Abstract
Background:
Organic anion-transporting polypeptides (OATPs) are influx transporters that mediate intracellular uptake of selective endogenous and xenobiotic compounds. Identification of new molecular targets and discovery of novel targeted therapies is top priority for pancreatic cancer, which lacks any effective therapy.
Materials and methods:
We studied expression of OATP 1A2, 1B1, and 1B3 in pancreatic cancer tissue and in cell lines. Formalin-fixed paraffin-embedded biopsy material of 12 human pancreatic cancers was immunohistochemically assessed for protein expression of the three studied influx transporters. Immunohistochemistry was evaluated by experienced pathologists and quantified by use of an automated image analysis system. BxPC-3 and MIA PaCa-2 pancreatic cancer cell lines were used to quantify transcripts of OATP 1B1 and 1B3.
Results:
OATP 1A2, 1B1, and 1B3 proteins were found ubiquitously expressed in all studied cases. Quantification performed by HistoQuest system revealed that mean intensity was 53 for 1A2, 45 for 1B1, and 167 for OATP 1B1/1B3 on a range scale 0–250 units. At mRNA level, 1B1 and 1B3 were overexpressed in both studied cancer cell lines but not in normal pancreatic tissue.
Conclusion:
OATPs 1A2, 1B1, and 1B3 are highly expressed in pancreatic adenocarcinoma. We suggest that expression of these transporters in pancreatic cancer justify research efforts towards discovery of novel therapeutics targeting OATPs.
Keywords: organic anion-transporting polypeptides, targeted therapy, transporter
Introduction
Pancreatic carcinoma is the most deadly among cancers, ranked fourth as a cause of cancer-related deaths in the economically developed world.1,2 Characteristically, in Europe, new diagnoses of pancreatic cancer almost equaled the deaths caused by this disease in 2008.3
Surgery remains the gold standard for the treatment of pancreatic cancer, if diagnosed early. However, even in cases of surgically resected tumors, the outcome remains poor, and adjuvant therapy can offer marginal benefits.2,4 In advanced pancreatic cancer, the outlook is even worse. Extensive research has failed to produce any therapy efficient enough to substantially extend the median survival of treated patients beyond 6 months. Currently available therapies remain palliative on their intent.5–7 Therefore, identification of new molecular targets and discovery of novel targeted therapies is top priority for pancreatic cancer research.
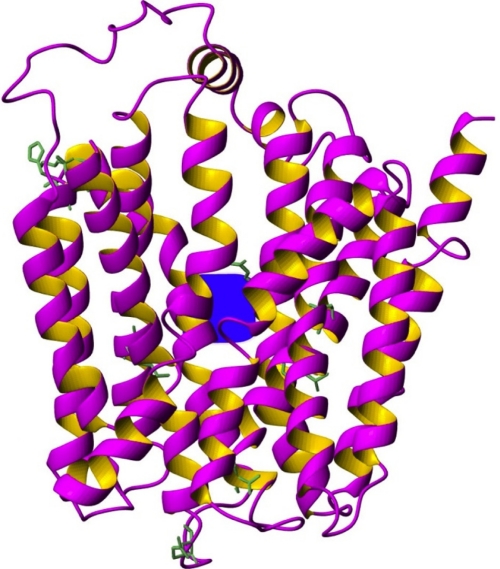
The organic anion transporting polypeptides (OATPs) superfamily comprises 11 polypeptide molecules that share a largely common structure with 12 putative transmembrane regions and a large extracellular loop between the 9th and 10th transmembrane domains8 (Figure 1). They operate as influx transporters that mediate the transmembrane uptake of various endogenous and xenobiotic anion compounds. Besides their characteristic expression in normal tissues, OATPs have been found overexpressed in several cancers, and it was such data that prompted us to undertake investigation of these transporters as potential therapeutic targets.9 Regarding pancreatic cancer, Abe et al, showed expression of OATP 1B3 in human pancreatic cancer on mRNA and protein level in a single case.10 To our knowledge, this is the first study that systemically assessed the expression profile of three OATPs (1A2, 1B1, and 1B3) in pancreatic cancer.
Figure 1.
Ribbon representation of the three-dimensional model of organic anion-transporting polypeptide 1B3. TM domains, a probable substrate binding site and the conserved amino acid side chains. The model was built by Modeller program (San Francisco, CA, USA) by using the structure template of the Escherichia coli glycerol-3-phosphate transporter (PDB 1pw4).24,25 In blue color the potential location of the substrate binding site (the putative translocation pathway) according to former mutagenesis studies is indicated.26 Conserved amino acid side-chains can also be seen on this figure.
Materials and methods
Tissue samples and anti-OATP antibodies
Formalin-fixed paraffin-embedded tissue samples of human pancreatic cancer were retrieved from the archives of the Department of Pathology, Chatzikosta General Hospital, Ioannina, Greece. The patients were diagnosed in the period 2000–2008. Their median age was 69 years; six were female and six male. Histologically, eight cases were diagnosed as poorly differentiated pancreas adenocarcinomas, and four cases had intermediate differentiation.
The samples were assessed for expression of OATP 1B1 and 1B1/1B3 by using the mESL and mMDQ antibodies respectively (PROGEN Biotechnik, Heidelberg, Germany). Expression of OATP 1A2 was evaluated in 11 samples by polyclonal anti-OATP 1A2 antibody (Atlas Antibodies AB, Stockholm, Sweden). A polyclonal anti-OATP 1B3 antibody (Atlas Antibodies AB, Stockholm, Sweden) was also used that recognizes C-terminal region of OATP 1B3, on the aim to monitor the expression of the 1B3 transporter as a single entity. All antibodies were diluted with Dako REAL™ Antibody Diluent (DAKO, Code S2022) to the final working concentration (Table 1). The DAKO Autostainer/PT link system was used for the immunostaining process.
Table 1.
Antibodies and technical data used for immunohistochemistry
| Ab symbol | OATP target | Clonality | Host species | Immunoglobulin subclass | Working dilution | Incubation time (minutes) | Epitope target | Company | Cat # |
|---|---|---|---|---|---|---|---|---|---|
| mMDQ | 1B1/1B3 | Monoclonal | Mouse | IgG1 | 1:10 | 70 | MDQHQHLNKTAESASSEKKKTRRC for 1B3 (N-terminus) MDQNQHLNKTAEAQPSENKKTRYC for 1B1 (N-terminus) |
Progen Biotechnik | 651140 |
| mESL | 1B1 | Monoclonal | Mouse | IgM | 1:5 | 70 | ESLNKNKHFVPSAGADSETHC (C-terminus) | Progen Biotechnik | 651139 |
| p1A2 | 1A2 | Polyclonal | Rabbit | IgG | 1:250 | 60 | SSVVGINTSYEGIPQDLYVENDIFADCNVDCNCPSKIWDP VCGNNGLSYLSACLAGCETSIGTGINMVFQNCS CIQTSG NSSAVLGLCDKGPDC (C-terminus) |
Atlas Antibodies | HPA027537 |
| p1B3 | 1B3 | Polyclonal | Rabbit | IgG | 1:250 | 60 | QGKDTKASDNERKVMDEANLEFLNNGEHFV PSAGTDSKTCNLD MQDNAAA (C-terminus) |
Atlas Antibodies | HPA004943 |
Abbreviation: OATP, organic anion-transporting polypeptide.
Cell lines
Two pancreatic cancer cell lines, BxPC-3 (CRL-1687TM) and MIA PaCa-2 (CRL-1420TM), were obtained from the American Tissue Culture Collection (ATCC, Manassas, VA) to be used in this study. MIA PaCa-2 originated from a male14 and BxPC-3 from a female donor.15 Cells were routinely cultured in RPMI-1640 medium (PAN Biotech, Aidenbach, Germany) supplemented with 10% fetal calf serum (Invitrogen Life Technologies, Paisley, Scotland) and 1% penicillin/streptomycin (Invitrogen) under standard culture conditions.
Immunohistochemistry
Tissue sections of 3–4 μm width were cut using a microtome and applied on microscope slides. Slides were incubated overnight at 65°C to enable optimal tissue–glass adhesion. Next, slides were immersed in DAKO’s PT-Link containing preheated (65°C) target retrieval solution, at pH 9 (DAKO Code S2375), treated at 93°C for 20 minutes in order to achieve deparaffinization, rehydration, and heat-induced epitope-retrieval (HIER). After cooling back to 65°C, slides were inserted in DAKO Autostainer system for the rest of the immunohistochemistry procedure. The mESL and mMDQ antibodies were applied on slides for 70 minutes, while for the polyclonal anti-OATP1A2 and anti-OATP1B3 antibodies incubation time was set to 60 minutes. The endogenous peroxidase was blocked using Daco REAL peroxidase blocking solution (Code S2023) for 10 minutes. DAKO’s special engineered Dextran backbone enriched with peroxidase molecules and goat secondary antibody molecules against rabbit and mouse immunoglobulins (Dako REAL™ EnVision™/HRP, Rabbit/Mouse ENV, Code Code K5007) was applied on slides for 20 minutes followed by a 5 minute Dako REAL™ DAB+ Chromogen (DAKO, Code K5007) detection system and a 2 minute hematoxylene (QS H-3404 Vector Laboratories) treatment. Finally, sections were dehydrated with alcohol/xylene baths and stabilized with mounting medium.
Immunohistochemistry evaluation
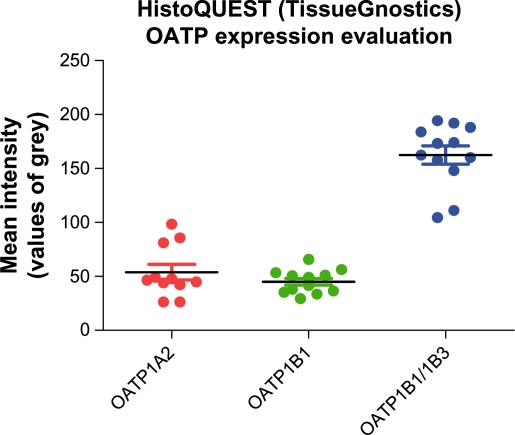
Immunostaining intensity was assessed by two experienced pathologists, who graded it, using endothelial cells and tissue macrophages as internal controls (Table 2). Quantitation was done by HistoQUEST (TissueGnostics, Vienna, Austria) system in values of gray from 0 to 25011,12 (Figures 2 and 3).
Table 2.
Immunohistochemical expression of OATPs. Score scale (0, 1+, 2+, 3+) reflecting antibody product expression intensity
| Patient number | Sex | Age | Differentiation | p1A2 | mESL | mMDQ |
|---|---|---|---|---|---|---|
| 1 | F | 71 | Intermediate | + | + | +++ |
| 2 | F | 67 | Poor | ++ | + | +++ |
| 3 | F | 74 | Poor | +++ | + | ++ |
| 4 | M | 56 | Poor | +++ | + | ++ |
| 5 | M | 80 | Intermediate | ++ | + | ++ |
| 6 | F | 55 | Poor | ++ | + | ++ |
| 7 | F | 78 | Poor | +++ | + | ++ |
| 8 | F | 70 | Poor | +++ | + | +++ |
| 9 | M | 72 | Intermediate | +++ | + | ++ |
| 10 | M | 67 | Poor | ++ | + | ++ |
| 11 | M | 65 | Poor | ++ | + | ++ |
| 12 | M | 70 | Intermediate | NA | + | ++ |
Abbreviations: F, female; M, male; NA, not applicable; OATP, organic anion-transporting polypeptide.
Figure 2.
OATP immunohistochemical expression intensity as was assessed by HistoQUEST (TissueGnostics) automated immunohistochemistry analysis system. The Y-axis represents mean intensity measured in values of grey scale from 0 to 250.
Abbreviation: OATP, organic anion-transporting polypeptide.
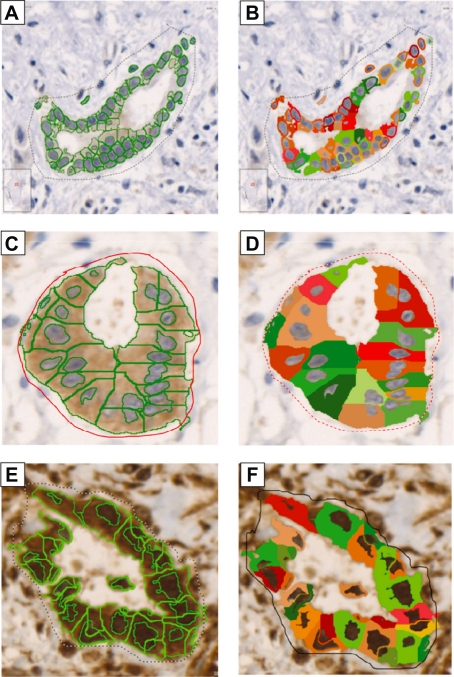
Figure 3.
HistoQUEST (TissueGnostics) expression analysis. Pictures A, C, and E represent the identification and segmentation of cells and nuclei. Pictures B, D, and F demonstrate the diaminobenzidine signal intensity for each cell in the studied area.
SLCO1A2, SLCO1B1, and SLCO1B3 mRNA expression quantification
Total RNA was isolated from both cell lines grown to subconfluency using the Trizol reagent (Invitrogen). We also used human liver RNA (purchased from Stratagene La Jolla, USA) and human pancreas RNA (purchased from Clontech, Saint-Germain-en-Laye, France) for control purposes.
The concentration, purity, and integrity of RNA samples were determined on a Nanodrop ND-1000 (Kisker-Biotech, Steinfurt, Germany) and by agarose gel electrophoresis.
Reverse transcription of total RNA to cDNA (2 μg) was done by using the high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, USA) as recommended by the manufacturer. The quantification of SLCO/OATP mRNA transcripts was done by using TaqMan® real-time PCR method (Applied Biosystems). The following TaqMan assays were used: hs00272374_m1 for OATP 1A2 and hs00251986_m1 for OATP 1B3. For accurate normalization of real time RT-PCR data we included four reference genes in the analysis, namely 18S rRNA (PN 4310893E; Applied Biosystems), ACTB (PN 4326315E; Applied Biosystems), CYC1 (PrimerDesign Ltd, Southampton, UK), and UBC (PrimerDesign Ltd). The target gene amplification mixture contained 5 μL 2× TaqMan® Gene Expression PCR Master Mix (Applied Biosystems), 0.5 μL of the appropriate Gene Expression Assay, 10 ng template cDNA diluted in 2.5 μL nuclease free water, and 2 μL nuclease free water. 5′–3′ exonuclease activity of the DNA polymerase was measured with the ABI 7900HT Fast real time PCR system was equipped with SDS 2.3 software (Applied Biosystems). All samples were amplified in duplicates. Results were imported into Prsim GraphPad for further analysis. Specimens exhibiting cycle threshold (Ct) values higher than 38 were considered to be negative, and comparable cDNA amounts in the experimental samples were calculated according to Hellemans et al.13 Values are expressed as n-fold difference according to a calibrator set to value 1. As a calibrator, RNA pooled from a panel of human tissue samples and cell lines analyzed was taken, and expression values in the calibrator were set to 1.
Results
Immunohistochemistry
The three studied polypeptides were found ubiquitously expressed in all studied biopsy cases. Both methods used confirmed extensive immunostaining of the entire cancer cell tissue with the antibodies used (Table 2; Figures 2 and 4).
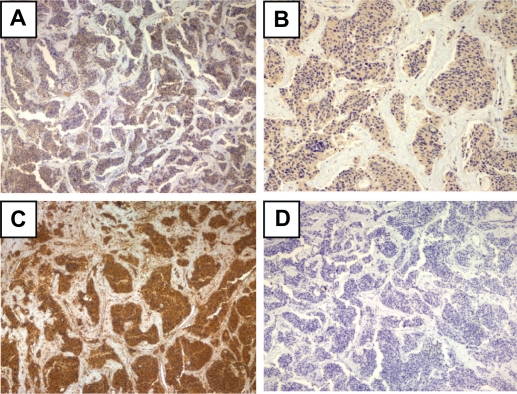
Figure 4.
Immunohistochemical staining of pancreatic adenocarcinoma tissue sample from a 77-year-old female patient diagnosed with poorly differentiated adenocarcinoma of the head of the pancreas. A) p1A2 antibody (×40), B) mESL antibody (×100), C) mMDQ antibody (×200), D) p1B3 antibody (×40).
Specifically, OATP 1A2 expression signal was weak in one sample and moderate to strong in all others. The HistoQUEST quantification analysis returned a mean intensity of 53.88 units with a standard error (SE) of 7.19. OATP 1B1 was found to be weakly expressed in all 12 cases with mean intensity 45.10 units (SE 3.15). Immunostaining for mMDQ (OATP 1B1/1B3) was proved the most intense. Nine cases demonstrated moderate expression, three cases stained strong, and HistoQUEST assessment showed a mean intensity of 167.90 units (SE 8.27). Interestingly, the anti-OATP 1B3 p1B3 antibody failed to detect expression of its nominal target in the same cancer tissue material (Figure 4), although it stained normal human liver tissue, which was used in this case as a positive control for p1B3 antibody (Figure 5).
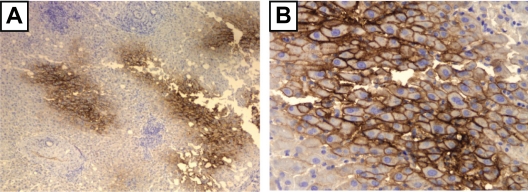
Figure 5.
Immunostaining with p1B3 of normal liver tissue with moderate steatosis of a 75-year-old individual who underwent subtotal hepatectomy because of a car accident trauma, shows cell membrane signal with increasing intensity towards central vein regions. A) ×40, B) ×200.
Cell lines
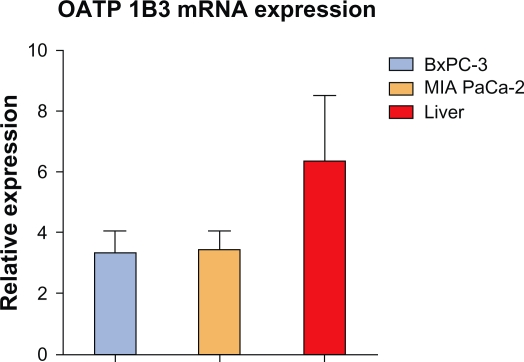
OATP 1B1 and 1B3 mRNA expression in the two cell lines was comparable to that in normal liver, which was taken as a control, because both these transporters are considered “liver-specific”. Their mRNA expression, however, in normal pancreas was either undetectable (OATP 1B1) or 30–60 times lower than in normal liver (OATP 1B3) (Figure 6).
Figure 6.
OATP 1B3 mRNA expression in BxPC-3, MIA PaCa-2 cancer cell lines and in human liver. Values are expressed as n-fold difference to the calibrator (pooled from the human tissue samples and cell lines) taken as value 1.
Abbreviation: OATP, organic anion-transporting polypeptide.
Discussion
The need for discovery of new molecular targets and the development of innovative targeted therapeutics in pancreatic cancer is indisputably illustrated by the fact that of the 36 orphan-designated products for pancreatic cancer by the United States Food and Drug Administration, none received marketing approval on this indication during the last 25 years.16 We considered that expression of organic anion-transporting polypeptides in a wide range of cancers along with their unique capacity for energy-independent intracellular transport of xenobiotics makes them reasonable targets for potential development of novel cancer therapeutics against hard-to-treat cancers.17–20
We opted to investigate the expression of OATP 1A2, 1B1, and 1B3 in pancreatic cancer, because of their common and distinctive ability to facilitate intracellular uptake of microcystin cyclopeptides, which are natural toxins produced from cyanobacteria.21 Microcystins exert cytotoxic effects through depletion of glutathione, generation of reactive oxygen species, and strong inhibition of protein phosphatases 1 and 2A.22 We surmise that these natural toxins are of potential pharmacological interest because they offer opportunities to engineer analogs optimized to induce selective cancer toxicity in OATP expressing tumors.9
The OATPs investigated in this study were all found to be ubiquitously expressed in pancreatic cancer: OATP 1A2, 1B1, and 1B3 protein expression was documented in biopsy samples and OATP 1B1 and 1B3 mRNA in cell lines. Quantification analysis showed an increased expression of 1B1/1B3 compared with 1A2 and 1B1, which indicates enhanced expression potential for OATP 1B3 in these tumors. It should be noted that the inability to detect OATP 1B3 C-terminal region with p1B3 antibody opposes the strong positive mMDQ signal in the same samples, which confirmed the presence of the 24 amino acid N-terminal epitope of OATP 1B3. Interestingly, we obtained similar results in a colon cancer study in which we identified an abundant presence of 1B3 protein when studied with mMDQ monoclonal antibody but not with the p1B3 antibody.23 We speculate that these findings might possibly be related to unknown mutation near the C-terminal end of 1B3 polypeptide in cancers. This is currently under investigation in our laboratory.
Finally, we consider that demonstration of OATP 1B3 and 1B1 mRNA expression in BxPC-3 and MIA PaCa-2 human pancreatic cell lines flags them as appropriate candidates for in-vitro studying of OATP targeted anticancer compounds.
Acknowledgments
This research was funded by the “Heraklitos II” program of the Operational Program for Education and Initial Vocational Training of the Hellenic Ministry of Education under the 3rd Community Support Framework and the European Social Fund.
Special thanks are given to University of Ioannina Cancer Biobank Center and the Pathology Department of Hatzikosta General Hospital in Ioannina for supporting this research, and Ms Sofia Kokouva for most valuable help.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a US population-based study. Am J Gastroenterol. 2007;102(7):1377–1382. doi: 10.1111/j.1572-0241.2007.01202.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. New Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group trial E2297. J Clin Oncol. 2002;20(15):3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 6.Burris H, Moore M, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Briasoulis E, Pavlidis N, Terret C, et al. Glufosfamide administered using a 1-hour infusion given as first-line treatment for advanced pancreatic cancer. A phase II trial of the EORTC-new drug development group. Eur J Cancer. 2003;39(16):2334–2340. doi: 10.1016/s0959-8049(03)00629-4. [DOI] [PubMed] [Google Scholar]
- 8.Kullak-Ublick GA, Hagenbuch B, Stieger B, et al. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109(4):1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- 9.Sainis I, Fokas D, Vareli K, Tzakos A, Kounnis V, Briasoulis E. Cyanobacterial cyclopeptides as lead compounds to novel targeted cancer drugs. Marine Drugs. 2010;8(3):629–657. doi: 10.3390/md8030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe T, Unno M, Onogawa T, et al. LST-2, A human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120(7):1689–1699. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- 11.Steiner GE, Ecker RC, Kramer G, Stockenhuber F, Marberger MJ. Automated data acquisition by confocal laser scanning microscopy and image analysis of triple stained immunofluorescent leukocytes in tissue. J Immunol Methods. 2000;237(1–2):39–50. doi: 10.1016/s0022-1759(99)00240-9. [DOI] [PubMed] [Google Scholar]
- 12.Kramer G, Steiner GE, Neumayer C, et al. Over-expression of anti-CD75 reactive proteins on distal and collecting renal tubular epithelial cells in calcium-oxalate stone-forming kidneys in Egypt. BJU Int. 2004;93(6):822–826. doi: 10.1111/j.1464-410x.2003.04751.x. [DOI] [PubMed] [Google Scholar]
- 13.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8(2):R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fountzilas G, Gratzner H, Lim LO, Yunis AA. Comparative effects of selected drug combinations on the growth of a human pancreatic carcinoma cell line (MIA PaCa-2) J Natl Cancer Inst. 1986;76(1):37–43. [PubMed] [Google Scholar]
- 15.Tan MH, Nowak NJ, Loor R, et al. Characterization of a new primary human pancreatic tumor line. Cancer Invest. 1986;4(1):15–23. doi: 10.3109/07357908609039823. [DOI] [PubMed] [Google Scholar]
- 16.Braun MM, Farag-El-Massah S, Xu K, Coté TR. Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat Rev Drug Discov. 2010;9(7):519–522. doi: 10.1038/nrd3160. [DOI] [PubMed] [Google Scholar]
- 17.Bronger H, Konig J, Kopplow K, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65(24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 18.Liedauer R, Svoboda M, Wlcek K, et al. Different expression patterns of organic anion transporting polypeptides in osteosarcomas, bone metastases and aneurysmal bone cysts. Oncol Rep. 2009;22(6):1485–1492. doi: 10.3892/or_00000591. [DOI] [PubMed] [Google Scholar]
- 19.Muto M, Onogawa T, Suzuki T, et al. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer Sci. 2007;98(10):1570–1576. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276(13):9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- 21.Fischer WJ, Altheimer S, Cattori V, Meier PJ, Dietrich DR, Hagenbuch B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol Appl Pharmacol. 2005;203(3):257–263. doi: 10.1016/j.taap.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Bouaïcha N, Maatouk I. Microcystin-LR and nodularin induce intracellular glutathione alteration, reactive oxygen species production and lipid peroxidation in primary cultured rat hepatocytes. Toxicol Lett. 2004;148(1–2):53–63. doi: 10.1016/j.toxlet.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Kounnis V, Ioachim E, Svoboda M, et al. Investigations on organic anion-transporting polypeptides 1A2, 1B1 and 1B3 in colon cancer as potential targets for cancer therapy. EJC. 2010;S8(7):112. [Google Scholar]
- 24.Huang Y, Lemieux MJ, Song J, Auer M, Wang D-N. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301(5633):616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 25.Eswar N, Webb B, Marti-Renom MA, et al. Comparative Protein Structure Modeling Using MODELLER. John Wiley & Sons, Inc; 2001. [Google Scholar]
- 26.Gui C, Hagenbuch B. Amino acid residues in transmembrane domain 10 of organic anion transporting polypeptide 1B3 are critical for cholecystokinin octapeptide transport. Biochemistry. 2008;47(35):9090–9097. doi: 10.1021/bi8008455. [DOI] [PMC free article] [PubMed] [Google Scholar]