Abstract
The need to simultaneously target infections with epidemiological overlap in the population with a single vaccine provides the basis for developing combination vaccines. Vibrio cholerae ghosts (rVCG) offer an attractive approach for developing vaccines against a number of human and animal pathogens. In this study, we constructed a multisubunit vaccine candidate co-expressing the serovar D-derived Porin B and polymorphic membrane protein-D proteins of Chlamydia trachomatis and evaluated its ability to simultaneously induce broad-based chlamydial immunity and elicit a vibriocidal antibody response to the Vibrio carrier envelope. Intramuscular (IM) immunization with the vaccine candidate elicited high levels of antigen-specific genital mucosal and systemic Th1 cell-mediated and humoral immune responses against heterologous serovars and strains, including serovars E, F, G, H and L. Also, in addition to the multisubunit vaccine, the single subunit constructs conferred significant cross protection against the heterologous mouse strain, C. muridarum. Furthermore, all mice immunized with rVCG vaccine constructs responded with a significant rise in vibriocidal antibody titer, the surrogate marker for protection in cholera.These findings demonstrate the ability of the multisubunit vaccine to inducecross protectivechlamydial as well asvibriocidal immunityand establish the possibility of developing a broadly efficacious Chlamydia-cholera combination vaccine.
Keywords: Chlamydia, cross-protection, combination vaccine, cholera
1. Introduction
Chlamydia trachomatis genital infections constitute a major public health challenge due to the significant morbidity that includes pelvic inflammatory disease, ectopic pregnancy and infertility [1, 2]. Of the 15 serovars of C. trachomatis, serovars D to K are chiefly responsible for urogenital infections and of these serovars, D, E, and F account for approximately 60 to 70% of these infections worldwide [3-6]. Although Chlamydia genital infection can be treated with antibiotics, the frequent asymptomatic infection especially in women precludes early diagnosis and treatment, making clinical presentation of sequelae often the first indication of infection. In the USA alone more than $2 billion is spent annually in the management of chlamydial genital infections [7]. Consequently, it has been suggested that the development and administration of a prophylactic or therapeutic vaccine capable of protecting against infection or even ameliorating severe disease would be the most promising and effective strategy to control Chlamydia[7, 8]. However, there is currently no licensed vaccine against Chlamydia.
The current immunologic paradigms for designing and evaluating chlamydial vaccines include the obligatory requirement for a T-helper Type 1 (Th1) immune response [7, 9] and an accessory antibody response. Additional requirements include the selection of a suitable vaccine candidate capable of inducing the required immune effectors and the development of an effective delivery system with adequate immunostimulatory properties to boost immune responses. The use of whole chlamydial agents as vaccines is unattractive due to the potential existence of immunopathogenic components [10]. Also, there are currently no tools to genetically modify chlamydiae to produce safe, attenuated vaccine strains. Therefore, the development of vaccines based on chlamydial subunit components is the current focus of chlamydial vaccine design.In this regard, the major outer membrane protein (MOMP),a key determinant of chlamydial genus and species specificity [9], has been one of the leading subunit vaccine candidates to date.However, experience with purified or recombinant MOMP as a protective antigen in several animal models [11-13] indicated that MOMP alone is inadequate, suggesting a need for a multisubunit approach or a more effective delivery system that will optimize the protective immune response. Besides, the sequence diversity in the surface-exposed variable domains of MOMP from different C. trachomatis serovars imposes restrictions on the latitude of protective immunity elicited against heterologous serovars[14]. Moreover, since a broadly protective subunit chlamydial vaccine would be preferred, additional subunit vaccine candidates that will elicit both optimal and broad-based immunity are being sought. In this respect, among the recently predicted immunogenic proteins [15, 16], the polymorphic outer membrane proteins (POMPs or Pmps) [17, 18] and the conserved porin B (PorB) family of membrane proteins [19] are likely vaccine candidates.PmpD and PorB are evolutionarily conserved antigens on the surface of chlamydial elementary (EBs) and reticulate (RBs) bodies [10, 19, 20] each with potential togenerate broad-based protective immunity [21]. We have previouslyshown that the novel recombinant Vibrio cholerae ghost (rVCG) platform is an effective carrier and delivery system for cloned C. trachomatis proteins, accommodating multiple subunits, and supporting the elicitation of protective chlamydial-specific immune responses[12, 21, 22].
Cholera is an acute diarrheal disease caused by Vibrio choleraeand is still a major cause of morbidity and mortalityin many parts of the world [23]. The recent cholera outbreak in Haiti with an attendant high case fatality rate [24] underlines the urgent need for appropriate intervention strategies that can be used in both endemic regions and during epidemics.Although access to safe drinking water and provision of adequatesanitation systems are essential to the long-term control of cholera,this is a distant goal for many developing countries[25]. The availability of an effective cholera vaccine would therefore, be a realistic means to control the disease in endemic regions. The problems associated with live attenuated oral cholera vaccines [26] and the finding that in humans, protection is afforded, primarily by antibodies to LPS [27] have fueled the quest to develop nonliving oral cholera vaccines. This is supported by the fact that, whether immune responses to V. cholerae O1 are generated by vaccination or environmental exposure, the best indicator of immune status is the level of serum bactericidal antibody [28]. VCG possess the normal array of cell surface antigens of live bacteria, in particular those of greatest vaccine significance and have been proposed as an alternative to heat or chemically killed cholera vaccines [29]. Indeed, previous studies have shown that anti-VCG sera produced in rabbits were highly protective against challenge with live bacteria [30, 31]. Considering the epidemiological overlap of endemic cholera and incidence of oculogenital chlamydial infections, the development of aneffective combination vaccine against Chlamydia and cholera will be highly desirable.
In this communication we report the development of a self-adjuvanting chlamydial vaccine candidate that was designed to include multiple antigens (PmpD and PorB) each with capacity to induce adequate protective immunity against infection.We demonstrated that the immune effectors generated in mice following IM immunization cross-reacted with different chlamydial serovars andprotected mice againstheterotypic infection with C. muridarum.Furthermore, immunized mice responded with a significant rise in vibriocidal antibody titer against the Vibrio carrier envelope. These results may have major implications in the rational design and development of broadly protective chlamydial as well as combination vaccines targeted for human use.
2. Materials and Methods
2.1.Construction of vaccine vectorsand expression ofvaccine antigens
PmpD and PorB cDNA were obtained from serovar D Chlamydia genomic DNA by PCR. The pKS-PmpD and pKS-PorB single vaccine vectors harboring the PmpD or PorB coding sequences were constructed by inserting the amplified PmpD or PorB PCR product,respectively between the E’ and L’ anchorsof vector pKSEL5-2 using restriction sites incorporated into the primer sets. The resultant plasmids were designated as pKS-PmpD and pKS-PorB, respectively. Also, the pKS-PmpD/PorB vaccine vector harboring the PmpD and PorB coding sequences was constructed by sequentially inserting the amplified PmpD and PorB PCR productsinto vector, pKSEL5-2 to generate plasmid pKS-PmpD/PorB (Fig. 1a) in which thePmpD protein is expressed from the C-terminal E’ anchor and PorB is expressed as an N-terminal-L’ fusion protein. For the expression of PorB and PmpD, sequences were subcloned into the pMAL-p2x and pRSET-A vectors, respectively, and protein expression was detected by SDS-PAGE and immunoblotting analysis.
Figure 1.
Construction of membrane targeting plasmids pKS-PmpD/pPorB and pKS-gD2 and expression of recombinant chlamydial proteins. (a) The amplified PmpD and PorB genes were genetically fused to and in frame with the membrane spanning domains of genes E’ and L’ of phages PhiX174 and MS2, respectively. The HSV-2 gD2 gene was similarly inserted between LacZ’ and L’ in pKSEL5-2. In both constructs, PmpD/PorB and gD2 are under the transcriptional control of the lacPO promoter. Restriction and sequencing analysis confirmed the correct orientation and size of the cloned genes. (b) For the expression of PorB and PmpD, sequences were subcloned into the pMAL-p2x and pRSET-A vectors, respectively, and protein expression was detected using mAb to MBP (PorB-MBP) and Anti-Xpress™ (PmpD-XE), respectively due to the absence of antibodies to these proteins. (A). Lanes 1-2, rPorB-MBP expressed from plasmid pMAL-PorB at 2 and 3 h, respectively; lane 3, purified MBP; (B).Lane 1, MW markers; Lane 2, rPmpD-XE fusion protein expressed from plasmid, pRSET-PmpD.
2.2. Production of rVCG vaccines
The multisubunit vaccine candidate consisted of recombinant VCG expressing the chlamydial porin B (PorB) and polymorphic membrane (PmpD) proteins while the single candidates contained either PmpD or PorB. An rVCG construct expressing glycoprotein D from HSV-2, a chlamydial irrelevant antigen (rVCG-gD2) was used as an antigen control. Production of the rVCG vaccines was carried out by gene E-mediated lysis essentially as described previously [12]. Lyophilized VCGs were weighed and the number of CFU per milligram of VCG was estimated based on the total number of CFU in the culture medium at the highest absorbance attained before lysis. Ghost preparations were stored at room temperature until use.
2.3. Chlamydia stocks and antigens
In stock preparations of C. trachomatis serovars(D, E, F, G, H, L and MoPn) were used in this study. All stocks were previously titrated on HeLa cell monolayers followed by purification of EBs over renografin gradients and stored at -70°C. Chlamydial antigens were prepared by UV-inactivation of EBs for 3 h and stored at -70°C until used.
2.4. Mice
All mice used in these studies were of the C57BL/6 strain (female, aged 6 to 8 weeks) from The Jackson Laboratory (Bar Harbor, ME). They were housed in the animal facility of Morehouse School of Medicine and animal study protocols were performed in compliance with institutional IACUC and federal guidelines.
2.5. Immunization, challenge and analysis of protective immunity
Groups of mice were immunized intramuscularly (IM) with 50 μl PBS containing 2 mg of lyophilized rVCG-PmpD/PorB or rVCG-gD2 control (rVCG expressing glycoprotein D from HSV-2; a chlamydial irrelevant antigen) on days 0, 14 and 28 as previously described [22]. Also, two groups of mice were similarly immunized with rVCG-PmpD or rVCG-PorB. A dose of 1 mg of lyophilized rVCG corresponds to about 2 × 109cfu. Animals were anesthetized by intraperitoneal injection of 200 μl of a 5% sodium pentobarbital solution (Sigma-Aldrich, Milwaukee, WI) before vaccine administration.Two weeks after the last immunization, animals designated for efficacy studies were administered Depo Provera (2.5 mg/mouse; UpJohn Co., Kalamazoo, MI) to stabilize the estrous cycle and facilitate a productive infection and challenged intravaginally one week later with1.0 ×104 inclusion forming units (IFUs) of live C. muridarum (MoPn), the heterologous mouse strain, to assess cross protection. After challenge, mice were observed twice daily to monitor health status, such as clinical signs of adverse reaction to infection. To assess the level of infection, cervicovaginal swabs were collected from each animal every 3 days following the challenge and chlamydiae were isolated from swabs in tissue culture by standard methods[11]. Experiments were repeated to contain 10-12 mice per group.
For immunogenicity studies, additional sets of 8 mice per group were intramuscularlyimmunized with the multisubunit vaccine construct and control and challenged 2 weeks after with1.0 ×105 IFU of serovar D. Ninety-eight days after initial primary challenge, a time when mice vaginally infected with live chlamydiae are usually susceptible to reinfection, mice were rechallenged with 2.5 × 104 IFU of serovar D per mouse. Serum and vaginal lavage samples were collected at different time points and stored as previously described [32].
2.6. Purification of CD4+ T cells
Eight weeks after immunization, animals designated for immunogenicity studies were sacrificed and the genital tract (GT), iliac lymph nodes (ILN) draining the genital tract and spleens (SPL) for systemic draining were harvested. Immune T cell-enriched cells were prepared from tissues of immunized and control mice by the nylon wool enrichment procedure described previously [33]. T cells were then purified by the Midi magnetic bead-activated cell sorting (MidiMACS) purification system, by positive selection of CD4+ T cells, using CD4-specific MACS microbeads (Miltenyi Biotech, Auburn, CA). The purity of the CD4+ T cell population was determined to be at least >95% by flow cytometry using an APC-conjugated anti-CD4 monoclonal antibody (Pharmingen, San Diego, CA). A separate pool of splenocytes prepared from naive animals and treated with mitomycin C (25 μg/107 cells) for 20 min or γ-irradiated (3,000 rads) was used as a source of antigen-presenting cells (APCs).
2.7. Detection of cytokine production by ELISA
The level of Chlamydia-specific Th1 and Th2 response was assayed by measuring the antigen-specific Th1 (IFN-γ) and Th2 (IL-4, IL-5) cytokine production by each cell population, respectively.Briefly, purified CD4+ T cellswere platedin triplicate wells of 96-well tissue culture plates at 2 × 105 cells/well and cultured with APCs (2 × 105) and 10 μg/ml of the respective chlamydial serovar antigen for 5days. Control cultures containedAPCsand T cells without antigen. At the end of the incubation period, supernatants were harvested and assayed forcytokines using the Bio-Plex cytokine assay kit in combination with the Bio-Plex Manager software (Bio-Rad, Hercules, CA). Themean and SD ofall replicate cultures were calculated.
The experiment was repeated three times.
2.8. Measurement of T cell proliferation
Purified immune T cells were assessed for their ability to proliferate in response to in vitro restimulation in culture with MoPn or serovar D (positive control) chlamydial antigen using the 5-Bromo-2’-deoxy-uridine (BrdU) cell proliferation assay procedure described previously[34]. T cells cultured in the absence of chlamydial antigen served as internal control.
2.9. Antibody and antibody isotype determinations
Blood samples were collected by retro-orbital plexus puncture and vaginal lavage was obtained by flushing the vaginal vault with 100 μl of PBS before immunization and on week8 after the last boost. The amount of specific antibodies (IgG2a and secretory IgA) in sera and vaginal washes against different chlamydial serovar antigens was measured by a standard ELISA procedure described previously [32]. Plates were incubated with HRP-conjugated goat anti-mouse IgA or IgG isotype (Southern Biotechnology Associates, Inc., Birmingham, Ala.) for 1 h and developed with 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS). The optical density was measured at 490 nm on a Microplate reader. Results, generated simultaneously with a standard curve, display data sets corresponding to absorbance values as mean concentrations (ng/ml) ± SD and represent the mean values from triplicate experiments.
2.10. Bactericidal (Vibriocidal antibody titer)
Serum samples from mice immunized with rVCG-PmpD/PorB and rVCG-gD2 or PBS controlswere tested for the presence of IgG antibodies and antibodies with complement-dependent bactericidal activity, using an assay described previously[30]. Bactericidal endpoints (vibriocidal titers) represent the serum dilutions capable of lysing 50% of the indicatorbacteria.
2.11. Statistical analysis
The levels of Th1/Th2 cytokines, secretory IgA, and IgG2a in the ILN and serum samples from different experiments as well as the level of protection conferred by the two vaccine constructs were compared by Student's t test. The results were expressed as mean ± standard deviation (SD). Tests were performed using SigmaStat software (SPSS Inc.). A value of p≤ 0.05 was considered significant.
3. Results
3.1. Construction of vaccine vectors and expression of vaccine antigens
The single vaccine vectors, pKS-PmpD and pKS-PorB were constructed to contain the coding sequences for the mature PmpD and PorB proteins and in frame with the E’ and L’ membrane anchors, respectively. Also, the multisubunit vaccine expression vector, pKS-PmpD/PorB, was constructed such that the respective antigen coding regions were inserted between and in frame with the E’ and L’ membrane anchors under the transcriptional control of the lac promoter (Fig. 1a). Sequencing results confirmed that the cloned genes were in frame with the fusion anchors and that the integrity of the newly generated plasmid constructs was maintained. Following transformation of V. cholerae 01strain H1 with the subcloned plasmid vectors, expression of the recombinant proteins (rPmpD and rPorB) was confirmed by Western immunoblotting analysis using mAb to MBP (PorB-MBP) and Anti-Xpress™ (PmpD-OM), respectively (Fig. 1b) due to the absence of antibodies to these proteins. Production of rVCG expressing vaccine antigens was achieved by gene E-mediated lysis as described previously [30]. Lyophilized ghost preparations were stored at room temperature until used.
3.2. Induction of cross-reactivechlamydial-specific genital mucosal Th1/Th2 cytokineresponses by the multisubunit vaccine candidate
Immune T cells purified from the genital tract draining iliac lymph nodes (ILN) ILN of immunized mice at week 8 after immunization were analyzed for chlamydial-specific Th1/Th2 cell responses by measuring the amount of Th1 and Th2 cytokine secretion upon restimulation with a panel of different chlamydial serovar antigens. The results revealed that the multisubunit vaccine candidate induced the secretion of high levels of genital mucosal Th1-type cytokine responsesagainst the different C. trachomatisserovar antigens tested;IFN-γ levels were significantly higher (p< 0.05) than IL-5 levels, indicating the preferential induction of antigen-specific Th1 cells in the genital mucosa (Fig. 2). Significantly higher (p< 0.05) amounts of Chlamydia-specific IFN-γ were produced by immune T cells following restimulation with all the serovar antigens tested compared to T cells derived from rVCG-gD2 (control)-immunized mice (data not shown).Also, comparatively higher levels of the inflammatory cytokine, IL-17 were produced by immune T cells in relation to IL-10 after restimulation with the different chlamydial serovar antigens, suggesting thatthis inflammatory cytokinemay be a factor in protection against genital chlamydial infection.
Figure 2.
Cross-protective chlamydial-specific genital mucosal Th1/Th2 cytokineresponses. Groups of mice were immunized and boosted as described in the materials and methods section. At week 8 postimmunization, T cells were purified and pooled from the ILNs of immunized mice and controls and restimulated in vitro with MoPn and a panel of human chlamydial serovar antigens (UV-irradiated EBs; 10 mg/ml). The amount of local mucosal Th1 (IFN-γ) and Th2 (IL-5) as well as IL-10 and IL-17 cytokines contained in supernatants of culture-stimulated cells was measured using Bio-Plex cytokine assay kit in combination with the Bio-Plex Manager software. The concentration of the cytokines in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (±?S.D.) for triplicate cultures for each experiment. The controls (cultures without antigen and from rVCG-gD2 antigen control) did not contain detectable levels of cytokine and so the data were excluded from the results. The results are from two independent experiments. Significant differences between Th1 and Th2 cytokines (IFN-γ and IL-5) are indicated by asterisk (P <0.05).
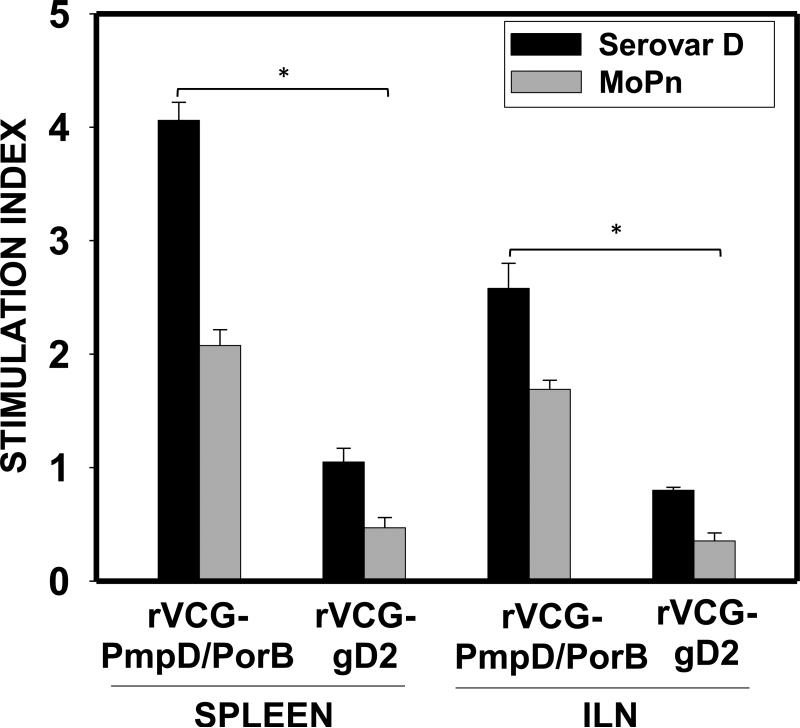
To confirm the cross-reactivity of serovar D-activated T cells with other chlamydial strains, purified immune T cells from vaccinated mice were also assessed for their ability to proliferate in response to in vitro restimulation with the heterologous C. muridarum by the BrdU incorporation assay. The result showed that the serovar D multisubunit vaccine-derived immune T cells proliferated in response to restimulation with boththe homologous serovar D and theheterologous C. muridarum (MoPn) antigens, indicating cross reactivity (Fig. 3). Also, the vaccine immunized-micehad significantly higher (p< 0.05) T cell proliferative responses in both the mucosal and systemic compartments evaluated compared to controls [SI, 2.58-4.06 (serovar D) and 1.69-2.08 versus 0.354-0.47 (MoPn)].
Figure 3.
Antigen-specific proliferative response. Eight weeks after the last boost, genital tract and splenic CD4+ T cells from groups of immunized mice were restimulated in vitro with C. trachomatis serovar D orC.muridarum (MoPn) antigen for 5 days. The antigen-specific proliferative response was determined using the BrdU incorporation assay; incorporation was detected by addition of ABTS substrate and the optical density was read at 405 nm. Results are expressed as the stimulation index (SI), the ratio between absorbance values of stimulated and non-stimulated cells and the bars represent the mean and S.D. of three independent experiments. *p<0.05 (rVCG-PmpD/PorB versus rVCG-gD2 control).
3.3. Induction of cross-reactive chlamydial-specific genital mucosal IgA and gG2a antibody responsesby the multisubunit vaccine candidate
Specific antibody responses, elicited 8 weeks after immunization with rVCG-PmpD/PorB or rVCG-gD2 (control),were measured by titrating the serum and vaginal secretions of vaccinated mice against a panel of different chlamydial serovar antigens, using an ELISA assay. Significant (P< 0.05) levels of IgA and the Th1-associated IgG2a antibodies to the different chlamydial serovars were detected in vaginal wash and serum of mice immunized with the multisubunit vaccine candidate compared to controls (Fig. 4). In addition, IgG2a levels in serum against all the serovar antigens tested were always higher than those from vaginal secretions.
Figure 4.
Chlamydial-specific IgA and IgG2a antibody responses induced 8 weeks postimmunization. Groups of mice were immunized and boosted as described above. Vaginal lavage and serum samples were obtained 8 weeks post immunization and pooled for each group. The concentration of antibodies elicited in genital lavage (mucosal, A) and serum (systemic, B) samples was assessed by an antibody ELISA. Results generated simultaneously with a standard curve, display data sets corresponding to absorbance values as mean concentrations (ng/ml) ± SD of triplicate cultures for each experiment. The results are from two independent experiments. *Statistically significant (p< 0.05) differences between IgG2a and IgA antibodies.
To assess the specific memory antibody responses, the levels of mucosal and systemic IgA and IgG2a antibody responses were measured two weeks after challenge (2.5 × 104 IFU/mouse) of previously immunized mice. The results (Fig. 5) showed that high levels of secretory IgA and IgG2a antibodies to the different chlamydial serovar antigens tested were detected in vaginal washes and serum 2 weeks after challenge, indicating that the infection driven recall responses were cross-reactive as well. Although in general, post-challenge mucosal and systemic IgA and IgG2a antibody levels were comparable to those obtained at week 8 postimmunization, the systemic IgG2a responses to serovars D and F elicited 2 weeks postchallenge were lower than post-week 8 levels (Fig. 4 and 5). In addition, the amount of the Th1-associated IgG2a antibodies secreted in serum 2 weeks after the primary challenge was significantly higher (p< 0.05) than that secreted into the vaginal mucosa.
Figure 5.
Memory antibody responses elicited 2 weeks postchallenge. Groups of mice were challenged intravaginally with live chlamydiae 2 weeks after immunization and vaginal lavage and serum samples obtained 14 days after were pooled. The amount of memory antibodies elicited in genital lavage (A) and serum (B) samples was assessed by an antibody ELISA. Results generated simultaneously with a standard curve display data sets corresponding to absorbance values as mean concentrations (ng/ml) ± SD of triplicate cultures for each experiment. The results are from two independent experiments. *Statistically significant (p< 0.05) differences between IgG2a and IgA antibodies.
To further establish if the long-term mucosal and systemic humoral recall responses are cross reactive, serum and vaginal secretions obtained from immune mice rechallenged 98 days after the initial primary challenge were evaluated for the magnitude of antibodies elicited. The results showed that high levels of secretory IgA and IgG2a antibodies to different chlamydial serovars were detected in vaginal wash and serum, 14 days after rechallenge indicating that the recall responses were cross-reactive (Fig. 6). Also, there were significantly (p< 0.05) higher levels of both mucosal and systemic IgA and IgG2a antibodies compared to controls (Fig. 6). Furthermore, both mucosal and systemic IgG2a levels were significantly higher (p< 0.05) than IgA levels (Fig. 6).
Figure 6.
Long-term memory antibody responses elicited 2 weeks after genital chlamydial reinfection. Previously immunized and challenged mice were rechallenged intravaginally 98 days after (i.e. after mice have completely resolved the primary infection) with live chlamydiae. Vaginal lavage (A) and serum (B) samples collected on day 14 after the rechallenge infection were pooled and used to determine chlamydial-specific antibody levels against whole chlamydial antigen by ELISA. Results were generated simultaneously with a standard curve, and display data sets corresponding to absorbance values as mean concentrations (ng/ml) ± SD of triplicate cultures from two independent experiments. Statistically significant (p< 0.05) differences between IgG2a and IgA antibodies are indicated by asterisk.
3.4. Immunization with serovar D-derived chlamydial antigens induces cross protection against heterologous MoPn (murine strain) challenge infection
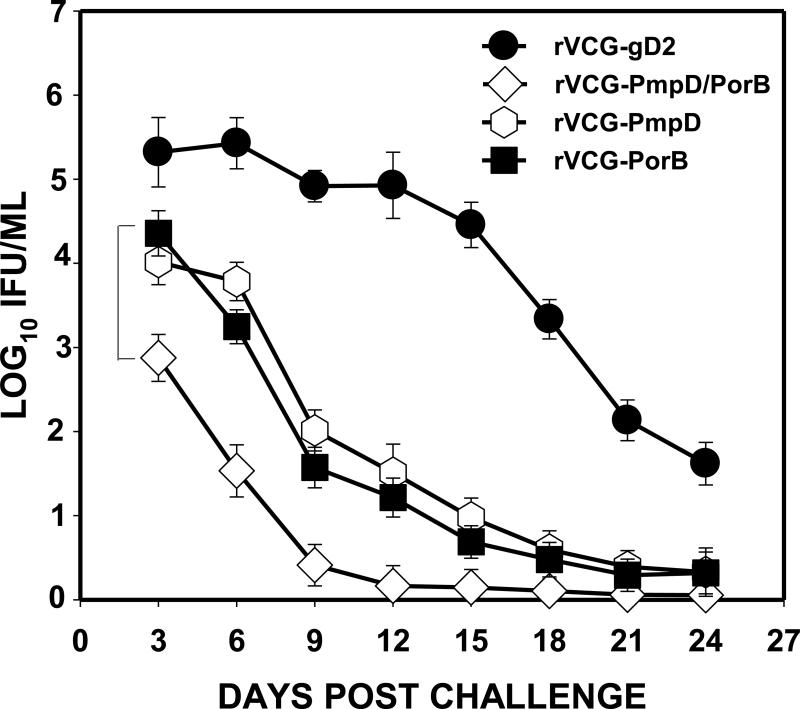
Since the ultimate goal of vaccination is to provide vaccinees protection against infection by different chlamydial serovars,animals immunized with the serovar D-derived vaccine constructs were challenged with the heterologous C. muridarum (MoPn) strain two weeks after the last immunization and periodically monitored for clearance. Figure 7 shows that mice immunized with the single and multisubunit vaccine constructs were protected against intravaginal challenge with the heterologous C. muridarum strain. The results also showed thatmice immunized with the multisubunit candidate vaccine shed about 2.5-log lower chlamydial IFUs than the controls (rVCG-gD2) as early as 3 days postchallenge and at least 1-log lower IFUs than the single subunit constructsup to 15 dayspostchallenge.The degree of prevention of bacterial colonization by the two single subunit vaccines was comparable.
Figure 7.
Cross protection of vaccine-immunized mice against genital challenge with C. muridarum (MoPn). Groups of immunized mice were challenged intravaginally with 1.0 × 105 IFU of live chlamydiae 2 weeks after the last immunization. One week prior to challenge, mice were administered Depo Provera to stabilize the estrous cycleand facilitate a productive infection. Infections were monitored by cervicovaginal swabbing of individual animals every 3 days and Chlamydia was isolated from swabs in tissue culture and enumerated. The data show the mean recoverable IFUs expressed as log10 IFU/ml ± S.D. Differences between control and experimental groups were compared by Student's t test at p< 0.05. The experiment was repeated to contain 10-12 mice per group.
3.5. Induction of bactericidal antibodies
Pre- and post-immunization sera from rVCG vaccine- and control-immunized mice weretitrated for Vibrio-specific IgG antibodies against heat-inactivated whole cell vibrios (WCV) and bactericidal activity against the homologous H1strain ofV. cholerae O1. IgG antibodies to WCV were detected in the sera of all immunized mice. No Vibrio-specific IgG antibodies were detected in preimmune sera. A greater than 4-fold increase in specific IgG antibody between pre- and post-immune sera 8 weeks after the last boost was observed. In addition, all post-immunization sera showed strong bactericidal activity against the homologous O1 serogroup of V. cholerae with titers of 5,120-20,480 compared to preimmune sera (titer, <10).
Discussion
This study describes the development of a broadly protective Chlamydia-cholera combination vaccine candidate based on the novel rVCG delivery platform. In addition to an effective delivery system, the development of a broadly protective chlamydial vaccine requires the identification ofsuitable immunogenic antigen(s) that will elicit the immune effectors capable of cross-reacting with different chlamydial serovars. The design of the multisubunit vaccine described here is based on the capacity of VCG to simultaneously deliver multiple vaccine antigens to the immune system andprovides protection based on two different chlamydial antigens.Simultaneous expression of multiple subunit antigens increases the opportunity for epitope diversity for a broad coverage of different chlamydial serovars and enhanced immunogenicity. On the other hand, the single subunit vaccines provide a straightforward approach to demonstrate that each of the antigens in the vaccine is individually sufficient to afford a high level of protective immunity. The chlamydial PmpD and PorB were selected as vaccine targets in this study because they are highly immunogenic antigens on the surface of elementary and reticulate bodies [20, 21, 35, 36] and unlike MOMP, could generate both species- and genus-specific neutralizing antibodies. Also, both proteins are evolutionarily conserved and involved in chlamydial attachment to host cells [19, 37], suggesting epitope conservation and the likelihood that immunity induced by PmpD and PorB may offer protection against all C. trachomatis serovars.
Immunologic evaluation revealed that IM immunization of mice with the multisubunit vaccine candidate induced local mucosal and systemic CD4+ T cells,which secreted high levels of IFN-γ and IL-17, and cross-reacted with a panel of heterologous human and mouse chlamydial serovar antigens.Also the immune T cells proliferated in response to in vitro restimulation with the heterologous C. muridarum antigen. This shows that the serovar D-derived rVCG vaccine elicited chlamydial-specific IFN-γ- and IL-17-secreting T cells, which responded torestimulation with different chlamydial serovar antigens. The importance of IFN–γ– secreting CD4+ T cells during Chlamydia infection has previously been demonstrated in both human clinical and experimental animal model studies [7, 9, 38]. Th17 cells are independently regulated CD4+ T cells characterized by the production of IL-17 and IL-22 and have been shown to play a rolein protection against certain intracellular pathogens[39, 40], including C. muridarum respiratory infections [41-43]. In vivo neutralization of IL-17 wasrecently reported to significantly enhance replication of C. muridarum and decreased mouse survival[41, 43]. Furthermore, a recent study reportedhigherconcentrations of IL-17and IL-22 in the humangenital mucosa after infection with C. trachomatiscompared to IFN-γ, indicating that IL-17 and IL-22 might be more specific of local C. trachomatis infection than IFN-γ [44]. Our results showing the secretion of high levels of IL-17 by vaccine-induced immune CD4+ T cells (but not control T cells) support these reports and suggest a role for Th-17 in protection against genital chlamydial infection.However, the role of IL-17 in immunity is yet unfolding and not completely understood. In addition, while the significance of the dramatically high IL-17 response observed with serovar L is unclear, it may likely be related to the invasiveness of serovar L relative to the other human serovars.Immunization with the multisubunit vaccine also elicited significant local mucosal and systemic IgA and IgG2a antibody responses, detectable in vaginal secretions and serum that cross-reacted with a panel of heterologous human and mouse chlamydial serovar antigens. Although likely important, the precise role that antibodies play in protection against Chlamydia, as well as whether they are involved in cross-protection against heterologous chlamydial serovars, remains unclear. However, previous reports suggest that the presence of specific neutralizing antibodies of IgG2a and IgA isotypes might be beneficial in controlling genital chlamydial infection [45]. In addition, antibody may contribute to protection by blocking the initial attachment of Chlamydia to epithelial cells thereby limiting dissemination to distant sites and enhancing chlamydial clearance. Besides, other studies suggest that the predominant role of antibodies in chlamydial clearance is in resistance to re-infection by enhancing rapid Th1 activation [46-48].
Efficacy analysis of the serovar D-derived vaccine constructs against the heterologous mouse strain, MoPn showed substantial reduction in vaginal shedding of Chlamydia in immunized mice compared to controls, with the multisubunit vaccine showing a protective advantage. Notably, each of the single subunit constructs individually afforded a sufficiently high level of protection against infection.A previous study reported that vaccination with a C. trachomatis-derived recombinant chlamydial protease-like activity factor (rCPAF) induced significant protection against C. muridarum infection[49]. However, the addition of rMOMP and/or rIncA did not significantly enhance the rCPAF+ IL-12-induced effect on bacterial clearance. This finding is in contrast to the results reported here as well as our previous resultsreporting the protective advantage of rVCG-based multisubunit constructs [21, 22]. While the reason for this difference is unknown, it maypossiblybe related to the nature of the delivery systememployed. Also, the significant reduction in the number of recoverable chlamydial IFUs and shortening of the time taken to clear the heterologous challenge infection underlines the potential of the rVCG-based multisubunit vaccine to induce broad-based protection against infection with different chlamydial serovars.
Another finding of the present study is the ability of the multisubunit vaccine to induce substantial genital tract immunity in the absence of external adjuvants.These results are consistent with our previous findings that VCGs represent a novel adjuvant system for inducing protective immunity against microbial infection at mucosal surfaces [12, 21, 22, 32]. These results are significant since subunit vaccines are often poorly immunogenic and require an adjuvant to function optimally.The adjuvanticity of rVCG may be due to a number of factorsrelatedto the surface characteristics of the Vibrio envelope complex.VCGs retain all the major immune stimulating constituents of the envelope, including lipopolysaccharide, peptidoglycan,flagellinand certain lipoproteins. These pathogen-associated molecular patterns trigger innate immune responses that ultimately lead to adaptive immunity. Moreover, intact nonliving bacterial cell envelopes are nontoxic [50] and are components of existing and acceptable nonliving human vaccines [51, 52]. The use of nonliving bacterial cell envelopes as delivery vehicles has a number of potential advantages: Since VCGs are empty cell envelopes they pose no pathogenic threats and unlike live attenuated vaccines present no horizontal gene transfer hazards. Consequently, VCG-based vaccines should be relatively safe compared to conventional vaccines. In addition, VCGs are environmentally stable at ambient temperatures when lyophilized, and unlike conventional vaccines have no cold chain requirements.
This study was also designed to test the ability of the chlamydialvaccine to exhibitserum vibriocidal activity against live V. cholerae. We hypothesized that immunization with a VCG-based chlamydial vaccine should in addition to providing protection against Chlamydia also provide protection against cholera. The results showed that immunization with the vaccine elicited substantial IgG antibody response against Vibrio surface components as well as strong vibriocidal activity in all vaccine-immunized mice. The vibriocidal titers reported in the present study are similar in magnitude to those obtained in our previous studies using VCG alone that protected mice/rabbits against Vibrio challenge [30, 31]. Previous studies have shown that antibodies to LPS are sufficient to mediate passive immunity in the infant mouse cholera model (IMCM) [53]. Thus, the observed bactericidal activity is likely to be mediated mainly by antibodies to LPS.Evidence indicates that secretory IgA (sIgA) antibodies directed to a single LPS carbohydrate epitope may be sufficient to protect against disease [54]. Serum vibriocidal antibodies rise in parallel with intestinal sIgA anti-LPS antibodies [55]. Vibriocidal antibodies are also inversely related to the risk of V. cholerae O1 diarrhea [56] and are thus surrogate markers for protection. Indeed, the potential to elicit serum antibodies with complement dependent bactericidal activity has proven to be the most reliable indicator of the protective efficacy of orally administered cholera vaccines [28]. The results indicate that VCG-based vaccines providing protection against Chlamydiacan also provide protection against cholera.
In summary, we have shown that IM immunization witha serovar D-derivedrVCG-basedmultisubunit chlamydial vaccine candidateprotectedmice against C. muridarum genital infection. The ability of the vaccine to induce immune responses against a panel of different human chlamydial serovars provides evidence that the development of a broad-based “universal” chlamydial vaccine is possible. The vaccine also elicited serum vibriocidal antibodiesthat are known to be surrogate markers of protection against cholera. These findings underscore the potential of VCG as a potent vaccine delivery system for developing combination vaccines.
Table 1.
Bactericidal responses induced by immunization with rVCG-PmpD/PorB and rVCG-gD2
| aSerum IgG antibody response: | bBactericidal titer of: | ||||
|---|---|---|---|---|---|
| Mouse | rVCG-PmpD/PorB (vaccine) | rVCG-gD2 (control) | Preimmune sera | rVCG-PmpD/PorB (vaccine) | rVCG-gD2 (control) |
| 1 | 0.35 | 0.52 | < 10 | 5,120 | 10,480 |
| 2 | 0.51 | 0.46 | <10 | 20,480 | 10,240 |
| 3 | 0.45 | 0.43 | <10 | 10,240 | 10,240 |
| 4 | 0.48 | 0.32 | <10 | 20,480 | 5,120 |
| 5 | 0.34 | 0.42 | <10 | 5,120 | 5,120 |
| 6 | 0.42 | 0.52 | <10 | 10,240 | 20,480 |
| 7 | 0.40 | 0.46 | <10 | 5,120 | 10,240 |
| 8 | 0.48 | 0.51 | <10 | 10,240 | 20,480 |
Serum IgG antibody response of mice to heat-killed whole cell vibrios (WCV) after IM immunization with rVCG vaccines. Results were normalized by subtracting preimmune absorbance values from those of immune sera. Serum samples were diluted 1: 100.
Values show bactericidal (vibriocidal) titers before (pre) and after (post) immunization with rVCG vaccine and control, against the homologous O1 serogroup, strain H1.
5. Acknowledgements
This work was supported by a Public Health Service grant AI41231 from the National Institutes of Health. The investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant #1 C06 RR18386 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Schachter J, Grayston JT. Epidemiology of Human Chlamydial Infections. In: Stephens RS, Byrne GI, Christiansen G, Clarke IN, Grayston JT, Rank RG, et al., editors. Chlamydial Infections. Berkeley; San Francisco, CA: 1998. pp. 3–10. [Google Scholar]
- 2.Brunham RC, Zhang DJ. Transgene as vaccine for Chlamydia. Am Heart J. 1999;138:S519–S22. doi: 10.1016/s0002-8703(99)70291-7. [DOI] [PubMed] [Google Scholar]
- 3.Singh V, Salhan S, Das BC, Mittal A. Predominance of Chlamydia trachomatis Serovars Associated with Urogenital Infections in Females in New Delhi, India. J Clin Microbiol. 2003;41(6):2700–2. doi: 10.1128/JCM.41.6.2700-2702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Chen X-S, Yin Y-P, Zhong M-Y, Shi M-Q, Wei W-H, et al. Distribution Study of Chlamydia trachomatis Serovars among High-Risk Women in China Performed Using PCR-Restriction Fragment Length Polymorphism Genotyping. J Clin Microbiol. 2007;45(4):1185–9. doi: 10.1128/JCM.02076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrovay F, Balla E, Nemeth I, Gonczol E. Genotyping of Chlamydia trachomatis from the endocervical specimens of high-risk women in Hungary. J Med Microbiol. 2009;58(6):760–4. doi: 10.1099/jmm.0.008607-0. [DOI] [PubMed] [Google Scholar]
- 6.Lima HEM, Oliveira MBP, Valente BGM, Afonso DAFP, DaRocha WDP, Souza MCMdP, et al. Genotyping of Chlamydia trachomatis From Endocervical Specimens in Brazil. Sexually Transmitted Diseases. 2007 September;34(9):709–717. doi: 10.1097/01.olq.0000258399.27873.d9. [Article] [DOI] [PubMed] [Google Scholar]
- 7.Igietseme JU, Eko FO, Black CM. Contemporary approaches to designing and evaluating vaccines against Chlamydia. Expert Rev Vaccines. 2003;2(1):129–46. doi: 10.1586/14760584.2.1.129. [DOI] [PubMed] [Google Scholar]
- 8.Stagg AJ. Vaccines against Chlamydia: approaches and progress. Mol Med Today. 1998;4(4):166–73. doi: 10.1016/s1357-4310(98)01232-5. [DOI] [PubMed] [Google Scholar]
- 9.Morrison R, Caldwell H. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70(6):2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunham RC, Peeling RW. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1994;3:218–33. [PubMed] [Google Scholar]
- 11.Su H, Parnell M, Caldwell HD. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against genital tract infection. Vaccine. 1995;13(11):1023–32. doi: 10.1016/0264-410x(95)00017-u. [DOI] [PubMed] [Google Scholar]
- 12.Eko F, Lubitz W, McMillan L, Ramey K, Moore T, Ananaba GA, et al. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine. 2003;21:1694–703. doi: 10.1016/s0264-410x(02)00677-1. [DOI] [PubMed] [Google Scholar]
- 13.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis Mouse Pneumonitis Major Outer Membrane Protein Can Elicit a Protective Immune Response against a Genital Challenge. Infect Immun. 2001;69(10):6240–7. doi: 10.1128/IAI.69.10.6240-6247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q, M-Sobrido L, Eko FO, Palese P, Garcia-Sastre A, Lyn D, Okenu D, Bandea C, Ananaba GA, Black CM, Igietseme JU. Live-attenuated influenza viruses as delivery vectors for Chlamydia vaccines. Immunology. 2007;122(1):28–37. doi: 10.1111/j.1365-2567.2007.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282(5389):754–9. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 16.Stephens RS. Chlamydial Genomics and Vaccine Antigen Discovery. J Infect Dis. 2000;181(Suppl 3):S521–S3. doi: 10.1086/315631. [DOI] [PubMed] [Google Scholar]
- 17.Grimwood J, Olinger L, Stephens RS. Expression of Chlamydia pneumoniae Polymorphic Membrane Protein Family Genes. Infect Immun. 2001;69(4):2383–9. doi: 10.1128/IAI.69.4.2383-2389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niessner A, Kaun C, Zorn G, Speidl W, Türel Z, Christiansen G, et al. Polymorphic membrane protein (PMP) 20 and PMP 21 of Chlamydia pneumoniae induce proinflammatory mediators in human endothelial cells in vitro by activation of the nuclear factor-kappaB pathway. J Infect Dis. 2003;188(1):108–13. doi: 10.1086/375827. [DOI] [PubMed] [Google Scholar]
- 19.Kubo A, Stephens RS. Characterization and functional analysis of PorB, a Chlamydia porin and neutralizing target. Molecular Microbiology. 2000;38(4):772–80. doi: 10.1046/j.1365-2958.2000.02167.x. [DOI] [PubMed] [Google Scholar]
- 20.Crane DD, Carlson JH, Fischer ER, Bavoil P, Hsia R-c, Tan C, et al. From the Cover: Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. PNAS. 2006;103(6):1894–9. doi: 10.1073/pnas.0508983103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ifere G, He Q, Ananaba G, Lyn D, Lubitz W, Kellar K, et al. Immunogenicity and protection against genital Chlamydia infection and its complications by a multisubunit candidate vaccine. J Microbiol Immunol Infect. 2007;40:188–200. [PubMed] [Google Scholar]
- 22.Eko FO, He Q, Brown T, McMillan L, Ifere GO, Ananaba GA, et al. A Novel Recombinant Multisubunit Vaccine against Chlamydia. J Immunol. 2004;173(5):3375–82. doi: 10.4049/jimmunol.173.5.3375. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter C. The treatment of cholera: clinical science on the bedside. J Infect Dis. 1992;166:2–14. doi: 10.1093/infdis/166.1.2. [DOI] [PubMed] [Google Scholar]
- 24.Ivers LC, Farmer P, Almazor CP, Leandre F. Five complementary interventions to slow cholera: Haiti. Lancet. 2010 Dec 18;376(9758):2048–51. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 25.Mekalanos J, Waldor M, Gardel C, Coster T, Kenner J, Killeen K, et al. Live cholera vaccines: perspectives on their construction and safety. Bull Inst Pasteur. 1995;93:255–62. [Google Scholar]
- 26.Grandjean C, Boutonnier A, Dassy B, Fournier JM, Mulard LA. Investigation towards bivalent chemically defined glycoconjugate immunogens prepared from acid-detoxified lipopolysaccharide of Vibrio cholerae O1, serotype Inaba. Glycoconj J. 2009 Jan;26(1):41–55. doi: 10.1007/s10719-008-9160-6. [DOI] [PubMed] [Google Scholar]
- 27.Attridge S, Voss E, Manning P. Pathogenic and vaccine significance of toxin-coregulated pili of Vibrio cholerae El Tor. J Biotechnol. 1999;73(2-3):109–17. doi: 10.1016/s0168-1656(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 28.Levine M, Tacket C. Recombinant live cholera vaccines. In: Wachsmuth I, Blake P, Olsvik O, editors. Vibrio cholerae and cholera Molecular to global perspectives. ASM Press; Washington: 1994. pp. 395–413. [Google Scholar]
- 29.Eko FO, Witte A, Huter V, Kuen B, Furst-Ladani S, Haslberger A, et al. New strategies for combination vaccines based on the extended recombinant bacterial ghost system. Vaccine. 1999;17:1643–9. doi: 10.1016/s0264-410x(98)00423-x. [DOI] [PubMed] [Google Scholar]
- 30.Eko FO, Mayr UB, Attridge SR, Lubitz W. Characterization and immunogenicity of Vibrio cholerae ghosts expressing toxin-coregulated pili. JBiotechnol. 2000;83:115–23. doi: 10.1016/s0168-1656(00)00315-1. [DOI] [PubMed] [Google Scholar]
- 31.Eko FO, Schukovskaya T, Lotzmanova EY, Firstova VV, Emalyanova NV, Klueva SN, et al. Evaluation of the protective efficacy of Vibrio cholerae ghost (VCG) candidate vaccines in rabbits. Vaccine. 2003 Sept 8;21(25-26):3663–74. doi: 10.1016/s0264-410x(03)00388-8. [DOI] [PubMed] [Google Scholar]
- 32.Macmillan L, Ifere G, He Q, Igietseme J, Kellar K, Okenu D, et al. A recombinant multivalent combination vaccine protects against Chlamydia and genital herpes. FEMS Immunol Med Microbiol. 2007;49:46–55. doi: 10.1111/j.1574-695X.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 33.Igietseme JU, Rank RG. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. InfectImmun. 1991;59:1346–51. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eko F, Ekong E, Okenu D, He Q, Ananaba G, Black C, et al. Induction of immune memory by a multisubunit chlamydial vaccine. Vaccine. 2011 Feb 4;29(7):1472–80. doi: 10.1016/j.vaccine.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawa DE, Stephens RS. Antigenic Topology of Chlamydial PorB Protein and Identification of Targets for Immune Neutralization of Infectivity. J Immunol. 2002;168(10):5184–91. doi: 10.4049/jimmunol.168.10.5184. [DOI] [PubMed] [Google Scholar]
- 36.Kawa DE, Schachter J, Stephens RS. Immune response to the Chlamydia trachomatis outer membrane protein PorB. Vaccine. 2004;22(31-32):4282–6. doi: 10.1016/j.vaccine.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 37.Wehrl W, Brinkmann V, Jungblut PR, Meyer TF, Szczepek AJ. From the inside out - processing of the Chlamydial autotransporter PmpD and its role in bacterial adhesion and activation of human host cells. Molecular Microbiology. 2004;51(2):319–34. doi: 10.1046/j.1365-2958.2003.03838.x. [DOI] [PubMed] [Google Scholar]
- 38.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal Vaccination with a Secreted Chlamydial Protein Enhances Resolution of Genital Chlamydia muridarum Infection, Protects against Oviduct Pathology, and Is Highly Dependent upon Endogenous Gamma Interferon Production. Infect Immun. 2007;75(2):666–76. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scriba TJ, Kalsdorf B, Abrahams D-A, Isaacs F, Hofmeister J, Black G, et al. Distinct, Specific IL-17- and IL-22-Producing CD4+ T Cell Subsets Contribute to the Human Anti-Mycobacterial Immune Response. J Immunol. 2008;180(3):1962–70. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitta MGR, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, et al. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. The Journal of Clinical Investigation. 2009;119(8):2379–87. doi: 10.1172/JCI38813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol. 2009 Jul 15;183(2):1291–300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Chen Q, Moore J, Kolls JK, Halperin S, Wang J. Critical role of the interleukin-17/interleukin-17 receptor axis in regulating host susceptibility to respiratory infection with Chlamydia species. Infect Immun. 2009 Nov;77(11):5059–70. doi: 10.1128/IAI.00403-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, et al. IL-17/Th17 Promotes Type 1 T Cell Immunity against Pulmonary Intracellular Bacterial Infection through Modulating Dendritic Cell Function. J Immunol. 2009 Nov 1;183(9):5886–95. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 44.Jha R, Srivastava P, Salhan S, Finckh A, Gabay C, Mittal A, et al. Spontaneous secretion of interleukin-17 and -22 by human cervical cells in Chlamydia trachomatis infection. Microbes and Infection. 2011;13(2):167–78. doi: 10.1016/j.micinf.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Igietseme JU, Uriri IM, Kumar SN, Ananaba GA, Ojior OO, Momodu IA, et al. Route of Infection That Induces a High Intensity of Gamma Interferon-Secreting T Cells in the Genital Tract Produces Optimal Protection Against Chlamydia trachomatis Infection in Mice. Infect Immun. 1998;66(9):4030–5. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore T, Ananaba GA, Bolier J, Bowers S, Belay T, Eko FO, et al. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105(2):213–21. doi: 10.1046/j.0019-2805.2001.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of T-cell immunity: implications for vaccine strategies against intracellular pathogens. Expert Review of Vaccines. 2004;3(1):23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 48.Morrison S, Morrison R. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005 Dec 1;175(11):7536–42. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, Guentzel MN, Seshu J, Zhong G, Murthy AK, Arulanandam BP. Induction of Cross-Serovar Protection against Genital Chlamydial Infection by a Targeted Multisubunit Vaccination Approach. Clin Vaccine Immunol. 2007 December 1;14(12):1537–44. doi: 10.1128/CVI.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mader HJ, Szostak MP, Hensel A, Lubitz W, Haslberger AG. Endotoxicity does not limit the use of bacterial ghosts as candidate vaccines. Vaccine. 1997;15(2):195–202. doi: 10.1016/s0264-410x(96)00141-7. [DOI] [PubMed] [Google Scholar]
- 51.Clemens J, Sack D, Harris vL, F, Chakraborty J, Ahmed F, Rao M, et al. Field trial of oral cholera vaccines in Bangladesh: results from three year follow-up. Lancet. 1990;335:270–3. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 52.Anh DD, Canh DG, Lopez AL, Thiem VD, Long PT, Son NH, et al. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine. 2007;25(6):1149–55. doi: 10.1016/j.vaccine.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 53.Attridge SR, Rowley D. Prophylactic significance of non-lipopolysaccharide antigens of Vibrio cholerae. J Infect Dis. 1983;148:931–9. doi: 10.1093/infdis/148.5.931. [DOI] [PubMed] [Google Scholar]
- 54.Winner L, III, Mack J, Weltzin R, Mekalanos J, Kraehenbuhl J-P, Neutra M. New model for analysis of mucosal immunity:intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–82. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jertborne M, Svennerholm A-M, Holmgren J. Saliva, breast milk and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986;24:202–9. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clemens J, van Loon F, Sack D, Chakraborty J, Rao M, Ahmed F, et al. Field trial of oral cholera vaccines in Bangladesh: serum vibriocidal and antitoxic antibodies as markers of the risk of cholera. J Infect Dis. 1991;163:1235–42. doi: 10.1093/infdis/163.6.1235. [DOI] [PubMed] [Google Scholar]