Abstract
Serotonin (5-hydroxytryptamine; 5-HT) is thought to regulate neurodevelopmental processes through maternal-fetal interactions that have long-term mental health implications. Dogma states that beyond fetal 5-HT neurons, there are significant maternal contributions to fetal 5-HT during pregnancy1,2, but this has not been tested empirically. To examine putative central and peripheral sources of embryonic brain 5-HT, we used the Pet-1−/− mice in which most dorsal raphe (DR) neurons lack 5-HT3. Measures of 5-HT revealed previously unknown differences in accumulation between the fore- and hindbrain during early and late fetal stages, through an exogenous source of 5-HT. We show that this source is not of maternal origin. Using additional genetic strategies, a new technology for studying placental biology ex vivo, and direct manipulation of placental neosynthesis, we investigated the nature of this exogenous source and uncovered a placental 5-HT synthetic pathway from a maternal tryptophan precursor, in both mice and humans. This study reveals a new, direct role for placental metabolic pathways in modulating fetal brain development and implicates novel maternal-placental-fetal interactions that could underlie the pronounced impact of 5-HT on long-lasting mental health outcomes.
Fetal 5-HT dysfunction is implicated in developmental programming by altering brain circuit formation4, which later translates into abnormal adult behaviors5,6. In humans there are risk alleles in genes involved in 5-HT function that combine with early adverse experiences during development to impact adult-onset mental illnesses7. Furthermore, polymorphisms in the 5-HT transporter (Slc6a4; SERT) and 5-HT receptors, which are expressed early in brain development8, are associated with neurodevelopmental disorders such as autism spectrum disorder and schizophrenia9,10. A puzzling issue regarding the role of 5-HT in fetal brain development is that receptors, transporters and degrading enzymes for 5-HT often appear before the development of 5-HT innervation11 itself, suggesting the existence of an exogenous source of 5-HT at early stages of development. The most biologically influential source of exogenous 5-HT is claimed to be maternal in origin, but there is a lack of experimental data to support such a mechanism of developmental programming.
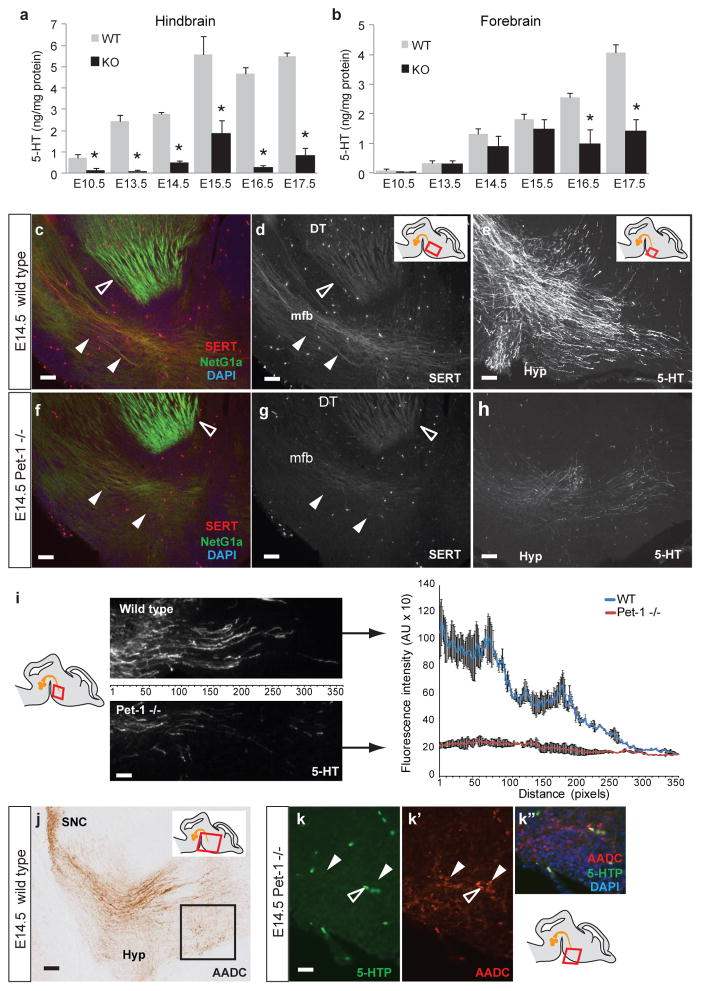
The analysis of fetal Pet-1−/− mice provides an opportunity to assess potential extraembryonic sources of 5-HT, as only ~30% of DR 5-HT neurons can be detected in null mice, which also express low level of TPH2, AADC, SERT and other markers of the serotonergic phenotype3. To assess if DR neurons are also the sole source of brain 5-HT during development, we compared the concentration of 5-HT in embryonic brains harvested from Pet-1−/− and wild type littermates from embryonic day (E)10.5, the onset of 5-HT synthesis in DR neurons, to E17.5, when 5-HT axons are fully deployed throughout the forebrain. HPLC was used to measure the concentration of 5-HT in the mid/hindbrain region (termed ‘hindbrain’), which contains 5-HT cell bodies and proximal axons, and in the forebrain, which contains only distal 5-HT axons12,13. Consistent with DR neurons providing the main source of 5-HT in the hindbrain, 5-HT concentration is lower in Pet-1−/− hindbrain compared to wild type mice at every age tested (Fig. 1a). Surprisingly, in the Pet-1−/− forebrain, 5-HT levels are statistically indistinguishable from wild type at E10.5 to E15.5; however, large differences emerge at E16.5 (Fig. 1b), which is consistent with DR axons being the major source of forebrain 5-HT at this and later ages, but not earlier (Supplementary Fig. 2 and ref. 3). Remarkably, even before the arrival of 5-HT axons in the ventral forebrain (E10.5–12.5), low levels of 5-HT are detected (Fig. 1b). Normally, over the next three embryonic days, progressively more 5-HT axons grow into the forebrain13 (Fig. 1c–e). In the Pet-1−/− forebrain, however, there is a dramatic reduction in 5-HT axon density compared to wild type (Fig. 1c–i), even though total tissue 5-HT concentrations are comparable. The density and distribution of thalamocortical axons, which also express SERT and can uptake 5-HT14, are similar in the Pet-1−/− and wild type (Fig. 1c, f). These results reveal a complex regulation of 5-HT in the fetal brain, with DR serotonergic neurons and axons representing the major source of 5-HT in the hindbrain and at later embryonic stages in the forebrain, but not the main source of 5-HT in the early developing forebrain.
Figure 1. Comparison of fetal 5-HT concentrations in the hindbrain and forebrain of Pet-1−/− and wild type embryos from E10.5 to E17.5.
a, 5-HT concentration in the Pet-1−/− (KO) hindbrain is significantly lower than in wild type (WT) littermates at every age tested. b, In contrast 5-HT concentrations in the Pet-1−/− forebrain are not significantly different from wild type littermates from E10.5 to E15.5 but become significantly lower at E16.5 and E17.5 (n>=6 embryos per genotype per age; *, p<0.005; one-way ANOVA; data are presented as means ± s.e.m.). c–h, Serotonergic axons (SERT+) and dorsal thalamic (DT) axons (NetG1a+) immunostained on sagittal sections at E14.5 in wild type (c) and Pet-1−/− (f) embryos (regions shown correspond to the red box in the drawings). In wild type E14.5 embryos (d) SERT+ axons grow ventrally into the forebrain through the medial forebrain bundle (mfb, white arrowheads); SERT also labels DT axons at this age (open arrowhead). In comparison, very few SERT+ axons remain in the Pet-1−/− (g). The pattern and density of SERT+ DT axons appear unaffected. Scale bars: 50 μm. The rostral-most extent of ingrowing serotonergic axons immunolabeled with 5-HT in the wild type (e) shows numerous 5-HT+ axons, some of which diverge toward the hypothalamus (Hyp). In contrast, only few 5-HT+ axons remain in the Pet-1−/− forebrain (h). Scale bars: 20 μm. i, Densitometric analysis of 5-HT+ axons in the most rostral part of the mfb at E14.5 (region indicated in the right panel) confirms fewer axons in the Pet-1−/−. j, AADC staining identifies DA neurons in the substantia nigra pars compacta (SNC) along with their and serotonergic axons coursing through the ventral forebrain at E14.5, and also AADC+ catecholaminergic neurons present in the hypothalamus (Hyp, black box). Scale bar: 100 μm. k-k″, AADC+ neurons in the hypothalamic region (red box in bottom right drawing; k′, white arrowheads) are 5-HTP-negative. Open arrowheads indicate fluorescence from blood vessels. Scale bar: 25 μm.
The greater decrease in total tissue 5-HT concentration in the hindbrain than in the forebrain in Pet-1−/− mice suggests a differential contribution of non-DR sources in these regions. Alternatively, since 5-HT degradation enzyme (monoamine oxydase A; MAO-A) activity is higher in the hindbrain than in the forebrain at early stages of development15, a differential degradation of 5-HT across the two brain regions may account for the difference. Consistent with this possibility, 5-hydroxyindoleacetic acid (5-HIAA) concentration in the E14.5 sMAOA−/− (which lacks MAO-A enzymatic activity and cannot efficiently degrade 5-HT16) is decreased 3.4 fold in the forebrain, but 6.1 fold in the hindbrain compared to wild type littermates (Supplementary Fig. 3). In contrast, at E16.5 5-HIAA concentrations are decreased to a similar extent in the sMAOA−/− forebrain and hindbrain (3.6 and 3.1 fold respectively). A down regulation of MAO-A activity in the Pet-1−/− forebrain before E16.5 could explain the normal concentrations of 5-HT measured in the region. In order to test this possibility, we quantified MAO-A - activity in the forebrain at E14.5, and show that MAO-A activity is not different in Pet-1−/−, Pet-1+/− and Pet-1+/+ forebrains (Supplementary Table 2). Furthermore, 5-HIAA concentrations are not different in the Pet-1−/− and Pet-1+/+ forebrains at E12.5 and E14.5 (Supplementary Fig. 3C). The data demonstrate that MAO-A activity is not down regulated in the Pet-1−/− forebrain.
The non-DR origin of 5-HT in the early fetal forebrain could arise from multiple sources. Because adult forebrain catecholaminergic (CA) and DA neurons express the aromatic l-amino acid decarboxylase (AADC) enzyme, these cells can ectopically synthesize 5-HT, albeit after administration of large doses of the precursor 5-HTP17. We tested the possibility that embryonic AADC-expressing neurons could ectopically produce 5-HT in the early Pet-1−/− forebrain. Consistent with measures of unaltered DA concentration (Supplementary Fig. 1), AADC immunostaining reveals normal CA/DA neuron and axon density in the Pet-1−/− forebrain (Fig. 1j). Furthermore, CA neurons present in the Pet-1−/− hypothalamus do not exhibit ectopic 5-HTP or 5-HT immunoreactivity (Fig. 1k-k″), consistent with no local cellular source of 5-HT in the Pet-1−/− forebrain.
In the developing forebrain, E10.5–E15.5 corresponds to the period of pronounced neurogenesis and axon growth. As 5-HT modulates both processes4,10, it is essential that its availability be regulated during this time. It is remarkable, therefore, that over this time period, even in the absence of 5-HT axons, the concentration of 5-HT in the Pet-1−/− forebrain is normal. Possible exogenous sources include the embryonic gut, the maternal blood through the placenta, or the placenta itself. We ruled out the embryonic gut as a source because expression of the 5-HT biosynthetic enzyme tryptophan hydroxylase (TPH1), which provides blood 5-HT, begins late (E15.5) in fetal enterochromaffin cells1,18. To test the possibility that maternal 5-HT is transferred to the fetal brain, we examined brains from fetuses of SERT knockout (SERT−/−) dams; total blood and platelets in these dams contain virtually no 5-HT19. This absence of blood 5-HT is attributed to a failure of uptake by platelets, and both rapid degradation of the remaining free plasma 5-HT in the liver and compensatory uptake by other transporters in the gut19. Despite this, the concentration of 5-HT is not different in the forebrain of SERT+/− E12.5 embryos from SERT−/− or wild type dams (Supplementary Table 1), indicating that maternal blood 5-HT, is not the main source of fetal blood and forebrain 5-HT at early stages of development.
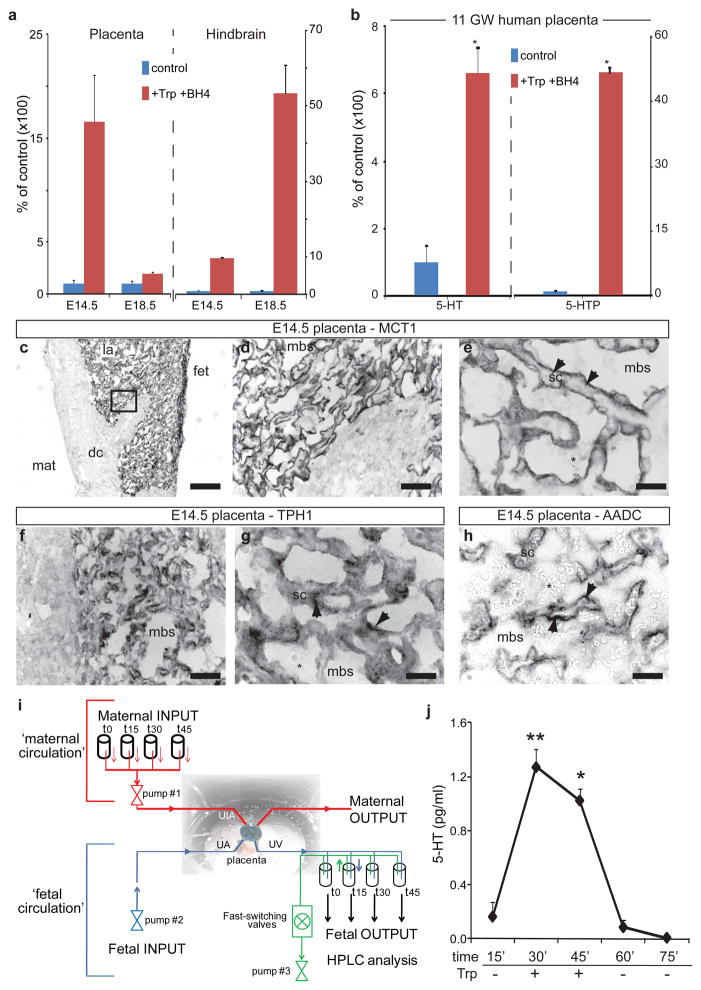
An alternative we considered is that the essential amino acid tryptophan, originating from the pregnant dam, would be converted to 5-HT in the placenta and delivered to the fetal circulation. Injection of tryptophan in pregnant dams increases 5-HT concentration in the fetal brain20, but the precise location of the synthetic conversion has never been identified. qRT-PCR of placental tissue detected transcripts encoding TPH1 and AADC, but not Pet-1 (Supplementary Fig. 4A). Immunocytochemistry confirms that TPH1 and AADC proteins are expressed in the syncytiotrophoblastic cell layer of the placenta at E10.5–E14.5 (Fig. 2c–h; Supplementary Fig. 4E–J). The placenta thus has the necessary machinery to synthesize 5-HT. We tested for placental 5-HT neosynthesis directly, and show that both 5-HTP and 5-HT neosyntheses occur in placenta and fetal hindbrain extracts incubated with tryptophan (Fig. 2a) as early as E10.5 (Supplementary Fig. 4B). Interestingly, placental 5-HT synthesis capacity is greater at E14.5 than at E18.5, whereas the converse is true in the hindbrain, consistent with our observed changes in TPH1 transcript expression (data not shown). The capacity for placental 5-HT synthesis at E14.5 is not affected by the absence of embryonic Pet-1 gene expression (Supplementary Fig. 4C). This synthetic capacity is not unique to mice, as human placental fetal villi at 11 weeks of gestation show robust 5-HT neo-synthesis (Fig. 2b), suggesting that a placental source of 5-HT is important for human fetal development.
Figure 2. Placental synthesis of 5-HT in vitro and ex vivo.
a, Placental extracts were incubated with the co-factor tetrahydrobiopterin (BH4; control) or BH4 and tryptophan (+Trp+BH4) and 5-HT neosynthesis measured after 30 min. b, Similar experiments conducted using human placenta tissue collected at 11 weeks of gestation (11 GW) show that human fetal villi synthesize 5-HTP and 5-HT (statistical significance versus control analyzed by Student’s t test; *, p<0.005; n=3; data are presented as means ± s.e.m.). c, Immunostaining for the monocarboxylate transporter MCT1, a marker of syncytiotrophoblastic cells (sc) in the labyrinth (la) region on the fetal side (fet) of an E14.5 mouse placenta. On the maternal side (mat) the decidua (dc) is devoid of staining. Asterisks indicate red blood cells of maternal origin. d–h, Higher magnifications of the region boxed in c; MCT1 is expressed on the apical side (arrows) of syncytiotrophoblasts facing the maternal blood space (mbs). The 5-HT synthetic enzymes TPH1 (f, g) and AADC (h) are expressed in overlapping patterns in the cytoplasm of syncytiotrophoblastic cells (arrows). i, Schematics of the ex-vivo dual perfusion system for the mouse placenta; UtA: uterine artery, UA umbilical artery, UV umbilical vein. g, 5-HT neosynthesis in ex vivo dually-perfused mouse placentas at E17.5. L-tryptophan (100 μM) was injected through the UtA. 5-HT, neo-synthesized from maternal tryptophan and released into the UV, is evident within 15 min of precursor injection (statistical significance of 5-HT levels variation across time was analyzed by one-way ANOVA; *, p<0.05; **, p<0.01; data from 3 independent experiments are presented as means ± s.e.m.).
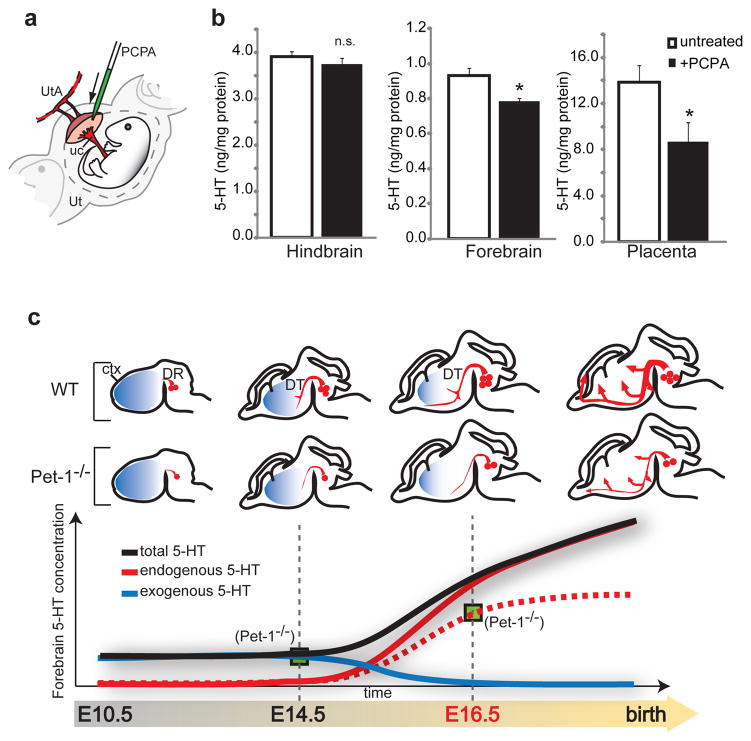
The data are consistent with the possibility, but do not prove neosynthesis and transport of 5-HT from maternal tryptophan precursor in an intact placenta. We addressed this with two strategies. First, we developed a novel ex vivo technology for regulating placental organ perfusion, allowing for the presentation of maternal precursor and collection of fetal perfusate in intact, live murine placentas (Fig. 2i). Within 15 minutes of tryptophan injection through the maternal uterine artery, there is a large accumulation of newly synthesized 5-HT that passes through the fetal placental circulation (Fig. 2j), demonstrating that the live placenta is able to convert tryptophan to 5-HT, and release the neurotransmitter into the fetal circulation. In contrast, in experiments in which 5-HT (1.5 nM) was injected into the uterine artery, only 0.32 ± 0.16 % of the maternal free 5-HT was transferred to the fetal umbilical vein during a 30 minutes perfusion period. Second, we specifically blocked placental TPH1 enzymatic activity by microinjecting small volumes of the TPH inhibitor p-chlorophenyalanine (PCPA) directly into the labyrinth zone of E14.5 placentas in utero (Fig. 3a). In order to minimize non-specific effects due to diffusion of the drug into the maternal and fetal blood compartments, placental and fetal brain tissues were harvested after a short 30 minutes period of drug exposure. This pharmacological manipulation reduces 5-HT levels in the placenta but does not reduce 5-HT in the fetal hindbrain, indicating that exposure to PCPA was too short to inhibit TPH2 activity in DR serotonergic neurons (Fig. 3b). Yet, remarkably, the brief exposure to PCPA results in a significant decrease in fetal forebrain 5-HT levels (Fig. 3b). The data demonstrate directly that an exogenous source of 5-HT produced in the placenta is required to maintain normal levels of forebrain 5-HT during early stages of forebrain development (Supplementary Figure 5).
Figure 3. HPLC measures of 5-HT concentrations in E14.5 hindbrain, forebrain and placenta of in utero PCPA-injected mice.
a, Illustration of the in vivo injection procedure: PCPA or vehicle solution was injected into the labyrinth zone of the placenta. After 30 min, dams were euthanized and tissue collected and processed for HPLC. UtA: uterine artery; uc: umbilical cord; Ut: uterus. b, Compared to vehicle injection (untreated, n=6), placental PCPA injection (+PCPA, n=4), has no significant (n.s.) effect on 5-HT concentration in the hindbrain, whereas it significantly lowers 5-HT concentration in the forebrain and the placenta (statistical significance versus 5-HT levels in untreated tissue was analyzed by Student’s t test; *, p<0.05; data are presented as means ± s.e.m.). c, Model of the progressive switch of the source of 5-HT in the fetal forebrain, from an early exogenous (placental, blue line) to a later endogenous (5-HT axons, red line) source. The green boxes represent the amount of 5-HT measured in the Pet-1−/− mice, which lack most of the endogenous neuronal source.
The concept that 5-HT from maternal blood could be transferred to the fetal circulation after crossing the placenta is widely accepted1,2, but direct or indirect transfer of the molecule has never been demonstrated. Although uptake of exogenous 5-HT by syncytiotrophoblasts in the human and mouse placenta was demonstrated in vitro2,21, the concomitant high level of MAO-A expression suggests a mechanism by which the placenta would prevent the vasoconstrictive effect of any free maternal blood 5-HT (a small fraction of total blood 5-HT19), rather than transfer it to the fetus21. This potentially lethal bioactivity and quasi-absence of maternal blood 5-HT transfer to the fetus has been demonstrated experimentally20,22 and in the present results. A recent study concluded that maternal 5-HT is crucial for early fetal development (prior to E111) based on abnormal phenotypes that emerge in embryos of TPH1−/− pregnant dams. Indirect effects of the TPH1−/− maternal mutation, however, cannot be excluded since TPH1−/− mice are diabetic23, a pathological condition that affects fetal development independent of a direct maternal 5-HT effect24. Although the importance of a maternal source of 5-HT before and during placentation remains an open question, our results provide the first direct evidence that maternal influences on fetal brain development can occur through a precursor that is metabolized directly by the placenta. Given our demonstration of synthetic capability in early human placenta, it will be important clinically to define the extent of the specific time period during human pregnancy for this placental influence on brain development. The present results also place an emphasis on the need to examine fetal and placental tryptophan availability. Mutations in tryptophan 2,3-dioxygenase degrading enzymes (TDO1/2, which are expressed in the placenta25) affect neurogenesis, produce anxiety-related behavior in mice26 and are associated with increased risk for schizophrenia, bipolar disorder and autism27,28. Our results provide a mechanism through which alterations of tryptophan metabolic pathways in the placenta would consequently affect placental 5-HT synthesis and fetal forebrain development.
The current study provides a new framework for understanding the complexity of maternal-fetal relationships that can influence brain structure and function. We focused on the 5-HT system and show that there is a progressive switch from an early dependence on an exogenous (placental) source of 5-HT to a later endogenous brain source (Fig. 3c). The exogenous source of 5-HT is provided to the forebrain during developmental epochs that include cortical neurogenesis, migration and initial axon targeting10. These events can be negatively impacted by disrupting 5-HT signaling; we demonstrated that a forebrain-specific disruption of 5-HT signaling in vivo impacts axon guidance mechanisms, leading to abnormal thalamocortical axons trajectories4. This phase in the mouse corresponds to the 1st and early 2nd trimesters in the human, prenatal periods that are associated with greater risk for mental illnesses due to maternal perturbations29,30. Thus, translation of our findings to those corresponding periods in the human will be of significant clinical relevance.
Methods Summary
Animals and reagents
Timed-pregnant CD-1 mice were purchased from the Charles River Laboratory. Plug date was considered E0.5 and the age of individual embryos confirmed by measuring the crown-rump length and checking for developmental landmarks such as digits and eye formation. Pet-1 (ref. 3) knockout (−/−), heterozygotes (+/−) and wild type (+/+) littermate embryos were generated by crossing Pet-1+/− males and females. SERT+/− embryos were obtained by crossing SERT−/− females19 with wild type C57Bl6 males. The Pet-1 and SERT knockout lines have been backcrossed on the C57Bl/6J background for >10 generations. The MAO-A spontaneous knockout mouse (sMAOA KO) was described earlier16. All procedures using mice were approved by the Institutional Animal Care and Use Committee at University of Southern California and conformed to NIH guidelines. Unless otherwise noted, all reagents and antibodies were purchased from Sigma (USA). Protocols for HPLC measure, tissue staining, in vitro 5-HT synthesis assays, MAOA enzymatic activity assays, in vivo synthesis inhibition assays and ex vivo placental perfusions are described in Supplementary Methods.
Human placenta samples
Normal human placental villi samples were obtained from women undergoing elective pregnancy terminations at the Reproductive Options Clinic at the Los Angeles County (LAC) + University of Southern California (USC) Medical Center. For the purposes of this study, the tissue was carefully dissected by the pathologist and immediately flash-frozen in liquid nitrogen. No patient characteristics other than gestational age were recorded. The University of Southern California Health Science Campus Institutional Review Board approved this study.
Supplementary Material
Acknowledgments
We thank Drs Hsiao-Huei Wu and Kathie Eagleson for discussions and comments on the manuscript. We thank Raymond Johnson (Vanderbilt HPLC core facility), Le Zhang for technical help and Ellis Meng for discussions. This work was supported by the NICHD (grant 5R21HD065287 to A.B.) and the NIMH (grant R01MH39085 to J.C.S. and 1P50MH078280A1 to R.D.B. and P.L.).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Authors Contribution A.B. conducted the experiments with assistance from N.G. in the placenta studies, K.C., J.C.S., in the providing of mutant mouse strains and MAO-A enzymatic assays, R.D.B. and E.S.D. in the providing of mutant mouse strains and M.W. and J.K. in the providing of human tissue. A.B. and P.L. conceived this study, interpreted the data and wrote the manuscript. All authors commented on the paper.
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nature.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
References
- 1.Cote F, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yavarone MS, Shuey DL, Sadler TW, Lauder JM. Serotonin uptake in the ectoplacental cone and placenta of the mouse. Placenta. 1993;14:149–161. doi: 10.1016/s0143-4004(05)80257-7. [DOI] [PubMed] [Google Scholar]
- 3.Hendricks TJ, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 4.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 5.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 6.Gross C, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SE, et al. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–794. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 9.Anderson GM, et al. Serotonin transporter promoter variants in autism: functional effects and relationship to platelet hyperserotonemia. Mol Psychiatry. 2002;7:831–836. doi: 10.1038/sj.mp.4001099. [DOI] [PubMed] [Google Scholar]
- 10.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 11.Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- 12.Aitken AR, Tork I. Early development of serotonin-containing neurons and pathways as seen in wholemount preparations of the fetal rat brain. J Comp Neurol. 1988;274:32–47. doi: 10.1002/cne.902740105. [DOI] [PubMed] [Google Scholar]
- 13.Lidov HG, Molliver ME. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- 14.Lebrand C, et al. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 15.Lebrand C, et al. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- 16.Scott AL, Bortolato M, Chen K, Shih JC. Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. Neuroreport. 2008;19:739–743. doi: 10.1097/WNR.0b013e3282fd6e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynn-Bullock CP, Welshhans K, Pallas SL, Katz PS. The effect of oral 5-HTP administration on 5-HTP and 5-HT immunoreactivity in monoaminergic brain regions of rats. J Chem Neuroanat. 2004;27:129–138. doi: 10.1016/j.jchemneu.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- 19.Chen JJ, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howd RA, Nelson MF, Lytle LD. L-tryptophan and rat fetal brain serotonin. Life Sci. 1975;17:803–811. doi: 10.1016/0024-3205(75)90538-x. [DOI] [PubMed] [Google Scholar]
- 21.Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. J Biol Chem. 1989;264:2195–2198. [PubMed] [Google Scholar]
- 22.Robson JM, Senior JB. The 5-Hydroxytryptamine Content of the Placenta and Foetus during Pregnancy in Mice. Br J Pharmacol Chemother. 1964;22:380–391. doi: 10.1111/j.1476-5381.1964.tb02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jawerbaum A, White V. Animal Models in Diabetes and Pregnancy. Endocr Rev. 2010;31(5):680–701. doi: 10.1210/er.2009-0038. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, et al. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J. 2001;355:425–429. doi: 10.1042/0264-6021:3550425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai M, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller CL, et al. Two complex genotypes relevant to the kynurenine pathway and melanotropin function show association with schizophrenia and bipolar disorder. Schizophr Res. 2009;113:259–267. doi: 10.1016/j.schres.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabi R, Serajee FJ, Chugani DC, Zhong H, Huq AH. Association of tryptophan 2,3 dioxygenase gene polymorphism with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:63–68. doi: 10.1002/ajmg.b.20147. [DOI] [PubMed] [Google Scholar]
- 29.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.