (91.3) | 32 (94.1) | 0.68 |
Laboratory Measures
Fasting blood samples were collected from each subject for a complete blood count (CBC), plasma lipid panel (total and high-density lipoprotein cholesterol (HDL-C) and triglycerides), homocysteine, lipoprotein(a), glucose, C-reactive protein (CRP), and fibrinogen. Low-density lipoprotein cholesterol (LDL-C) was calculated as previously described [14]. Patients were excluded if they reported an illness, hospitalization, or surgery within the prior 2 months or a blood transfusion or splenectomy within the prior 3 months.
Data Analysis
Since the participants’ age range was wide, values were transformed to z-scores based on population norms for age and gender (when such data were available in the literature) to allow comparisons across all age ranges [15]. Population norms were extracted from data available from the Third National Health and Nutrition Examination Survey (NHANES III) [16-18]. z-Scores were based on population mean and standard deviation or were estimated from the population 50th and 90th percentile values for each variable when the standard deviation was not provided. Since the NHANES III study did not require children age 4–11 to fast, LDL-C and triglyceride norms are not available for these ages. We therefore extrapolated the 50th and 90th percentile values from the 12 to 15 years age category to the younger children for these two variables [16]. Mann–Whitney tests were used to compare all variables between the groups with intact spleen and with prior splenectomy. Significance level was set at 0.05.
RESULTS
Table II shows the comparison of the laboratory variables between the groups with intact spleen and those with prior splenectomy. These data are presented as z-scores, where appropriate, as well as absolute values for reference.
TABLE II.
Comparison of Laboratory Variables in the Two Patient Groups
| Median (25th, 75th percentiles) |
|||
|---|---|---|---|
| Laboratory test | Group 1: no splenectomy (n = 23) | Group 2: splenectomy (n = 34) | P-value |
| Hemoglobin concentration | |||
| z-Score g/dl |
−1.05 (−2.21, −0.26) 12.2 (11.4, 13.2) |
0.86 (0.12, 1.53) 14.4 (13.6, 15) |
<0.001 NA |
| WBC count | |||
| z-score × 103/mm3 |
−0.11 (−0.51, 0.51) 7.0 (6.3, 8.4) |
1.23 (0.50, 1.83) 9.8 (8.1, 11.1) |
<0.001 NA |
| Platelet count | |||
| z-score × 103/mm3 |
−1.10 (−1.53, −0.26) 240 (193, 276) |
2.49 (1.69, 5.53) 456 (381, 525) |
<0.001 NA |
| Total cholesterol | |||
| z-score mg/dl |
−1.75 (−2.22, −0.76) 112 (95, 138) |
−0.59 (−1.28, −0.06) 164 (142, 197) |
0.001 NA |
| LDL cholesterol | |||
| z-score mg/dl |
−1.03 (−1.92, −0.55) 57 (42, 87) |
−0.50 (−0.95, 0.15) 104 (83, 128) |
0.042 NA |
| HDL cholesterol | |||
| z-score mg/dl |
−0.68 (−1.28, −0.09) 40 (36, 45) |
−0.48 (−0.79, −0.13) 43 (38, 47) |
0.162 NA |
| Triglycerides | |||
| z-score mg/dl |
−0.12 (−0.25, 0.12) 80 (65, 95) |
−0.36 (−0.49, 0.28) 83 (55, 127) |
0.680 NA |
| Total serum bilirubin (mg/dl) | 2.00 (1.50, 2.90) | 0.75 (0.50, 0.90) | <0.001 |
| Reticulocyte count (%) | 6.10 (4.70, 8.50) | 1.90 (1.40, 2.65) | <0.001 |
| Fibrinogen (mg/dl) | 273 (228, 312) | 335 (276, 370) | 0.002 |
| Homocysteinea (μ mol/L) | 6.0 (4.3, 7.8) | 9.0 (7.0, 11.0) | 0.004 |
| Lipoprotein(a)a (mg/dl) | 5.0 (2.0, 45.0) | 22.0 (4.0, 55.0) | 0.343 |
| C-reactive protein (mg/dl) | 1.18 (0.25, 3.90) | 1.61 (0.70, 3.35) | 0.757 |
Variables expressed as z-score and absolute value where appropriate.
n = 16 in Group 1 and n = 22 in Group 2.
Hematologic Data and Bilirubin Levels
As expected, the hemoglobin concentration, white blood cell count, and platelet count were much higher in the group following splenectomy (P < 0.001 for each). Also, not surprisingly, the total serum bilirubin and reticulocyte counts were significantly lower in that group (P < 0.001 for each). Figure 1A-C display these data as the percentage of subjects falling within categorical ranges of z-scores.
Fig. 1.
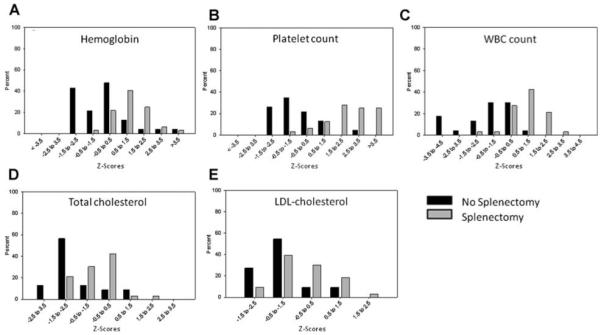
Percentage of patients whose values fall in each category for z-scores, demonstrating the distribution around the mean (z-score equal to 0). Variables shown are hemoglobin concentration (A), platelet count (B), white blood cell count (C), total cholesterol (D), and LDL-C (E) in the two groups: No splenectomy (black shading) and splenectomy (gray shading).
Lipid Profiles
Total cholesterol and LDL-C levels were significantly reduced in patients with intact spleen and chronic hemolysis. Although these cholesterol values were relatively higher in patients who had had splenectomy, they were at or slightly below the population norms (i.e., the median z-scores remained below zero). Figure 1D,E display the total cholesterol and LDL-C data by percentage of subjects falling within categorical ranges of z-scores. No differences were seen in HDL-C or triglyceride levels between the two groups.
Other Markers of Cardiovascular Risk
Both fibrinogen and homocysteine concentrations in plasma were significantly higher in patients with prior splenectomy. Lipoprotein(a) and CRP levels, although higher in the group with prior splenectomy, did not differ significantly between the two groups.
Secondary Analyses
When adults (≥18 years of age) were analyzed separately, the results were similar to the results found for the entire study population, although power was lost due to small numbers in each group (Supplemental Table). The only exception was that lipoprotein(a) was significantly higher in the adults with prior splenectomy compared to the adults with intact spleen (P = 0.028). This difference was not seen in the analysis when adults and children were analyzed together.
DISCUSSION
The first comprehensive report of a potential increased risk of arteriosclerotic events in patients with HS and prior splenectomy was made by Schilling [11]. Medical record reviews and interviews were conducted in 184 patients with HS over age 40 years to determine the rate of arteriosclerotic events (defined as stroke, myocardial infarction, and coronary or carotid artery surgery). Patients with HS and prior splenectomy were found to be 5.6 times more likely to have had an event than patients with HS and intact spleens. A follow-up report compared the rate of arteriosclerotic events in 129 patients with HS and intact spleen to 145 family members unaffected by HS [12]. The relative risk of a first arteriosclerotic event in patients with HS and intact spleens was one-fifth the risk of unaffected family members, suggesting a protective effect of hemolysis when the spleen was present. However, the rates of arteriosclerotic events in persons in this study were not significantly elevated until after the 7th decade of life. A third report by Schilling et al. [4] evaluated all three groups together (HS with intact spleen, HS with prior splenectomy and unaffected family members) to determine the relative risk of arterial thrombotic events in each group. Again it was found that those patients with HS and an intact spleen had lower rates of vascular events than unaffected family members. Moreover, persons with HS and prior splenectomy were at significantly higher risk for arterial events than those with HS and an intact spleen. However, no significant increased risk of thrombosis was observed for those with HS and prior splenectomy over their unaffected family members. The majority of the arterial events again occurred in patients >70 years of age.
The data reported by Schilling and colleagues are extremely intriguing, but the major limitation of these studies is that the information was collected retrospectively or from patient interviews, and no objective data were provided to support these findings. Therefore, our intent in the current study was to provide further laboratory data to support or refute the epidemiologic finding that splenectomy alters the risk of arterial events in persons with HS.
Our results prospectively confirm that, following splenectomy, persons with HS have higher hemoglobin, white blood cell count, and platelet count values than those having an intact spleen. These blood count measurements in asplenic HS subjects are also higher than normal population means for age and gender (Fig. 1) [13,19]. This finding is interesting in that the Framingham Heart Study has demonstrated that even a small increase in hemoglobin concentration poses an increased risk for vascular complications [20,21]. Elevated white blood cell counts have also been associated with increased risk of cardiovascular disease [22-27]. The persistently elevated platelet counts seen in asplenic patients such as in our current study could further predispose individuals to thrombotic events, although this has yet to be proven [28-30] and clearly needs further investigation.
In this prospective study plasma total cholesterol, LDL-C, fibrinogen, and homocysteine levels were significantly higher in the asplenic HS patients than in those patients with an intact spleen. However, compared to population norms, HS patients with an intact spleen had markedly reduced levels of total cholesterol and LDL-C. Previous reports have also noted low cholesterol levels in various forms of chronic hemolytic anemia [31-38]. It has been suggested that ongoing hemolysis leads to consumption of cholesterol by its integration into red blood cell membranes due to increased cell turnover [35]. These lower lipid levels in HS patients prior to splenectomy may thus convey a protective influence of hemolysis on development of atherosclerosis. Although the hypolipidemia abates following splenectomy, cholesterol levels do not increase to higher than normal values (Fig. 1D,E) and therefore may not pose additional cardiovascular risk. In contrast, fibrinogen level, also associated with an increased incidence of arteriothrombotic events [39,40], was significantly higher in HS patients following splenectomy. The median fibrinogen concentration in the HS patients with prior splenectomy (335 mg/dl) was at a level which has previously been associated with a twofold increased incidence of vascular events compared to subjects with fibrinogen level in the lowest tertile [41]. Elevated fibrinogen concentrations following splenectomy have previously been reported in mice [42], although other studies in human subjects have failed to show a significant difference in fibrinogen concentrations pre- and post-splenectomy in various conditions [43-45]. To our knowledge no prior studies have specifically examined patients with HS. Plasma homocysteine levels, another potential marker of arteriothrombosis risk [39], were also significantly higher in our HS patients following splenectomy than in those with intact spleens. However, the median value was still within the normal range so this test result might not be expected to confer increased risk of vascular complications.
In addition to the lower total and LDL cholesterol levels reported here, the elevated bilirubin concentration in the HS patients prior to splenectomy may also be beneficial, explaining at least partially Schilling et al. [4,12] report of a relatively low risk of vascular complications in individuals with HS who have not had splenectomy. There are several possible mechanisms for a protective effect of hyperbilirubinemia against thrombosis. These include the potent antioxidant effects of bilirubin as well as its ability to inhibit both proliferation of vascular smooth muscle cells and endothelial activation [46-51]. Moreover, the lower hemoglobin levels and white blood cell counts prior to splenectomy may also reduce blood viscosity [26].
There are several limitations to our study. First, an age discrepancy exists between the pre- and post-splenectomy groups. While we attempted to control for this by normalizing the data with z-scores, population norms based on age and gender were not available for every measurement and for every age group and could have skewed the results, especially for homocysteine and lipoprotein(a) level. Second, since we utilized our pediatric patients as a “window” to identify adults with HS, our cohort emphasizes only patients with likely autosomal dominant disease and therefore may not be generalizable to all patients with HS. Lastly, as many of variables tested are hereditary the results in this small sample of patients may be skewed since many of the subjects were related to one another. Given the small sample size we were not able to formally test this statistically.
In conclusion, lipid profiles and other measures of cardiovascular risk appear to be affected by splenectomy in individuals with HS. This could represent a pathophysiologic mechanism to explain an increased risk of vascular complications in such patients. Future investigation of cardiovascular complications following splenectomy will require larger numbers of subjects to more clearly define the potential benefits of chronic hemolysis in the setting of a functioning spleen, as well as the deleterious effects of splenectomy with and without continued hemolysis. These necessary epidemiologic studies will require additional assessment of other known risk factors for cardiovascular disease, including family history, blood pressure, smoking history, and diabetes in addition to surrogate laboratory measures which we have examined.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the North and Central Texas Clinical and Translational Science Initiative grants 5 KL2 RR024983-02 and 5 UL1 RR024982-02 (to S.E.C.) and Doris Duke Charitable Foundation (to S.T.). The authors would like to thank Roberto Torres for his assistance recruiting participants and Cindy E. Neunert, MD for her thoughtful review of the manuscript.
Grant sponsor: North and Central Texas Clinical and Translational Science Initiative; Grant numbers: 5 KL2 RR024983-02, 5 UL1 RR024982-02; Grant sponsor: Doris Duke Charitable Foundation.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Bolton-Maggs PH, Stevens RF, Dodd NJ, et al. Guidelines for the diagnosis and management of hereditary spherocytosis. Br J Haematol. 2004;126:455–474. doi: 10.1111/j.1365-2141.2004.05052.x. [DOI] [PubMed] [Google Scholar]
- 2.King H, Shumacker HB., Jr. Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952;136:239–242. doi: 10.1097/00000658-195208000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crary SE, Buchanan GR. Vascular complications following splenectomy for hematologic disorders. Blood. 2009;114:2861–2868. doi: 10.1182/blood-2009-04-210112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schilling RF, Gangnon RE, Traver MI. Delayed adverse vascular events after splenectomy in hereditary spherocytosis. J Thromb Haemost. 2008;6:1289–1295. doi: 10.1111/j.1538-7836.2008.03024.x. [DOI] [PubMed] [Google Scholar]
- 5.Robinette CD, Fraumeni JF., Jr. Splenectomy and subsequent mortality in veterans of the1939-45 war. Lancet. 1977;2:127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 6.Mohren M, Markmann I, Dworschak U, et al. Thromboembolic complications after splenectomy for hematologic diseases. Am J Hematol. 2004;76:143–147. doi: 10.1002/ajh.20018. [DOI] [PubMed] [Google Scholar]
- 7.Bonderman D, Jakowitsch J, Adlbrecht C, et al. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2005;93:512–516. doi: 10.1160/TH04-10-0657. [DOI] [PubMed] [Google Scholar]
- 8.Hoeper MM, Niedermeyer J, Hoffmeyer F, et al. Pulmonary hypertension after splenectomy? Ann Intern Med. 1999;130:506–509. doi: 10.7326/0003-4819-130-6-199903160-00014. [DOI] [PubMed] [Google Scholar]
- 9.Jais X, Ioos V, Jardim C, et al. Splenectomy and chronic thromboembolic pulmonary hypertension. Thorax. 2005;60:1031–1034. doi: 10.1136/thx.2004.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gladwin MT, Kato GJ. Cardiopulmonary complications of sickle cell disease: Role of nitric oxide and hemolytic anemia. Hematology (Am Soc Hematol Educ Program) 2005:51–57. doi: 10.1182/asheducation-2005.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schilling RF. Spherocytosis, splenectomy, strokes, and heart attacks. Lancet. 1997;350:1677–1678. doi: 10.1016/s0140-6736(05)64276-6. [DOI] [PubMed] [Google Scholar]
- 12.Schilling RF, Gangnon RE, Traver M. Arteriosclerotic events are less frequent in persons with chronic anemia: Evidence from families with hereditary spherocytosis. Am J Hematol. 2006;81:315–317. doi: 10.1002/ajh.20566. [DOI] [PubMed] [Google Scholar]
- 13.Troendle SB, Adix L, Crary SE, et al. Laboratory markers of thrombosis risk in children with hereditary spherocytosis. Pediatr Blood Cancer. 2007;49:781–785. doi: 10.1002/pbc.21319. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Gravetter FJ, Wallnau LB. Statistics for the behavioral sciences. Wadsworth Publishing; Belmont, CA: 2009. [Google Scholar]
- 16.Hickman TB, Briefel RR, Carroll MD, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: Data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27:879–890. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 17.Appendix III-A distributions of total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides in the U.S. adult population, NHANES III data (1988–1994) (Serum) Circulation. 2002;106:3237–3240. [Google Scholar]
- 18.Hollowell JG, van Assendelft OW, Gunter EW, et al. Hematological and iron-related analytes—Reference data for persons aged 1 year and over: United States, 1988–94. Vital Health Stat. 2005;11:1–156. [PubMed] [Google Scholar]
- 19.Schilling RF. Hereditary spherocytosis: A study of splenectomized persons. Semin Hematol. 1976;13:169–176. [PubMed] [Google Scholar]
- 20.Gagnon DR, Zhang TJ, Brand FN, et al. Hematocrit and the risk of cardiovascular disease—The Framingham study: A 34-year follow-up. Am Heart J. 1994;127:674–682. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Gordon T, Wolf PA, et al. Hemoglobin and the risk of cerebral infarction: The Framingham Study. Stroke. 1972;3:409–420. doi: 10.1161/01.str.3.4.409. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Anderson K, Wilson PW. White blood cell count and cardiovascular disease. Insights from the Framingham Study. JAMA. 1992;267:1253–1256. [PubMed] [Google Scholar]
- 23.Gillum RF, Ingram DD, Makuc DM. White blood cell count, coronary heart disease, and death: The NHANES I Epidemiologic Follow-up Study. Am Heart J. 1993;125:855–863. doi: 10.1016/0002-8703(93)90181-8. [DOI] [PubMed] [Google Scholar]
- 24.Brown DW, Giles WH, Croft JB. White blood cell count: An independent predictor of coronary heart disease mortality among a national cohort. J Clin Epidemiol. 2001;54:316–322. doi: 10.1016/s0895-4356(00)00296-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee CD, Folsom AR, Nieto FJ, et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: Atherosclerosis risk in communities study. Am J Epidemiol. 2001;154:758–764. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 26.Yarnell JW, Baker IA, Sweetnam PM, et al. Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease. The Caerphilly and Speedwell collaborative heart disease studies. Circulation. 1991;83:836–844. doi: 10.1161/01.cir.83.3.836. [DOI] [PubMed] [Google Scholar]
- 27.Prentice RL, Szatrowski TP, Fujikura T, et al. Leukocyte counts and coronary heart disease in a Japanese cohort. Am J Epidemiol. 1982;116:496–509. doi: 10.1093/oxfordjournals.aje.a113434. [DOI] [PubMed] [Google Scholar]
- 28.Griesshammer M, Bangerter M, Sauer T, et al. Aetiology and clinical significance of thrombocytosis: Analysis of 732 patients with an elevated platelet count. J Intern Med. 1999;245:295–300. doi: 10.1046/j.1365-2796.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 29.Boxer MA, Braun J, Ellman L. Thromboembolic risk of post-splenectomy thrombocytosis. Arch Surg. 1978;113:808–809. doi: 10.1001/archsurg.1978.01370190030004. [DOI] [PubMed] [Google Scholar]
- 30.Hirsh J, Dacie JV. Persistent post-splenectomy thrombocytosis and thrombo-embolism: A consequence of continuing anaemia. Br J Haematol. 1966;12:44–53. doi: 10.1111/j.1365-2141.1966.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 31.Fessas P, Stamatoyannopoulos G, Keys A. Serum-cholesterol and thalassemia trait. Lancet. 1963;1:1182–1183. doi: 10.1016/s0140-6736(63)92478-4. [DOI] [PubMed] [Google Scholar]
- 32.Rifkind BM, Gale M. Hypolipidaemia in anaemia. Implications for the epidemiology of ischaemic heart-disease. Lancet. 1967;2:640–642. doi: 10.1016/s0140-6736(67)90685-x. [DOI] [PubMed] [Google Scholar]
- 33.Asai K, Kuzuya M, Naito M, et al. Effects of splenectomy on serum lipids and experimental atherosclerosis. Angiology. 1988;39:497–504. doi: 10.1177/000331978803900602. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki J, Waterman MR, Buchanan GR, et al. Plasma and erythrocyte lipids in sickle cell anaemia. Clin Lab Haematol. 1983;5:35–44. doi: 10.1111/j.1365-2257.1983.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 35.Shalev H, Kapelushnik J, Moser A, et al. Hypocholesterolemia in chronic anemias with increased erythropoietic activity. Am J Hematol. 2007;82:199–202. doi: 10.1002/ajh.20804. [DOI] [PubMed] [Google Scholar]
- 36.Sugihara T, Yawata Y. Observations on plasma and red cell lipids in hereditary spherocytosis. Clin Chim Acta. 1984;137:227–232. doi: 10.1016/0009-8981(84)90182-7. [DOI] [PubMed] [Google Scholar]
- 37.Shores J, Peterson J, VanderJagt D, et al. Reduced cholesterol levels in African-American adults with sickle cell disease. J Natl Med Assoc. 2003;95:813–817. [PMC free article] [PubMed] [Google Scholar]
- 38.Westerman MP. Hypocholesterolaemia and anaemia. Br J Haematol. 1975;31:87–94. doi: 10.1111/j.1365-2141.1975.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 39.Feinbloom D, Bauer KA. Assessment of hemostatic risk factors in predicting arterial thrombotic events. Arterioscler Thromb Vasc Biol. 2005;25:2043–2053. doi: 10.1161/01.ATV.0000181762.31694.da. [DOI] [PubMed] [Google Scholar]
- 40.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: A meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 41.Maresca G, Di Blasio A, Marchioli R, et al. Measuring plasma fibrinogen to predict stroke and myocardial infarction: An update. Arterioscler Thromb Vasc Biol. 1999;19:1368–1377. doi: 10.1161/01.atv.19.6.1368. [DOI] [PubMed] [Google Scholar]
- 42.Miko I, Nemeth N, Sipka S, Jr., et al. Hemorheological follow-up after splenectomy and spleen autotransplantation in mice. Microsurgery. 2006;26:38–42. doi: 10.1002/micr.20208. [DOI] [PubMed] [Google Scholar]
- 43.Butler MJ, Britton BJ, Hawkey C, et al. The coagulation and fibrinolytic response to splenectomy. Surg Gynecol Obstet. 1976;142:731–736. [PubMed] [Google Scholar]
- 44.Robertson DA, Simpson FG, Losowsky MS. Blood viscosity after splenectomy. Br Med J (Clin Res Ed) 1981;283:573–575. doi: 10.1136/bmj.283.6291.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adar T, Ben-Ami R, Elstein D, et al. Aggregation of red blood cells in patients with Gaucher disease. Br J Haematol. 2006;134:432–437. doi: 10.1111/j.1365-2141.2006.06199.x. [DOI] [PubMed] [Google Scholar]
- 46.Vitek L, Novotny L, Sperl M, et al. The inverse association of elevated serum bilirubin levels with subclinical carotid atherosclerosis. Cerebrovasc Dis. 2006;21:408–414. doi: 10.1159/000091966. [DOI] [PubMed] [Google Scholar]
- 47.Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: A meta-analysis of published studies. Exp Biol Med (Maywood) 2003;228:568–571. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 48.Ishizaka N, Ishizaka Y, Takahashi E, et al. High serum bilirubin level is inversely associated with the presence of carotid plaque. Stroke. 2001;32:580–583. doi: 10.1161/01.str.32.2.580-b. [DOI] [PubMed] [Google Scholar]
- 49.Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 50.Ollinger R, Bilban M, Erat A, et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005;112:1030–1039. doi: 10.1161/CIRCULATIONAHA.104.528802. [DOI] [PubMed] [Google Scholar]
- 51.Kawamura K, Ishikawa K, Wada Y, et al. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


